Harmonization of red blood cell distribution width (RDW): an attainable target?
Introduction
The red blood cell distribution width (RDW) is an indirect measure of anisocytosis, which is receiving increasing interest as a diagnostic and prognostic factor in a vast array of human disorders (1). The main advantage of the RDW is attributable to the fact that it does not require direct measurement, but can be calculated (and automatically reported) by the vast majority of commercially available hematological analyzers by simply dividing the standard deviation (SD) of the mean corpuscular volume (MCV) by the MCV. The resulting value is then multiplied by 100 to express data as a percentage (%), which is the measuring unit most widely used for reporting RDW data in clinical and laboratory practice (1). Therefore, unlike many other predictive biomarkers, the RDW is a clinically useful index which can be simply and rapidly generated on all blood samples, and essentially is without added cost.
Regardless of the increasing clinical significance of anisocytosis in health and disease (2), previous evidence showed that harmonization of RDW values obtained with different hematological analyzers is still an unmet target (3). Despite many ongoing efforts for several laboratory tests (4), the standardization of calculated parameters in laboratory medicine is a difficult target, since only laboratory methods, and not calculations, may be standardized by recalibration. Therefore, a different strategy should be employed for these indices, substantially based on harmonization rather than on standardization (5,6). One potential approach entails the recalculation of single instrument values by means of reliable coefficients after the measurement has been completed. Ideally, this can be achieved by measuring some reference or “standard” sample(s) with different instrumentation, followed by calculation of a regression line (when values are linearly distributed) or a polynomial curve (when values are non-linearly distributed) which interpolate the different measured values. The coefficients of regression line or polynomial curve could then be used for recalculating data and hopefully then achieve a major degree of harmonization. In the current study, we followed this strategy to investigate whether a major degree of harmonization may be achieved for RDW measurements.
Methods
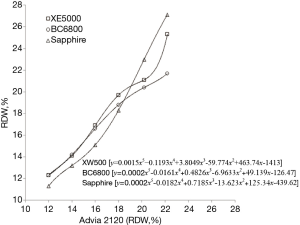
Six inpatient blood samples collected in evacuated blood tubes containing K2EDTA (Becton Dickinson Italia S.p.A., Milan, Italy) were arbitrarily selected from those measured with an Advia 2120 (Siemens Advia 2120, Diagnostic Solutions, Milan, Italy), in order to cover the clinical significant range of RDW values in health and disease (i.e., 12.0%, 14.0%, 16.0%, 18.0%, 20.0%, 22.0%). The six samples were gently mixed, and each was then divided in four identical aliquots. One aliquot was measured again on Advia 2120, whereas the three other aliquots were concomitantly measured with three different hematological analyzers (Sysmex XE5000, Dasit SpA, Cornaredo, Italy; Mindray BC6800, Medical Systems S.p.A., Genova, Italy; Abbott Sapphire, Abbott Diagnostics Division Italia, Roma, Italy). The data obtained by measuring the aliquots of the six reference samples with XE5000, BC6800 and Sapphire were then plotted against results generated by Advia 2120 on the same aliquots. As shown in Figure 1, the relationship between Advia 2120 RDW values and those generated by XE5000, BC6800 and Sapphire exhibited a substantial deviation from linearity, so that a polynomial curve needed to be constructed by interpolating the values on the plot. The resulting polynomial curves enabled a virtually perfect fitting versus Advia 2120 data (in all cases, the fitting of the polynomial curves was characterized by P<0.001).
The ensuing validation study was based on the measurement of 126 inpatient samples (mean age of the patients 57±22 years; 49 females and 77 males) referred to the local laboratory for routine hematological testing. These samples were also drawn in evacuated blood tubes containing K2EDTA (Becton Dickinson Italia S.p.A., Milan, Italy). Immediately after arrival in the laboratory, each blood sample was gently mixed and separated in four identical aliquots, which were then analyzed with each of the four hematological analyzers (Siemens Advia 2120, Sysmex XE5000, Mindray BC6800, Abbott Sapphire). All tests were completed within 2 hours after sample arrival in the laboratory. The technical principles of hematocrit, MCV and RDW measurement in the different analyzers has been described elsewhere (3). Briefly, in all cases the RDW value is extrapolated from the red blood cell (RBC) volume histogram curve, but using different statistical approaches.
