
Global epidemiology of occult HBV infection
Introduction
This review has been to a large extent previously published by Science Press, Beijing, China: Allain JP, Fu H, Li C, et al. Occult Hepatitis B infection. 2015; page 32-48. This updated review is published with the agreement of Science Press.
The detection of HBV DNA in patients with chronic liver disease (CLD) negative for hepatitis B surface antigen (HBsAg) was first noticed in 1985 (1). Until the mid-1990s, this observation remained controversial with nearly equal number of studies reproducing or not the initial discovery (2). Such discrepancies were attributed to differences in epidemiology and performance of the now archaic methods utilized for HBV DNA detection. Nevertheless, the concept of occult HBV infection (OBI) progressively rooted and triggered a large number of studies confirming the reality and scientific importance of this new feature of this ‘old’ viral infection. In 2008, an international workshop was convened in Taormina, Italy, where a group of specialists led by G Raimondo defined OBI as: the presence of HBV DNA in the liver (with detectable or undetectable HBV DNA in the serum) of individuals testing HBsAg negative by currently available assays (3). It added that the amount of HBV DNA in the serum is usually very low (<200 IU/mL). As a result, the definition clearly indicated that the diagnosis of OBI was closely dependent on the performance of the key assays: HBsAg and HBV DNA. In the past 15 years, the sensitivity of both assays has progressively increased, decreasing the number of OBI with increased sensitivity of HBsAg assays and increasing this number with increased sensitivity of HBV DNA assays.
Performance of HBsAg assays
Since the first assays available in 1970, HBsAg testing has been steadily improving until enzyme immunoassays utilizing monoclonal antibodies reached in 2002 a sensitivity of 0.13–0.62 ng/mL for licensed assays and 0.07–0.12 ng/mL for three investigational assays since released for use by FDA (4). In 2006, a comparative evaluation of 17 HBsAg CE marked assays indicated 0.018 to 0.1 IU/mL sensitivity range for serotype AD and 0.012–0.11 IU/mL for AY (5). Table 1 summarizes the performance of current HBsAg assays utilized in articles reporting on OBI. Since then, several assays with higher sensitivity were developed and clinically evaluated that used chemiluminescence enzyme immunoassay (CLEIA) or chemiluminescence immunoassay (CLIA) (6-8). The limit of detection (LOD) reached 0.025 ng/mL compared to 0.2 ng/mL for CLIA. A modified CLEIA claimed to reach a sensitivity of 0.005 IU/mL (9). These improved assays were developed for two main purposes: improve the detection of the early infection window period (4,8) and monitor antiviral treatment (7,9,10). The latest assays claim to be able to replace HBV DNA detection in monitoring treated patients. However none of these assays specifically compared HBsAg and DNA in the circumstances of OBI. As shown below, the hope of LOD similar to HBV DNA was defeated by the very nature of OBI.
Table 1
| HBsAg | LOD IU/mL | HBV NAT | 95% LOD in individual samples |
|---|---|---|---|
| PRISM ×12 | 0.03 | Ultrio ×24 | 13 |
| Murex ×4 | 0.03 | Ultrio Plus ×2 | 3–4 |
| AxSYM ×7 | 0.1 | Cobas Ampliscreen ×6 | 3.4–5 |
| Architect ×2 | 0.03 | Roche MPX ×6 | 4–6.7 |
| BioRad ×2 | 0.1 | Artus ×2 | 3.8–50 |
| BioMerieux ×6 | 0.1 | In-house 1 | 20 |
| Wantai ×2 | 2 | In-house 2 | <6 |
| Kehua | 0.2 | In-house 3 | 20 |
| Huakang | 0.2 | In-house 4 | 20 |
| Xiamen | 0.1 | In-house 5 | 5 |
| Dade | 0.1 | In-house 6 | 20 |
The number following the × sign indicates the number of studies utilizing each particular assay. NAT, nucleic acid testing
In a range of circumstances, the major hydrophobic region (MHR) of the HBV surface protein can be mutated with amino acid changes potentially affecting detection with HBsAg assays. This situation is particularly frequent in OBI of genotype A2-D, less so in genotypes A1 and E (11). In addition to sensitivity, ability to detect variants is critical for the diagnosis of OBI. It is recommended to retest HBV DNA positive samples, anti-HBc positive with an alternative sensitive HBsAg assay that may more effectively detect particular HBsAg variants.
Performance of HBV nucleic acid testing (NAT)
The detection of HBV DNA was clearly key to the identification of OBI and commercial assays with increased sensitivity became recently available (12). The LOD of assays reported in articles on OBI are shown in Table 1. The impact of sensitivity was particularly illustrated in a study conducted in Hong Kong comparing two commercial assays (Ultrio and Ultrio Plus, Grifols) from the same manufacturer with LOD of 13 and 3 IU/mL, respectively (13). The yield of both OBI and window period cases doubled with the more sensitive assay applied to random blood donor samples. In general, assays enabling to quantify HBV DNA are derived from the detection assays but the dynamic range of quantification is higher than the 3–5 IU/mL LOD so that a significant proportion of positive HBV DNA samples are below the limit of quantification (LOQ). Since then, a new real-time PCR based assay was developed by Roche (Cobas CTX) with a claimed 95% LOD of 1.6 IU/mL or 7.4 copies/mL and LOD 50% of 0.3 IU/mL or 1.6 copies/mL. Such sensitivity should considerably increase OBI detection but at the same time make the assay highly susceptible to contamination (14). Irrespective of this issue, all reactive results with any HBV NAT need to be confirmed with an alternative assay of similar sensitivity. Short of an alternative test with sensitivity matching the screening method, 5–10 repeats of the screening assay on the same or an alternative sample has been advocated (15) arguing of the Poisson distribution of HBV DNA template in OBI samples. There are algorithms allowing to transform the number of repeat reactive into viral load at very low concentration (16). In addition, HBV serology can be helpful, particularly in excluding samples without anti-HBc or anti-HBs as likely false positive (17). This strategy has its own limitations since several studies have identified OBIs in anti-HBs only or serologically negative samples. Ultimately, follow-up of individuals with uncertain diagnosis of HBV DNA yield cases can differentiate between window period and OBI, although fluctuating levels of OBI HBV DNA between low positive and undetectable is frequently encountered (18). In several studies targeting HBV DNA+/HBsAg− blood donors, follow-up testing for HBV markers showed that 20–80% of OBI cases (mean 50%) remained HBV DNA positive 1–3 months after the index sample was collected (17-22).
