
Challenges in the laboratory diagnosis and management of von Willebrand disease in South Africa
Introduction
According to the South African Haemophilia Foundation, patients with bleeding diatheses in South Africa are cared for in 20 haemophilia treatment centres (HTCs) nationwide. The function of HTCs, in collaboration with the South African National Department of Health, the South African Haemophilia Foundation (the national members’ organization) with its Medical and Scientific Advisory Council, and the National Haemophilia Nurses Committee, is to ensure optimal management of patients with bleeding disorders such as von Willebrand disease (VWD) (1).
The 2016 Global Survey of the World Federation of Haemophilia estimated that there are 632 patients diagnosed with VWD in South Africa; 375 female and 257 male. Of these patients, 431 have mild, 61 moderate and 42 severe VWD. Most of these patients are diagnosed between the ages of 14 and 44 years. VWD patients are predominantly diagnosed in five academic centres and a single central reference laboratory does the confirmatory testing that aids in the subclassification of VWD. This paper will concentrate on the reference laboratory’s findings regarding these statistics, as well as challenges in the laboratory diagnosis and management of VWD in South Africa.
Methods
Laboratory diagnosis and management of VWD
The VWD reference laboratory adopted and modified the guidelines for diagnosis and treatment of VWD in Italy (2,3) according to the algorithm outlined in Figure 1. In concordance with these guidelines the following diagnostic tests are offered at the reference laboratory: von Willebrand factor (VWF) antigen (VWF:Ag), ristocetin cofactor activity (VWF:RCo), collagen binding assay (VWF:CB), multimeric analysis, VWF propeptide levels (VWF:pp), factor VIII (FVIII) binding assay, ristocetin induced platelet agglutination (RIPA), and RIPA mixing studies to identify platelet type VWD (PT-VWD) (3).
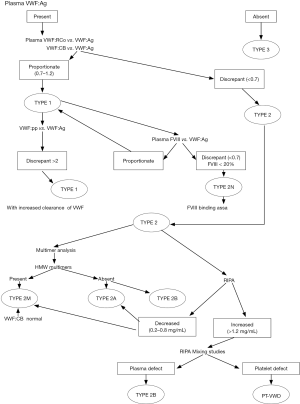
A proportional reductixon of both VWF:Ag and VWF:RCo with a RCo/Ag ratio ≥0.7 as well as a proportional reduction of both VWF:Ag and VWF:CB with a CB/Ag ratio ≥0.7 suggests type 1 VWD. If type 1 VWD is diagnosed, the clearance rate of VWF is determined and the VWF:pp then performed. If the ratio between the VWF:pp and the VWF:Ag is more than 2, an increased clearance rate of VWF is suspected for this patient (i.e., type 1 Vicenza).
If the RCo/Ag ratio and/or the CB/Ag ratio is <0.7, then type 2 VWD is diagnosed. Type 2B VWD can be identified with an enhanced RIPA (response to <0.8 mg/mL ristocetin). Type 2B VWD is distinguished from a PT-VWD (pseudo VWD) by performing RIPA mixing studies. Type 2A and 2M might show a reduced RIPA (response only to >1.2 mg/mL ristocetin). Multimeric analysis in plasma is necessary to distinguish between type 2A VWD (lack of largest and intermediate multimers) and type 2M VWD (all multimers are present). The multimeric distribution pattern of type 2M VWD often differs from that of normal plasma in the lower density of high molecular weight multimers and the higher density of lower molecular weight multimers. The VWF:CB is usually normal in type 2M VWD due to the presence of the high molecular weight multimers, except where a collagen binding defect is diagnosed in such patients. In type 1 VWD, the ratio between FVIII level and VWF:Ag is always concordant. When this ratio is discrepant (i.e., FVIII/Ag <0.7) with a FVIII level of less than 20%, type 2N VWD is suspected and this subtype can be confirmed by performing a FVIII binding assay (3).
This VWD reference laboratory is also the only laboratory in the country that performs the VWF:CB, the mutimeric analysis of VWF, the VWF:pp and the VWF:FVIII binding assay (3).