Data obtained using the different hematological analyzers were then analyzed with Student’s paired t-test and Pearson’s correlation. The bias was calculated using Bland-Altman plot analysis and the agreement at the arbitrary 15% RDW threshold was estimated by calculation of diagnostic agreement and kappa statistics. The statistical analysis was carried out with Analyse-it (Analyse-it Software Ltd, Leeds, UK). The study was carried out in accordance with the Declaration of Helsinki and under the terms of relevant local legislations. As the entire study was based on pre-existing and anonymized samples, specific informed consent or approval by ethical committees were deemed unnecessary according to local regulations.
Results
The comparison of RDW values before and after recalculation by means of polynomial curves is shown in Tables 1 and 2. The distribution of raw values obtained with the four hematological analyzers was always found to be significantly different, but in no case, did the differences remain statistically significant after RDW values were recalculated with the instrument-specific polynomial curves (Table 2). Interestingly, the man bias of RDW values obtained with the different hematological analyzers could be considerably reduced after recalculation of RDW values, in all cases except one (i.e., BC6800 versus Sapphire) displaying a mean bias very close to zero and a much narrower 95% confidence interval (95% CI). In all cases except two (Advia 2120 versus BC6800 and BC6800 versus Sapphire) the agreement at the 15.0% RDW threshold was always improved after values recalculation (Table 2). The results of the correlation before and after RDW value recalculation by the instrument-specific polynomial curves is shown in Table 3. In all cases except one (XE5000 versus BC6800) the slopes and the intercepts of the Deming’s fits were significantly improved after recalculation, suggesting that a major harmonization had been achieved through this approach. Notably, the Pearson’s correlation coefficients after RDW value recalculation were slightly improved in two cases, slightly worsened in two other cases, whereas they remained virtually unchanged in the remaining two cases.
Table 1
| Analyzer | Advia 2120 | XE5000 | BC6800 | Sapphire | |||||
|---|---|---|---|---|---|---|---|---|---|
| Before recalculation | After recalculation | Before recalculation | After recalculation | Before recalculation | After recalculation | ||||
| n | 126 | 126 | 126 | 126 | 126 | 126 | 126 | ||
| Mean ± SD (%) | 15.7±2.3 | 16.5±2.7 | 16.0±2.5 | 15.4±3.1 | 15.7±2.1 | 15.6±2.5 | 15.8±2.2 | ||
RDW, red blood cell distribution width; SD, standard deviation.
Table 2
| Analyzer | XE 5000 | BC6800 | Sapphire | |||||
|---|---|---|---|---|---|---|---|---|
| Before recalculation | After recalculation | Before recalculation | After recalculation | Before recalculation | After recalculation | |||
| Versus Advia 2120 | ||||||||
| Significance of difference (P) | <0.001 | 0.496 | 0.004 | 0.139 | 0.003 | 0.238 | ||
| Mean bias (95% CI) | 0.75% (0.60–0.90%) | −0.01% (−0.14–0.14%) | 0.26% (0.07–0.44%) | −0.11% (−0.30–0.09%) | −0.31% (−0.53–−0.09%) | 0.04% (−0.07–0.06%) | ||
| Agreement at 15% | 89% (kappa, 0.76; |
90% (kappa, 0.79; |
87% (kappa, 0.74; |
87% (kappa, 0.73; |
90% (kappa, 0.81; |
93% (kappa, 0.85; |
||
| Versus XE5000 | ||||||||
| Significance of difference (P) | – | – | <0.001 | 0.081 | <0.001 | 0.281 | ||
| Mean bias (95% CI) | – | – | −0.50% (−0.65–−0.34%) | −0.10% (−0.25–0.04%) | −1.06% (−1.22–−0.90%) | 0.04% (−0.10–0.07%) | ||
| Agreement at 15% | – | – | 89% (kappa, 0.76; |
91% (kappa, 0.81; |
79% (kappa, 0.59; |
89% (kappa, 0.77; |
||
| Versus BC6800 | ||||||||
| Significance of difference (P) | – | – | – | – | <0.001 | 0.060 | ||
| Mean bias (95% CI) | – | – | – | – | −0.57% (−0.79–−0.34%) | 0.31% (−0.03–0.09%) | ||
| Agreement at 15% | – | – | – | – | 87% (kappa, 0.75; |
87% (kappa, 0.75; |
||
RDW, red blood cell distribution width; CI, confidence interval.