Relation between HBsAg and HBV DNA levels
During the window period, two studies showed clearly the correlation between HBsAg and viremia (4,23). They also showed that below 300 IU/mL of HBV DNA, HBsAg was no longer detectable when tested with an assay with LOD 0.1 IU/mL. In this case, it appears that most of the detected HBsAg corresponds to full, infectious, complete virus Dane particles. In contrast, after chronic infection is established, there seem to be a switch in infected cells, enhancing S protein production in large but variable excess found in circulation as free or aggregated protein together with lipids forming pseudo-particles. These pseudo-particles are the majority of what is detected as HBsAg. As reported in several articles and shown in Figure 1, months post-infection, there is a poor correlation between HBsAg and HBV DNA that are both quantified in IU/mL against their respective international standard (24-28). Irrespective of genotype, but particularly frequent in genotype D, a substantial proportion of HBsAg positive samples (3–15%) carry no detectable HBV DNA. However, when increasing the NAT sensitivity, more of these samples are DNA positive, suggesting that the discrepancy is mostly related to assay sensitivity (14,29). In cases of OBI, among other mechanisms, specific amino acid substitutions in the S protein prevent the export of HBsAg explaining the lack of detection of HBsAg in circulation (30).
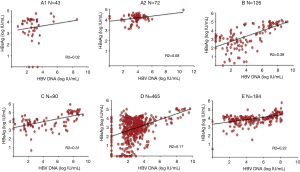
Further difficulties in assessing the viral load and determining the level of infectious virions is related to the co-circulation of spliced and unspliced (complete) HBV genomes in Dane particles (31). Spliced genomes are non-infectious but the percentages of these modified genomes vary according to individual, time in the infection course and genotype.
Prevalence of OBI in several populations (Table 2)
Table 2
| Author | Country | Year | Sample type | Number tested (%) | HBV DNA+/number tested (%) | LOD (IU/mL) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Whole population | Anti-HBc+ total | Anti-HBc+ Anti-HBs+ | Anti-HBs only | Anti-HBc only | ||||||
| Jilg (32) | Germany | 2001 | General pop | 5,305 | ||||||
| Anti-HBc | 544 (10.3) | 5/81 (6.2) | 20 | |||||||
| Song (33) | South Korea | 2009 | General pop | 1,091 | ||||||
| Anti-HBc | 364 (33.4) | 7 (0.6) | 2/364 (0.5) | 4/458 (0.9) | 0/22 (0) | 18-24 | ||||
| Kang (34) | South Korea | 2014 | General pop | 14,253 | ||||||
| Anti-HBc | 846 (5.9) | 27/571 (4.7) | 4–12 | |||||||
| Knöll (35) | Germany | 2006 | Hospital pop | 44/545 (8.1) | NA | |||||
| Vitale (36) | Italy | 2008 | Hospital pop | 6,544 | 5/85 (5.9) | 20 | ||||
| Launay (37) | France | 2011 | Hospital pop | 8/349 (2.3) | 350 | |||||
| Hui (38) | Hong Kong | 2005 | HSC donors | 124 | 19 (15.3) | 94 (17.0) | 3/30 (10.0) | 2 | ||
| Allain (39) | UK | 1999 | Bd | 103,869 | 0 (0) | ~260 | ||||
| 584 (0.6) | 0/584 | 0/69 (0) | ||||||||
| Chaudhuri (40) | India | 2003 | Bd | 6,159 | 48/230 (20.9) | 20 | ||||
| Bd | 3,304 | 40/147 (27.2) | ||||||||
| Kleinman (41) | USA | 2003 | Anti-HBc | 3,350 | 4/387 (1.0) | 10 | ||||
| Allain (42) | Ghana | 2003 | Bd | 576 | 22 (3.8) | 15–50 | ||||
| García-Montalvo (43) | Mexico | 2005 | Bd | 11,240 | ||||||
| Anti-HBc | 475 (4.2) | 13/158 (8.2) | 6–60 | |||||||
| Behzad (44) | Iran | 2006 | Bd | 2,000 | 16 (0.8) | |||||
| Anti-HBc | 131 (6.6) | 16 (12.2) | 6/85 (7.0) | 10/46 (21.7) | NA | |||||
| Manzini (45) | Italy | 2007 | 1st time bd | 6,313 | 16 (0.25) | 16 (0.25) | 0/39 (0) | 4.9 | ||
| Banerjee (46) | India | 2007 | Bd | 1,294 | 63 (4.9) | NA | ||||
| Anti-HBc | 303 (23.4) | 60/289 (20.8) | 21/118 (17.8) | 39/171 (22.8) | ||||||
| Bhatti (47) | Pakistan | 2007 | Bd | 966 | 4 (0.4) | 10 | ||||
| Anti-HBc | 190 (19.7) | 4/185 (2.2) | 1/162 (1.2) | 3/23 (13.0) | ||||||
| Asim (48) | India | 2010 | Bd | 2,175 | 31 (1.4) | NA | ||||
| Anti-HBc | 413 (19.0) | 31 (7.5) | 12/260 (4.6) | 19/153 (12.4) | ||||||
| Seo (49) | South Korea | 2011 | Bd | 12,461 | 2 (0.02) | |||||
| Anti-HBc | 1,674 (13.4) | 2 (0.12) | 1/1522 (0.07) | 1/152 (0.7) | 3.7 | |||||
| Mahgoub (50) | Sudan | 2011 | Anti-HBc | 145 | 4 (4.1) | 6/81 (7.4) | 0/64 (0) | 10 | ||
| Muselmani (51) | Syria | 2013 | Bd | 1,939 | 5 (0.26) | NA | ||||
| Anti-HBc | 215 (11.1) | 5 (2.3) | ||||||||
| Apica (52) | Uganda | 2016 | ER Popul | 314 | 90 (30) | NA | ||||
| Sondlane (53) | RS Africa | 2016 | HCW | 333 | 21 (6.7) | NA | ||||
| Sosa-Jurado (54) | Mexico | 2016 | Bd | 156 | 27 (17.3) | NA | ||||
| Alshayea (55) | Saudi Arabia | 2016 | Bd | 198 | 17 (8.6) | NA | ||||
| Hudu (56) | Malaysia | 2016 | Bd | 1,000 | 55 (5.5) | NA | ||||
| Blanco (57) | Argentina | 2017 | Bd | 168,215 | 3 (0.002) | 2.3–3.8 | ||||
Data are from HBsAg negative samples. It is assumed that the vast majority of OBIs is anti-HBc positive and can be equated to true prevalence. However, as shown in the Song study, anti-HBs only sample can be HBV DNA positive. Bd, blood donor; HCW, health care workers; Pop, population; LOD, limit of detection; HSC, hematopoietic stem cell; OBI; occult HBV infection; NA, not available.