The diagnostic screening tests for VWD include the VWF:Ag, WF:RCo, VWF:CB and the multimeric distribution of VWF. Confirmatory tests include RIPA and the VWF:FVIII binding assay. However, most other laboratories in South Africa, except for the reference laboratory, only perform the VWF:Ag and VWF:RCo assays (i.e., essentially screening for VWD).
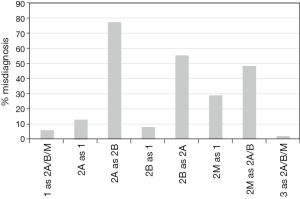
Retrospective data of 250 VWD cases were gathered and the percentage misdiagnoses were calculated, should only the VWF:Ag and VWF:RCo assays be used. The results are shown in Figure 2.
The relative distribution of the various VWD subtypes diagnosed by the VWD reference laboratory from 2011 to 2016 is outlined in Table 1. Laboratory statistics, as well as challenges in the laboratory diagnosis and management of VWD in South Africa were determined by the reference laboratory. Information regarding the available treatment modalities for VWD and challenges thereoff were gathered from all heamophilia treatment centers nationwide.
Table 1
| Subtype of VWD | Percentage [number] of total patients diagnosed, N=255 |
|---|---|
| Mild type 1 | 23 [59] |
| Moderate to severe type 1 | 13 [33] |
| Type 2A | 28 [73] |
| Type 2B | 12 [30] |
| Type 2M | 20 [50] |
| Type 2N | 0 [0] |
| Type 3 | 4 [10] |
| Platelet type | 0 [0] |
VWD, von Willebrand disease.
Results
Challenges in the laboratory diagnosis of VWD
By performing only the two most popular tests (VWF:Ag and VWF:RCo), 6% of type 1 VWD patients would have been misdiagnosed as type 2 VWD; 13% of type 2A patients would be classified as type 1 and 77% as type 2B disease; 8% of type 2B VWD patients would be misdiagnosed as type 1 and 55% as type 2B disease; 28% of type 2M VWD patients would be misdiagnosed as type 1 and 48% as type 2A or 2B VWD; 1% of type 3 VWD will be misdiagnosed as type 2 VWD. Moreover, even if the multimeric analysis were to be included in this diagnostic setup (i.e., together with the two most popular tests), still 20% of patients would still be misdiagnosed.
As previously published, the vast distances between referral laboratories and the reference laboratory leads to thermal sample compromise and testing challenges. When samples are stored at temperatures warmer than ‒70 °C, a cryoprecipite might be formed which greatly affects multimeric analysis (3). In the time period between 2012 and 2017, 8% of referred samples were rejected due to poor sample storage and transport conditions. The challenges posed by the geographically vast area of Southern Africa, as covered by the reference laboratory, and the previously described issues with specimen preparation, freezing and transport remain, and have furthermore been exacerbated by a general lack of dry ice for specimen transport (3).
Another challenge in the laboratory diagnosis of VWD, is the cost of the assays and the investigation. In the South African private medical sector, laboratory testing costs are covered by various medical aid schemes. However, the price that the medical aids are authorised to extend for these tests is often less than the costs of the latest test kits. Due to these cost constraints, the reference laboratory developed and validated in-house assays for more cost-effective VWD testing. These include the VWF:Ag and VWF:CB, the multimeric analysis and the propeptide assay (Meiring et al., 2017 unpublished data). The VWF:RCo has challenges of its own, since the sensitivity is reduced at low levels (<15%) (4). It is thus of utmost importance to perform all diagnostic tests to prevent misdiagnosis of the disorder.
VWD diagnostic statistics
The VWD reference laboratory is situated in Bloemfontein, the legislative capital of South Africa, in the central part of the country. The academic complex in Bloemfontein serves patients from the Free State and the Northern Cape provinces (a geographically vast area) with a total population of about 4 million (5). The VWD reference laboratory, however, receives patient samples country-wide for both initial screening and diagnosis, but especially for the classification of VWD. The relative distribution of the various VWD subtypes diagnosed by the VWD reference laboratory from 2011 to 2016 is outlined in Table 1. The reference laboratory still receives mostly type 2 VWD samples to be sub-classified, as also mentioned in 2011 (3). Still no type 2N VWD patients have been diagnosed. The reason might be the improbability of an autosomal recessive disorder in our diverse population or the possibility of misdiagnoses of type 2N with haemophilia carriers by the HTCs.