Table 3
| Analyzer | XE 5000 | BC6800 | Sapphire | |||||
|---|---|---|---|---|---|---|---|---|
| Before recalculation | After recalculation | Before recalculation | After recalculation | Before recalculation | After recalculation | |||
| Versus Advia 2120 | y = 1.17x − 1.89 (r=0.95) | y = 0.91x − 1.35 (r=0.95) | y = 1.11x − 1.52 (r=0.91) | y = 1.08x − 1.91 (r=0.91) | y = 1.38x − 6.35 (r=0.94) | y = 0.97x + 0.51 (r=0.96) | ||
| Versus XE5000 | – | – | y = 0.95x + 0.36 (r=0.94) | y = 1.21x − 3.44 (r=0.95) | y = 1.18x − 4.00 (r=0.96) | y = 1.06x − 0.92 (r=0.94) | ||
| Versus BC6800 | – | – | – | – | y = 1.25x − 4.63 (r=0.92) | y = 0.87x + 2.18 (r=0.91) | ||
Discussion
The results of this pilot study show that a major degree of harmonization of RDW values measured by different hematological analyzers may be simply achieved by recalculating values according to instrument-specific polynomial curves. The considerable reduction of bias is indeed the most important outcome of our investigation. Notably, when Advia 2120 was used as the reference instrumentation, the bias after RDW value recalculation decreased by 0.74% with XE5000, 0.15% with BC6800 and 0.27% with Sapphire, respectively. A considerable reduction of bias was also observed using XE500 as the reference analyzer (i.e., reduction of bias by 0.40% with BC6800 and 1.02% with Sapphire, respectively), as well as when comparing BC6800 with Sapphire (i.e., reduction of bias by 0.26%) (Table 2). The agreement at the 15.0% RDW cut-off was also improved in four out of six analyzers’ comparisons, whereas it remained virtually unchanged in the remaining two analyzers’ comparisons. Notably, the major degree of harmonization among RDW measures is finally attested by the Deming’s fit equation, which exhibited a consistent improvement of the relative coefficients (Table 3).
One of the leading problems challenging the clinical use of RDW in clinical practice is attributable to the fact that the various studies in the scientific literature used different instrumentation, so generating different cut-offs for diagnosing and monitoring various diseases (7). As a paradigmatic example, a critical review of the literature of studies exploring the association between RDW and mortality in patients with coronary artery disease reported that the risk threshold of RDW was dramatically broad across the different investigations, ranging from as low as 13.8% to as high as 15.7% (6). Despite our data need to be validated in larger clinical studies, encompassing the use of a greater number of hematological analyzers and samples, we can conclude that harmonization based on developing instrument-specific polynomial curves may be seen as a reliable strategy for improving the clinical usefulness of RDW across different laboratories (8).
Conclusions
Once universal consensus about a reference technique has been achieved, the uniform expression of RDW values could hence be achieved by either encouraging the different manufacturers to implement internal coefficients and adjusting their values against the reference method, but also by implementing instrument-specific coefficients in the laboratory information system (LIS) for automatically recalculating the RDW values according to the reference technique. If this is not achievable, then another utility would be within specific clinical studies where tests could be performed on different platforms and reported as both raw data and as ‘harmonized’ data.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob.2017.09.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). As the entire study was based on pre-existing and anonymized samples, specific informed consent or approval by ethical committees were deemed unnecessary according to local regulations.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Salvagno GL, Sanchis-Gomar F, Picanza A, et al. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci 2015;52:86-105. [Crossref] [PubMed]
- Lippi G, Plebani M. Red blood cell distribution width (RDW) and human pathology. One size fits all. Clin Chem Lab Med 2014;52:1247-9. [Crossref] [PubMed]
- Lippi G, Pavesi F, Bardi M, et al. Lack of harmonization of red blood cell distribution width (RDW). Evaluation of four hematological analyzers. Clin Biochem 2014;47:1100-3. [Crossref] [PubMed]
- Topic E, Nikolac N, Panteghini M, et al. How to assess the quality of your analytical method? Clin Chem Lab Med 2015;53:1707-18. [Crossref] [PubMed]
- Tate JR, Johnson R, Barth JH, et al. Harmonization of laboratory testing - A global activity. Clin Chim Acta 2014;432:1-3. [Crossref] [PubMed]
- Plebani M, Panteghini M. Promoting clinical and laboratory interaction by harmonization. Clin Chim Acta 2014;432:15-21. [Crossref] [PubMed]
- Montagnana M, Cervellin G, Meschi T, et al. The role of red blood cell distribution width in cardiovascular and thrombotic disorders. Clin Chem Lab Med 2011;50:635-41. [PubMed]
- Danese E, Lippi G, Montagnana M. Red blood cell distribution width and cardiovascular diseases. J Thorac Dis 2015;7:E402-11. [PubMed]
Cite this article as: Lippi G, Pipitone S, Favaloro EJ. Harmonization of red blood cell distribution width (RDW): an attainable target? Ann Blood 2017;2:15.