Prevalence studies have been conducted, often partially, in three types of populations: general population, hospital population and blood donors. Hospital populations are assumed to be biased towards higher prevalence of liver diseases, therefore of HBV infection. They are made of random samples coming to hospital laboratories. Blood donor populations are also biased because a variable percentage of samples come from repeat donors who have already been tested for HBV markers, lowering prevalence. Volunteer donors are also biased as they are often young adults and in many countries are 70–95% males. Only one study indicated testing first-time donors with gender distribution close to equivalent (45). In addition, the majority of the reported studies concentrated on samples carrying anti-HBc as only marker of HBV infection. The few studies testing for HBV DNA in all anti-HBc positive samples or in all samples carrying an HBV serological marker can be taken as a base to calculate OBI prevalence although some OBI are anti-HBc negative (anti-HBs only or primary OBI without serological markers). Finally, over time, and according to assays utilized, the NAT sensitivity varies considerably adding difficulties in comparing data sets.
Only three studies reporting on OBI prevalence in general populations have been published (32-34). A single study tested all 1,091 samples from random check-up seekers for HBsAg, anti-HBc, anti-HBs and HBV DNA. However HBV NAT was tested with Cobas MPX in pools of six samples reducing the clinical sensitivity to 18–24 IU/mL and therefore the detection of potential OBI (33). The distribution of markers was HBsAg+ 2%, anti-HBs+/anti-HBc+ 33.4%, anti-HBc only 2%, anti-HBs only 42% and no serological marker 18.6%. From these five categories, HBV DNA was detected in 7 samples (0.6%), 2 anti-HBc+/anti-HBs+, 4 anti-HBs only (presumably HBV vaccinated) and 1 negative for all markers. This unique observation draws serious doubts on the significance of the many studies conducted in anti-HBc or anti-HBc-only positive samples to assess the prevalence of OBI. Such was the case in the other two studies of populations that could be considered representative of Germans and Koreans, respectively where only samples presenting with the anti-HBc-only profile were tested for HBV DNA with assay LOD of 20 and 4–12 IU/mL, respectively. OBI with anti-HBs+/anti-HBc+ or anti-HBs-only or no HBV markers profiles were not included decreasing the true OBI prevalence by a factor of at least 2. Prevalence of OBI extrapolated to the total starting populations of 5,305 and 14,253 was 0.1% and 0.2%, respectively. Therefore the true prevalence of OBI is far from being reliably known. Such prevalence is assumed to increase with infection prevalence and possibly according to genotype but there is no evidence of either. Multiple studies have been conducted in various populations in which anti-HBc-only samples have been selected for HBV DNA testing. Among those samples, selected from biased hospital patients or laboratory populations, the percentage of HBV DNA positive samples ranges between 2.3% and 20.8%. The lowest was observed with a LOD of 350 IU/mL, the highest with an assay that did not confirm in 16/37 DNA positive samples, reducing the prevalence from 20.8% to 11.8%.
In ten studies (33,39,42,44-49) testing all anti-HBc positive/HBsAg negative samples for HBV DNA (sensitivity ranging between 4 and 230 IU/mL but 4/10 did not provide LOD), the prevalence of OBI ranged between 0.25% and 0.8% of total population (Table 2). No HBV DNA was found in a large UK study (39) but the sensitivity of the assay in 1998 was too low to detect OBI (median viral load 20 IU/mL). The low prevalence in the study by Seo contrasts with the Song data, which are clearly more representative of the general population of Korea (33,49). The high prevalence in Banerjee’s study (4.9%) might be biased by other unknown factors (46) when compared to another Indian study (Table 2) (48). The higher prevalence of OBI in Ghana is likely related to one of the highest anti-HBc prevalence in the world (76% in adult blood donors) and chronic HBV infection (15% HBsAg positive) (42).
OBI prevalence in 16 studies of samples carrying anti-HBc as unique marker of HBV ranges between 0 (2 studies) and >20% (4 reports). Studies from India and Pakistan indicate an average of 20.6% OBI in anti-HBc-only blood donor samples in areas where anti-HBc prevalence is approximately 20% (46-48). In six studies of OBI in samples carrying both anti-HBc and anti-HBs, the prevalence of OBI ranged between 0.07% and 17.8%. Here again studies by Seo and Banerjee appear exceptions, the others averaging at 4% (46,49). This data is in line with approximately 50% of OBIs in the northern hemisphere carrying anti-HBs and anti-HBc, the other 50% anti-HBc only. Recent studies conducted in Uganda and South Africa indicated very high prevalence of OBI in emergency patients and health care workers (30% and 6.7%, respectively (52,53).
Age and gender of OBI carriers
In the many reports on OBI, age and gender of individuals carrying the infection is not often given. When the information is provided, it is biased by the generally higher prevalence of HBV infection in men. In blood donor populations, particularly in developing countries with high prevalence of infection, males are by far predominant over female donors making calculation of prevalence according to gender difficult by lack of denominator. However blood donors in developed countries are most often with a male/female ratio close to 1 allowing a comparison of OBI prevalence according to gender. Figure 2 shows the distribution of OBI cases in blood donors from our group data reinforced with published data from the literature covering a total of 276 OBI cases from areas where genotype A1–E are prevalent. In areas of dominance of genotype A2 (Europe), B/C (South East Asia) and D (Mediterranean basin), median age, irrespective of gender, ranges between 45 and 55 years (17,18,58). In contrast, in South Africa where genotype A1 is dominant or in Ghana where genotype E is dominant, age of OBI carriers is considerably younger with a median around 30 years, irrespective of gender (59,60). It is not known whether this difference is related to genotype or to a less effective immune system of Africans. The duration span of the HBV infection acquired vertically or in early childhood is ranging between 15 and 60 years for African blood donors but a similar range is present in South East Asia where infection is mostly vertical mode. In Europe where many HBV infections occur later (older than 15 years) with IV drug use or sexually, it may take up to an older age to develop OBI.
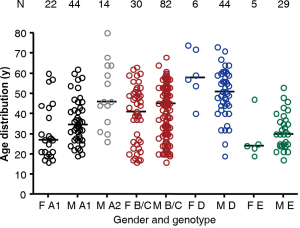
Figure 2 also shows that in areas where gender ratio in blood donors is approximately 1, 67% of OBI are males in South Africa (genotype A1), 100% males in Western Europe (on a small number of cases), 62% in South East Asia (genotype B/C) and 88% in Italy, Spain, Poland (genotype D). This difference might be related to the generally more efficient anti-viral activity of the female immune system but this hypothesis would require further studies to be supported by firm evidence.