Treatment of VWD in South Africa
Available treatment modalities include desmopressin (DDAVP), FVIII/VWF concentrates, antifibrinolytic drugs, topical therapies and hormonal treatment modalities for women. DDAVP is the treatment of choice in patients with type 1, 2A and 2M. Treatment with DDAVP is subject to a proven plasma response of FVIII levels and VWF:RCo activity.
Two intermediate purity FVIII/VWF concentrates are available in South Africa for the treatment of VWD: Haemosolvate® produced by the National Bioproducts Institute (NBI) from pooled fresh human plasma, and Virally Inactivated Factor VIII (VIAHF) produced by the Western Province Blood Transfusion Service (WPBTS) from small pools (5–6 bags) of cryoprecipitate. Both products are prepared from plasma obtained from voluntary non-remunerated donors after individual donation serologic and nucleic acid amplification and exclusion of human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV) infection. Both undergo viral inactivation steps which inactivate HIV, HBV and HCV: Haemosolvate® using a solvent-detergent process, and VIAHF via 80 °C heating for 72 hours. Haemosolvate® or VIAHF are considered the treatment of choice when DDAVP is not indicated or ineffective, as both concentrates contain high molecular weight VWF multimers (6). In a previous study by our laboraotry, we have proven VWF:Ag levels in Haemosolvate® to be as high as double that of FVIII, and our centre is also involved in continuous quality control monitoring of this product (3).
Tranexamic acid (Cyclokapron®) is available in capsule and intravenous formulations. Recent studies confirm the effectiveness of prophylactic tranexamic acid in reducing the number of mild and moderate bleeding episodes (7,8); however, this has not yet been explored at our centre. The use of tranexamic acid on an on-demand basis with bleeding episodes, is advocated locally.
Hormonal treatments for women with VWD include combined oral contraceptives and hormonal intra-uterine devices, which are readily available in our country.
Available topical therapy is limited to fibrin glue (Thromboseel® and Tisseel®, South African National Blood Service and Adcock Ingram Critical Care).
Challenges in the treatment of VWD in South Africa
In South Africa, the route of administration of DDAVP is limited to a single DDAVP intravenous formulation which can also be administered subcutaneously. The intranasal preparation of DDAVP, although available in South Africa for the treatment of enuresis (0.1 mg/mL), is not sufficient in concentration for use in VWD, where a concentration of 1.5 mg/mL is quoted in international guidelines (6). This is a challenging limitation in paediatric VWD care where intranasal DDAVP would be a convenient choice.
Although intermediate purity FVIII/VWF concentrates are relatively freely available locally, no high purity VWF concentrate is currently available in South Africa. When used to treat VWD, FVIII/VWF concentrates can result in markedly elevated FVIII levels (disproportionate to the VWF levels achieved with treatment) which has been associated with thrombosis (9). A high purity VWF product could prevent such disproportionate increases in FVIII levels, further improving treatment safety (6).
The Medical and Scientific Advisory Council of the South African Haemophilia Foundation has issued guidelines for the treatment of haemophilia in South Africa (10), but no formal guidelines focusing on the management of VWD in South Africa have been published—a significant obstacle to safe, standardised care. Locally tailored guidelines similar to the United Kingdom Haemophilia Centre Doctors Organization guideline approved by the British Committee for Standards in Haematology (11) or the National Heart, Lung, and Blood Institute guidelines (6) should ideally be drafted on a national level. Guidance on issues specifically applicable to resource constrained environments like South Africa, such as prophylaxis, genetic testing, and management of surgical interventions, may be particularly useful. Such guidelines may also be of value to other developing countries, where the use of American and British guidelines may not be appropriate.
Discussion
The probable underdiagnosis of VWD in South Africa, may be a consequence of poor physician awareness of the disease, which may be a result of inadequate undergraduate medical training on the bleeding disorders in general, and VWD in particular. VWD as a specific disease entity receives only approximately 20 minutes of dedicated lecture time in the 5-year undergraduate medical curriculum at the University of the Free State.