Identification of OBI in patients with CLD
OBI was first described in patients with CLD in 1985 and subsequently in patients with hepatocellular carcinoma (HCC). OBI in HCC literature is divided into two groups: without co-infection with hepatitis C virus (HCV) and with co-infection. Studies of patients with HCC negative for anti-HCV and HBsAg published since 2000 are shown in Table 3. The prevalence of HBV DNA ranges between 40.5% in Taiwan (90% genotype B) and 76.2% in Egypt (100% genotype D). Three of the studies tested for HBV DNA in liver tissue (prevalence 67.6–76.2%) and the other five studies in serum (47.6–75.4%). It is difficult to determine whether the difference in prevalence suggesting higher frequency of OBI when examining liver tissue is related to true increased prevalence or to increased sensitivity of assays over time. In few studies where a control population was included, the difference in prevalence was highly significant (P<0.001). In anti-HCV positive HCC, the prevalence of OBI ranges between 22% and 73.3% in studies conducted between 2002 and 2011 (69). The lower prevalence found in HCC negative for anti-HCV suggests an etiologic role of both viruses. The potential impact of OBI in the development of HCC in HCV co-infected patients has been debated. Several studies suggest that the incidence of HCC in co-infected patients is significantly higher than in OBI negative patients (70). However, the likelihood that patients have been HBV infected early in life (at birth or during childhood) and developed chronic LD is very high in both Japanese and Italian incidence studies. HCV infection was most likely acquired at a considerably later date after years or decade of asymptomatic HBV infection in the process of becoming OBI. The question really is: do individuals long-term infected with HBV possibly reaching the status of OBI who became co-infected with HCV are at an increased risk of HCC? So far, no long-term prospective studies have been conducted examining this side of the co-infection coin. However, several epidemiological studies conducted in Europe and Asia provided convincing evidence that OBI associated with either HCV infection or alcoholism-related CLD was a significant risk factor for the development of HCC (71). Such evidence was not confirmed in the USA (72). In addition, there was recent evidence that reactivation of OBI might take place after successful treatment of HCV with direct-acting antiviral drugs (73,74).
Table 3
| Study | Year | Country | Number of patients | Dominant genotype | Percentage of OBI (%) | Percentage OBI in controls (%) | P value |
|---|---|---|---|---|---|---|---|
| Yotsuyanagi (61) | 2000 | Japan | 42 | C | 47.6 | 2.4 | <0.001 |
| Shiota (62) | 2000 | Japan | 26 | C | 69.2 | ||
| Hsia (63) | 2003 | USA/Canada | 31 | A/C/D | 59.4 | ||
| Pollicino (64) | 2004 | Italy | 34 | D | 67.6 | ||
| Kew (65) | 2008 | South Africa | 118 | A1 | 75.4 | ||
| Fang (66) | 2009 | China | 135 | C/B | 70.4 | 10.6 | <0.001 |
| Chen (67) | 2009 | Taiwan | 222 | B | 40.5 | 8.0 | |
| Wong (68) | 2011 | Hong Kong | 33 | B/C | 72.7 | ||
| Hassan (69) | 2011 | Egypt | 21 | D | 76.2 |
HCC, hepatocellular carcinoma.
In studies comparing different populations of patients with CLD, the prevalence of OBI in HCC was significantly higher than in other types of CLD. Whether examined in areas where genotype A, B, C or D are prevalent, no difference in prevalence was observed (70). Molecular studies of tumor and non-tumor tissue showed that HBV DNA was significantly more found in tumor tissue of OBI HCC and that mutations and deletions in the Pre-S/S region were frequent (75).
OBI and hemodialysis
Relatively high prevalence of HBV infection identified by positivity of HBsAg was described in many cohorts of patients in chronic renal dialysis. It was largely recognized as being nosocomial. The availability of HBV DNA detection prompted many investigators to examine the prevalence of OBI in small and large cohorts of patients. In 24 studies from 11 countries the prevalence of OBI ranged between 0 and 26.6% (median 3%). In countries where multiple large studies were conducted such as in Italy, Turkey, Iran and Brazil, prevalence ranged between 0 and 26.6%, 1.3% and 12.4%, 0 and 3.1%, 1.5% and 15%, respectively. Only in Egypt, an area of moderate HBV infection prevalence, consistent OBI prevalence of approximately 4% was found in two studies. Such prevalence is quite close to what is observed in the general population of each of the countries involved. In the countries with discrepant data, differences in prevalence might be related to levels of infection risk prevention or to assay performance. These data were recently reviewed (76).
OBI and immunodeficiency
HBV remains detectable in the liver of virtually all infected individuals, whether with chronic or recovered infection. It is well known that anti-HBc positive patients receiving massive drug-induced immunosuppression for organ transplantation (OT) or bone marrow transplantation (BMT) are at high risk of reactivation of HBV replication, endangering patients’ lives. In many ways, OBI can be considered as an intermediary stage of the infection to a large extent dependent on the efficacy of the host immune system. In the relatively immunodeficient group of patients with leukemia, the prevalence of OBI was 10.5% compared to 2.9% in non-leukemic patients in China, most of them infected with HBV genotype C (77). Therefore, when immunodeficient either naturally or drug-induced, HBV infected patients were predicted to move from undetectable to detectable DNA without full reactivation indicated by HBsAg and high viral load. This situation explains the interest of hemodialysis centers in OBI detection since many of their patients undergo kidney transplantation and receive immunosuppressive treatment.
OBI and immunodeficiency is an issue for patients with lymphoma, leukemia or other types of cancer because they receive immunosuppressive drugs that might trigger the reactivation of these ‘dormant’ HBV infections. In such cases, the main factor of reactivation is the drug used. In a study of 127 patients with lymphoma, 32 patients were treated with rituximab and two of them reactivated HBV while none of 16 not receiving the drug reactivated (78). Such post-rituximab reactivation can be delayed for up to a year after discontinuation of the drug (79). In another study (80) of 80 patients with lymphoma, 46 were anti-HBc positive. Twenty-one of them were treated with CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) and rituximab with five reactivations but in 25 patients treated with CHOP alone no reactivation was observed. A new assay detecting HBV core antigen has been proposed to detect reactivation in patients with OBI on chemotherapy (81).
In patients who receive OT or BMT or hematopoietic stem cell transplantation (BMT or HSCT), the impact of OBI is seen at the donors level as well at the recipient level that might reactivate their own HBV infection or OBI depending on the drug regiment they receive or the OBI from the donor. For instance, in a study by Hui et al. in 2006, in 118 HCV positive patients receiving a liver transplantation, 41 patients carried OBI and 77 were HBV DNA negative. Of 90% received ciclosporin (82) reactivation occurred in 9.8% of OBIs and 1.3% of non-OBI patients. In another study, six BMT recipients were anti-HBc and anti-HBs positive, the four OBI patients reactivated and the two patients HBV DNA negative did not (83). The general conclusion of these studies is that among anti-HBc positive patients treated with immunosuppressive drugs, those carrying OBI are at higher risk of reactivation than those negative for HBV DNA but at lower risk than those with overt HBV infection indicated by detectable HBsAg.
Another situation of immunodeficiency is related to HIV infection that induces progressively increasing deterioration of the host immune system. Sub-Saharan Africa being an area of high prevalence of both HIV and HBV, several studies were conducted in that region. Few of the studies directly compared the prevalence of OBI in HIV infected and non-infected patients carrying anti-HBc as evidence of previous contact with HBV. In two studies where such comparison was made (84,85) the prevalence of OBI in HIV infected patients was significantly higher than in non-infected patients (Table 4). In other studies in Africa and other continents, prevalence in anti-HBc positive patients was higher than in non-infected population although not comparatively tested (Table 4). As in immunodeficiencies induced by drugs, HIV-1 infection seems to allow individuals who have recovered from HBV infection to move from that status to OBI and in some cases to overt HBV infection HBsAg positive (86). These data are indirect evidence that efficacy of the host immune system is to a large extent determining the status of OBI.
Table 4
| Author (year) | Country | HBV DNA positive/total in anti-HBc positive/HBsAg negative (%) | HIV+ no HBV marker | |
|---|---|---|---|---|
| HIV negative | HIV positive | |||
| Mphahlele, 2006 (84) | South Africa | 2/105 (1.9) | 31/142 (22.1) | |
| Bell, 2012 (86) | South Africa | 45/181 (24.9) | 0/79 | |
| Barth, 2011 (87) | South Africa | 6/62 (9.7) | ||
| Firnhaber, 2012 (88) | South Africa | 12/222 (5.4) | ||
| Compston, 2009 (85) | Ghana | 2/88 (2.3) | 28/238 (11.8) | |
| Pourkarim, 2011 (89) | Belgium | 27/175 (15.4) | ||
| Tramuto, 2013 (90) | Italy | 0.25 (Manzini) (45) | 14/159 (8.8) | |
| Morsica, 2009 (91) | Italy | 27/175 (15.0) | ||
| Bell, 2012 (86) | The Netherlands | 6/187 (3.2) | ||
| Khorvash, 2014 (92) | Iran | 0.8 (Behzad) (44) | 3/12 (25.0) | |
| Gupta, 2010 (93) | India | 1.4 (Asim) (48) | 24/42 (57.1) | |
| 4.9 (Banerjee) (46) | ||||
| Araujo, 2008 (94) | Brazil | 6/43 (14.0) | ||
| Oliveira, 2016 (95) | Brazil | 19/505 (3.8) | ||
Authors in parenthesis refer to data presented in Table 3 for comparison.
Conclusions
This review of some aspects of the epidemiology of HBV infection expressed as OBI is disappointing since there is a remarkable paucity of studies determining the true prevalence of OBI in representative general populations. Studies provide disparate results related to multiple biases whether in terms of assay performance, subject selection in terms of gender or age or health status (too healthy for blood donors, not enough for hospital populations). Epidemiologically adequate studies remain to be conducted to provide a reliable answer to this critical question.
One interesting question is whether OBI status is a new branch of the natural history of HBV infection in its own right as an intermediate state between recovery and chronicity or is it a secondary turn in the long-term history of HBV infection recovery or asymptomatic chronic infection. Only long-term careful studies of recent infection might provide the answer.
There is presently no evidence that individuals carrying OBI can be infectious vertically, horizontally or sexually. Only massive exposure to blood products prepared from blood of OBI carrier contains sufficient amount of infectious virions to cause infection. It is however possible that lower infectious doses might be infectious in severely immunodeficient individuals but no evidence was yet provided.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob.2017.06.01). JPA serves as an unpaid editorial board member of Annals of Blood from Dec 2016 to Dec 2018. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bréchot C, Degos F, Lugassy C, et al. Hepatitis B virus DNA in patients with chronic liver disease and negative tests for hepatitis B surface antigen. N Engl J Med 1985;312:270-6. [Crossref] [PubMed]
- Paterlini P, Gerken G, Nakajima E, et al. Polymerase chain reaction to detect hepatitis B virus DNA and RNA sequences in primary liver cancers from patients negative for hepatitis B surface antigen. N Engl J Med 1990;323:80-5. [Crossref] [PubMed]
- Raimondo G, Allain JP, Brunetto MR, et al. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J Hepatol 2008;49:652-7. [Crossref] [PubMed]
- Biswas R, Tabor E, Hsia CC, et al. Comparative sensitivity of HBV NATs and HBsAg assays for detection of acute HBV infection. Transfusion 2003;43:788-98. [Crossref] [PubMed]
- Scheiblauer H, Soboll H, Nick S. Evaluation of 17 CE-marked HBsAg assays with respect to clinical sensitivity, analytical sensitivity, and hepatitis B virus mutant detection. J Med Virol 2006;78:S66-70. [Crossref] [PubMed]
- Aoki M, Saito M, Watanabe H, et al. Clinical significance of a highly sensitive enzyme immunoassay of hepatitis B surface antigen using a novel electron spin resonance technique. J Med Virol 2002;66:166-70. [Crossref] [PubMed]
- Togashi H, Hashimoto C, Yokozawa J, et al. What can be revealed by extending the sensitivity of HBsAg detection below the present limit? J Hepatol 2008;49:17-24. [Crossref] [PubMed]
- Matsubara N, Kusano O, Sugamata Y, et al. A novel hepatitis B virus surface antigen immunoassay as sensitive as hepatitis B virus nucleic acid testing in detecting early infection. Transfusion 2009;49:585-95. [Crossref] [PubMed]
- Shinkai N, Matsuura K, Sugauchi F, et al. Application of a newly developed high-sensitivity HBsAg chemiluminescent enzyme immunoassay for hepatitis B patients with HBsAg seroclearance. J Clin Microbiol 2013;51:3484-91. [Crossref] [PubMed]
- Shen G, Zhang Y. Highly sensitive electrochemical stripping detection of hepatitis B surface antigen based on copper-enhanced gold nanoparticle tags and magnetic nanoparticles. Anal Chim Acta 2010;674:27-31. [Crossref] [PubMed]
- El Chaar M, Candotti D, Crowthers RA, et al. Impact of Hepatitis B virus surface protein mutations on the diagnosis of occult Hepatitis B virus infection. Hepatology 2010;52:1600-10. [Crossref] [PubMed]
- Enjalbert F, Krysztof DE, Candotti D, et al. Comparison of seven hepatitis B virus (HBV) nucleic acid testing assays in selected samples with discrepant HBV marker results from United States blood donors. Transfusion 2014;54:2485-95. [Crossref] [PubMed]
- Tsoi WC, Lelie N, Lin CK. Enhanced detection of hepatitis B virus in Hong Kong blood donors after introduction of a more sensitive transcription-mediated amplification assay. Transfusion 2013;53:2477-88. [Crossref] [PubMed]
- Cobas MPX: enhanced assay performance based on dual-target for HIV-1 and dual probe for HCV on the cobas 6800/800 system. Available online: https://www.cobas68008800.com/pages/news_and_events/poster_items/cobas-mpx-enhanced-assay-performance-based-on-dual-target-for-hiv-1-and-dual-probe-for-hcv-on-the-cobas-68008800-systems
- Vermeulen M, van Drimmelen H, Coleman C, et al. A mathematical approach to estimate the efficacy of individual-donation and minipool nucleic acid amplification test options in preventing transmission risk by window period and occult hepatitis B virus infections. Transfusion 2014;54:2496-504. [Crossref] [PubMed]
- Weusten J, van Drimmelen H, Vermeulen M, et al. A mathematical model for estimating residual transmission risk of occult hepatitis B virus infection with different blood safety scenarios. Transfusion 2017;57:841-9. [Crossref] [PubMed]
- Zheng X, Ye X, Zhang L, et al. Characterization of occult hepatitis B virus infection from blood donors in China. J Clin Microbiol 2011;49:1730-7. [Crossref] [PubMed]
- Candotti D, Lin CK, Belkhiri D, et al. Occult hepatitis B infection in blood donors from South East Asia: molecular characterisation and potential mechanisms of occurrence. Gut 2012;61:1744-53. [Crossref] [PubMed]
- Lin H, Zhao H, Tang X, et al. Serological Patterns and Molecular Characterization of Occult Hepatitis B Virus Infection among Blood Donors. Hepat Mon 2016;16:e40492. [Crossref] [PubMed]
- Keechilot CS, Shenoy V, Kumar A, et al. Detection of occult hepatitis B and window period infection among blood donors by individual donation nucleic acid testing in a tertiary care center in South India. Pathog Glob Health 2016;110:287-91. [Crossref] [PubMed]
- Guo Z, Fu P, Yin Y, et al. The characteristics of hepatitis B surface antigen (HBsAg)-negative hepatitis B virus (HBV) infection in Chinese blood donors: a follow-up study of donors tested negative for HBsAg and reactive for simultaneous nucleic acid testing of HBV, hepatitis C virus, and human immunodeficiency virus. Transfusion 2017;57:832-40. [Crossref] [PubMed]
- Liao H, Liu Y, Chen J, et al. Characterization of hepatitis B virus (HBV) preS/S gene mutations in blood donors with occult HBV infection in the Baoji area of North China. Transfusion 2017;57:857-66. [Crossref] [PubMed]
- Allain JP, Candotti D. Diagnostic algorithm for HBV safe transfusion. Blood Transfusion 2009;7:174-82. [PubMed]
- Kiely P, Margaritis AR, Seed CR, et al. Hepatitis B virus nucleic acid amplification testing of Australian blood donors highlights the complexity of confirming occult hepatitis B virus infection. Transfusion 2014;54:2084-91. [Crossref] [PubMed]
- Minegishi K, Yoshikawa A, Kishimoto S, et al. Superiority of minipool nucleic acid amplification technology for hepatitis B virus over chemiluminescence immunoassay for hepatitis B surface antigen screening. Vox Sang 2003;84:287-91. [Crossref] [PubMed]
- Kuhns MC, Kleinman SH, McNamara AL, et al. Lack of correlation between HBsAg and HBV DNA levels in blood donors who test positive for HBsAg and anti-HBc: implications for future HBV screening policy. Transfusion 2004;44:1332-9. [Crossref] [PubMed]
- Alghamdi A, Aref N, El-Hazmi M, et al. Correlation between hepatitis B surface antigen titers and HBV DNA levels. Saudi J Gastroenterol 2013;19:252-7. [Crossref] [PubMed]
- Lee HJ, Kim SY, Lee SM, et al. Elecsys hepatitis B surface antigen quantitative assay: performance evaluation and correlation with hepatitis B virus DNA during 96 weeks of follow-up in chronic hepatitis B patients. Ann Lab Med 2012;32:420-5. [Crossref] [PubMed]
- Vermeulen M, Coleman C, Mitchel J, et al. Sensitivity of individual-donation and minipool nucleic acid amplification test options in detecting window period and occult hepatitis B virus infections. Transfusion 2013;53:2459-66. [Crossref] [PubMed]
- Biswas S, Candotti D, Allain JP. Specific amino acid substitutions in the S protein prevent its excretion in vitro and may contribute to occult hepatitis B virus infection. J Virol 2013;87:7882-92. [Crossref] [PubMed]
- El Chaar M, El Jisr T, Allain JP. Hepatitis B virus DNA splicing in Lebanese blood donors and genotype A to E strains: implications for hepatitis B virus DNA quantification and infectivity. J Clin Microbiol 2012;50:3159-67. [Crossref] [PubMed]
- Jilg W, Hottentrager B, Weinberger K, et al. Prevalence of markers of hepatitis B in the adult German population. J Med Virol 2001;63:96-102. [Crossref] [PubMed]
- Song EY, Yun YM, Park MH, et al. Prevalence of occult hepatitis B virus in a general adult population in Korea. Intervirology 2009;52:57-62. [Crossref] [PubMed]
- Kang SY, Kim MH, Lee WI. Occult hepatitis B virus infection in Korean patients with isolated anti-HBc. Arch Virol 2014;159:227-33. [Crossref] [PubMed]
- Knöll A, Hartmann A, Hamoshi H, et al. Serological pattern "anti-HBc alone": characterization of 552 individuals and clinical significance. World J Gastroenterol 2006;12:1255-60. [Crossref] [PubMed]
- Vitale F, Tramuto F, Orlando A, et al. Can the serological status of ‘anti-HBc alone’ be considered a sentinel marker for detection of ‘occult’ HBV infection? J Med Virol 2008;80:577-82. [Crossref] [PubMed]
- Launay O, Masurel J, Servant-Delmas A, et al. High levels of serum hepatitis B virus DNA in patients with 'anti-HBc alone': role of HBsAg mutants. J Viral Hepat 2011;18:721-9. [Crossref] [PubMed]
- Hui CK, Sun J, Au WY, et al. Occult hepatitis B virus infection in hematopoietic stem cell donors in a hepatitis B virus endemic area. J Hepatol 2005;42:813-9. [Crossref] [PubMed]
- Allain JP, Hewitt PE, Tedder RS, et al. Evidence that anti-HBc but not HBV DNA testing may prevent some HBV transmission by transfusion. Br J Haematol 1999;107:186-95. [Crossref] [PubMed]
- Chaudhuri V, Nanu A, Panda SK, et al. Evaluation of serologic screening of blood donors in India reveals a lack of correlation between anti-HBc titer and PCR-amplified HBV DNA. Transfusion 2003;43:1442-8. [Crossref] [PubMed]
- Kleinman SH, Strong DM, Tegtmeier GG, et al. Hepatitis B virus (HBV) DNA screening of blood donations in minipools with the COBAS AmpliScreen HBV test. Transfusion 2005;45:1247-57. [Crossref] [PubMed]
- Allain JP, Candotti D, Soldan K, et al. The risk of hepatitis B virus infection by transfusion in Kumasi, Ghana. Blood 2003;101:2419-25. [Crossref] [PubMed]
- García-Montalvo BM, Farfán-Ale JA, Acosta-Viana KY, et al. Hepatitis B virus DNA in blood donors with anti-HBc as a possible indicator of active hepatitis B virus infection in Yucatan, Mexico. Transfus Med 2005;15:371-8. [Crossref] [PubMed]
- Behzad-Behbahani A, Mafi-Nejad A, Tabei SZ, et al. Anti-HBc & HBV DNA detection in blood donors negative for hepatitis B virus surface antigen in reducing risk of transfusion associated HBV infection. Indian J Med Res 2006;123:37-42. [PubMed]
- Manzini P, Girotto M, Borsotti R, et al. Italian blood donors with anti-HBc and occult hepatitis B virus infection. Haematologica 2007;92:1664-70. [Crossref] [PubMed]
- Banerjee A, Chandra PK, Datta S, et al. Frequency and significance of hepatitis B virus gene variants circulating among anti-HBc only’ individuals in Eastern India. J Clin Virol 2007;40:312-7. [Crossref] [PubMed]
- Bhatti FA, Ullah Z, Salamat N, et al. Anti-hepatitis B core antigen testing, viral markers, and occult hepatitis B virus infection in Pakistani blood donors: implications for transfusion practice. Transfusion 2007;47:74-9. [Crossref] [PubMed]
- Asim M, Ali R, Khan LA, et al. Significance of anti-HBc screening of blood donors & its association with occult hepatitis B virus infection: implications for blood transfusion. Indian J Med Res 2010;132:312-7. [PubMed]
- Seo DH, Whang DH, Song EY, et al. Prevalence of antibodies to hepatitis B core antigen and occult hepatitis B virus infections in Korean blood donors. Transfusion 2011;51:1840-6. [Crossref] [PubMed]
- Mahgoub S, Candotti D, El Ekiaby M, et al. Hepatitis B virus (HBV) infection and recombination between HBV genotypes D and E in asymptomatic blood donors from Khartoum, Sudan. J Clin Microbiol 2011;49:298-306. [Crossref] [PubMed]
- Muselmani W, Habbal W, Monem F. Significance of screening antibodies to hepatitis B virus core antigen among Syrian blood donors. Transfus Med 2013;23:265-8. [Crossref] [PubMed]
- Apica BS, Seremba E, Rule J, et al. High prevalence of occult hepatitis B infection in an African urban population. J Med Virol 2016;88:674-80. [Crossref] [PubMed]
- Sondlane TH, Mawela L, Razwiedani LL, et al. High prevalence of active and occult hepatitis B virus infections in healthcare workers from two provinces of South Africa. Vaccine 2016;34:3835-9. [Crossref] [PubMed]
- Sosa-Jurado F, Hilda Rosas-Murrieta N, Guzman-Flores B, et al. Prevalence of Serologic Hepatitis B Markers in Blood Donors From Puebla, Mexico: The Association of Relatively High Levels of Anti-Core Antibodies With the Detection of Surface Antigen and Genomic DNA. Hepat Mon 2016;16:e36942. [Crossref] [PubMed]
- Alshayea AI, Eid GE, El-Hazmi MM, et al. Prevalence and characterization of occult hepatitis B infection among blood donors in central Saudi Arabia. Saudi Med J 2016;37:1114-9. [Crossref] [PubMed]
- Hudu SA, Harmal NS, Saeed MI, et al. Molecular and serological detection of occult hepatitis B virus among healthy hepatitis B surface antigen-negative blood donors in Malaysia. Afr Health Sci 2016;16:677-83. [Crossref] [PubMed]
- Blanco S, Balangero MC, Valle MC, et al. Usefulness of nucleic acid testing to reduce risk of hepatitis B virus transfusion-transmitted infection in Argentina: high rate of recent infections. Transfusion 2017;57:816-22. [Crossref] [PubMed]
- Candotti D, Grabarczyk P, Ghiazza P, et al. Characterization of occult hepatitis B virus from blood donors carrying genotype A2 or genotype D strains. J Hepatol 2008;49:537-47. [Crossref] [PubMed]
- Allain JP, Belkhiri D, Vermeulen M, et al. Characterization of occult hepatitis B virus strains in South African blood donors. Hepatology 2009;49:1868-76. [Crossref] [PubMed]
- Zahn A, Li C, Danso K, et al. Molecular characterization of occult hepatitis B virus in genotype E-infected subjects. J Gen Virol 2008;89:409-18. [Crossref] [PubMed]
- Yotsuyanagi H, Shintani Y, Moriya K, et al. Virologic analysis of non-B, non-C hepatocellular carcinoma in Japan: frequent involvement of hepatitis B virus. J Infect Dis 2000;181:1920-8. [Crossref] [PubMed]
- Shiota G, Oyama K, Udagawa A, et al. Occult hepatitis B virus infection in HBs antigen-negative hepatocellular carcinoma in a Japanese population: involvement of HBx and p53. J Med Virol 2000;62:151-8. [Crossref] [PubMed]
- Hsia CC, Scudamore CH, Di Bisceglie AM, et al. Molecular and serological aspects of HBsAg-negative hepatitis B virus infections in North America. J Med Virol 2003;70:20-6. [Crossref] [PubMed]
- Pollicino T, Squadrito G, Cerenzia G, et al. Hepatitis B virus maintains its pro-oncogenic properties in the case of occult HBV infection. Gastroenterology 2004;126:102-10. [Crossref] [PubMed]
- Kew MC, Welschinger R, Viana R. Occult hepatitis B virus infection in Southern African blacks with hepatocellular carcinoma. J Gastroenterol Hepatol 2008;23:1426-30. [Crossref] [PubMed]
- Fang Y, Shang QL, Liu JY, et al. Prevalence of occult hepatitis B virus infection among hepatopathy patients and healthy people in China. J Infect 2009;58:383-8. [Crossref] [PubMed]
- Chen CH, Changchien CS, Lee CM, et al. A study on sequence variations in pre-S/surface, X and enhancer II/core promoter/precore regions of occult hepatitis B virus in non-B, non-C hepatocellular carcinoma patients in Taiwan. Int J Cancer 2009;125:621-9. [Crossref] [PubMed]
- Wong DK, Huang FY, Lai CL, et al. Occult hepatitis B infection and HBV replicative activity in patients with cryptogenic cause of hepatocellular carcinoma. Hepatology 2011;54:829-36. [Crossref] [PubMed]
- Hassan ZK, Hafez MM, Mansor TM, et al. Occult HBV infection among Egyptian hepatocellular carcinoma patients. Virol J 2011;8:90. [Crossref] [PubMed]
- Huang X, Hollinger FB. Occult hepatitis B virus infection and hepatocellular carcinoma: a systematic review. J Viral Hepat 2014;21:153-62. [Crossref] [PubMed]
- Pollicino T, Saitta C. Occult hepatitis B virus and hepatocellular carcinoma. World J Gastroenterol 2014;20:5951-61. [Crossref] [PubMed]
- Lok AS, Everhart JE, Di Bisceglie AM, et al. Occult and previous hepatitis B virus infection are not associated with hepatocellular carcinoma in United States patients with chronic hepatitis C. Hepatology 2011;54:434-42. [Crossref] [PubMed]
- De Monte A, Courjon J, Anty R, et al. Direct-acting antiviral treatment in adults infected with hepatitis C virus: Reactivation of hepatitis B virus coinfection as a further challenge. J Clin Virol 2016;78:27-30. [Crossref] [PubMed]
- Wang C, Ji D, Chen J, et al. Hepatitis due to reactivation of Hepatitis B virus in endemic areas among patients with hepatitis C treated with direct-acting antiviral agents. Clin Gastroenterol Hepatol 2017;15:132-6. [Crossref] [PubMed]
- Coppola N, Onorato L, Iodice V, et al. Occult HBV infection in HCC and cirrhotic tissue of HBsAg-negative patients: a virological and clinical study. Oncotarget 2016;7:62706-14. [PubMed]
- Fontenele AM, Filho NS, Ferreira AS. Occult hepatitis B in patients on hemodialysis: a review. Ann Hepatol 2013;12:527-31. [PubMed]
- Zhang Z, Zhang Y, Xu N, et al. High risk of occult hepatitis B virus infection in leukemia patients from China. Arch Virol 2017;162:349-57. [Crossref] [PubMed]
- Fukushima N, Mizuta T, Tanaka M, et al. Retrospective and prospective studies of hepatitis B virus reactivation in malignant lymphoma with occult HBV carrier. Ann Oncol 2009;20:2013-7. [Crossref] [PubMed]
- Nakaya A, Fujita S, Satake A, et al. Delayed HBV reactivation in rituximab-containing chemotherapy: How long should we continue anti-virus prophylaxis or monitoring HBV-DNA? Leuk Res 2016;50:46-9. [Crossref] [PubMed]
- Yeo W, Chan TC, Leung NW, et al. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol 2009;27:605-11. [Crossref] [PubMed]
- Seto WK, Wong DH, Chan TY, et al. Association of Hepatitis B Core-Related Antigen With Hepatitis B Virus Reactivation in Occult Viral Carriers Undergoing High-Risk Immunosuppressive Therapy. Am J Gastroenterol 2016;111:1788-95. [Crossref] [PubMed]
- Hui CK, Lau E, Monto A, et al. Natural history of patients with recurrent chronic hepatitis C virus and occult hepatitis B co-infection after liver transplantation. Am J Transplant 2006;6:1600-8. [Crossref] [PubMed]
- Knöll A, Boehm S, Hahn J, et al. Reactivation of resolved hepatitis B virus infection after allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplant 2004;33:925-9. [Crossref] [PubMed]
- Mphahlele MJ, Lukhwareni A, Burnett RJ, et al. High risk of occult hepatitis B virus infection in HIV-positive patients from South Africa. J Clin Virol 2006;35:14-20. [Crossref] [PubMed]
- Compston LI, Li C, Sarkodie F, et al. Prevalence of persistent and latent viruses in untreated patients infected with HIV-1 from Ghana, West Africa. J Med Virol 2009;81:1860-8. [Crossref] [PubMed]
- Bell TG, Makondo E, Martinson NA, et al. Hepatitis B virus infection in human immunodeficiency virus infected southern African adults: occult or overt--that is the question. PLoS One 2012;7:e45750. [Crossref] [PubMed]
- Barth RE, Huijgen Q, Tempelman HA, et al. Presence of occult HBV, but near absence of active HBV and HCV infections in people infected with HIV in rural South Africa. J Med Virol 2011;83:929-34. [Crossref] [PubMed]
- Firnhaber C, Chen CY, Evans D, et al. Prevalence of hepatitis B virus (HBV) co-infection in HBV serologically-negative South African HIV patients and retrospective evaluation of the clinical course of mono- and co-infection. Int J Infect Dis 2012;16:e268-72. [Crossref] [PubMed]
- Pourkarim MR, Lemey P, Amini-Bavil-Olyaee S, et al. Molecular characterization of hepatitis B virus strains circulating in Belgian patients co-infected with HIV and HBV: overt and occult infection. J Med Virol 2011;83:1876-84. [Crossref] [PubMed]
- Tramuto F, Maida CM, Colomba GM, et al. Prevalence of occult hepatitis B virus infection in a cohort of HIV-positive patients resident in Sicily, Italy. Biomed Res Int 2013;2013:859583.
- Morsica G, Ancarani F, Bagaglio S, et al. Occult hepatitis B virus infection in a cohort of HIV-positive patients: correlation with hepatitis C virus coinfection, virological and immunological features. Infection 2009;37:445-9. [Crossref] [PubMed]
- Khorvash F, Javadi A, Tayeri K, et al. Occult hepatitis B virus infection among human immunodeficiency virus-infected patients with isolated hepatitis B core antibody in Isfahan, Iran. J Res Med Sci 2014;19:S64-6. [PubMed]
- Gupta S, Singh S. Occult hepatitis B virus infection in ART-naive HIV-infected patients seen at a tertiary care centre in north India. BMC Infect Dis 2010;10:53-60. [Crossref] [PubMed]
- Araujo NM, Branco-Vieira M, Silva AC, et al. Occult hepatitis B virus infection in HIV-infected patients: Evaluation of biochemical, virological and molecular parameters. Hepatol Res 2008;38:1194-203. [PubMed]
- Oliveira MP, Lemes PS, Matos MA, et al. Overt and occult hepatitis B virus infection among treatment-naïve HIV-infected patients in Brazil. J Med Virol 2016;88:1222-9. [Crossref] [PubMed]
Cite this article as: Allain JP. Global epidemiology of occult HBV infection. Ann Blood 2017;2:7.