This may be difficult to address in light of the heavy demands placed on undergraduate medical curricula to accommodate vast amounts of information from different disciplines, but may be amenable to interventions at post-graduate level. Training interventions targeted at interns and other junior doctors may be effective in improving practice standards, as was recently shown locally in transfusion medicine (12,13). Such training interventions should ideally also target physicians that are most likely to be faced with VWD patients at presentation, such as gynaecologists, paediatricians, otorhinolarynologists and general practitioners.
Misdiagnosis (now thought to possibly be a bigger problem than previously suspected) may be reduced by routinely using a more extensive testing profile, which is currently not standardised in South Africa. This reiterates the need for national guidelines on both the laboratory testing and treatment of VWD, which are currently lacking. Product issues (lack of suitable intranasal DDAVP preparations and high-purity VWF concentrates) remain a challenge as well as a general lack of physician awareness and limited undergraduate training, which may contribute to underdiagnosis of the disease.
Conclusions
The advancement of VWD care in South Africa, and perhaps many other developing countries, would likely benefit most from the establishment of national guidelines, targeted postgraduate training interventions and improved undergraduate medical training, an expanded treatment product repertoire and standardised laboratory testing.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Emmanuel J. Favaloro) for the series “Diagnosis and Management of von Willebrand Disease: Diverse Approaches to a Global and Common Bleeding Disorder” published in Annals of Blood. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob.2017.11.01). The series “Diagnosis and Management of von Willebrand Disease: Diverse Approaches to a Global and Common Bleeding Disorder” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the manuscript and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the declaration of Helsinki (as revised in 2013). As the entire study was based on pre-existing and anoymized samples, specific informed consent were deemed unneccessary according to local regulations.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mahlangu JN. Haemophilia care in South Africa: 2004-2007 look back. Haemophilia 2009;15:135-41. [Crossref] [PubMed]
- Federici AB, Castman G, Mannucci PM. Guidelines for the diagnosis and management of von Willebrand disease in Italy. Haemophilia 2002;8:607-21. [Crossref] [PubMed]
- Meiring SM, Coetzee MJ, Kelderman M, et al. Laboratory diagnosis and management of von Willebrnad disease in South Africa. Semin Thromb Hemost 2011;37:576-80. [Crossref] [PubMed]
- Favaloro EJ, Bonar RA, Meiring M, et al. Evaluating errors in the laboratory identification of von Willebrand disease in the real world. Thromb Res 2014;134:393-403. [Crossref] [PubMed]
- Statistics South Africa, 2008. Commuity Survey 2007; Basis results – Municipalities. Statistical Release P301.1
- Nichols WL, Hultin MB, James AH, et al. von Willebrand disease (VWD): evidence-based diagnosis and management guidelines, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA). Haemophilia 2008;14:171-232. [Crossref] [PubMed]
- South African National Blood Service (SANBS), Western Province Blood Transfusion Service (WPBTS). Clinical guidelines for the use of blood products in South Africa 2014; 5th ed. Available online: http://www.wpblood.org.za/village/wpbnew/sites/default/files/clinical_guidelines_5th%20Edition_2014.pdf
- Eghbali A, Melikof L, Taherahmadi H, et al. Efficacy of tranexamic acid for the prevention of bleeding in patients with von Willebrand disease and Glanzmann thrombasthenia: a controlled, before and after trial. Haemophilia 2016;22:e423-6. [Crossref] [PubMed]
- Mannucci PM. Treatment of von Willebrand’s disease. New Engl J Med 2004;351:683-94. [Crossref] [PubMed]
- Mahlangu JN, Gilham AMedical and Scientific Advisory Council of the South African Haemophilia Foundation. Guideline for the Treatment of Haemophilia in South Africa. S Afr Med J 2008;98:126-40. [PubMed]
- Laffan MA, Lester W, O’Donnell JS, et al. The diagnosis and management of von Willebrand disease: a United Kingdom Haemophilia Centre Doctors Organization guideline approved by the British Committee for Standards in Haematology. Brit J Haematol 2014;167:453-65. [Crossref] [PubMed]
- Joubert S, Bosman M, Joubert G, et al. The utilization of red cell concentrates at Kimberley Hospital Complex, Northern Cape Province, South Africa. Transfus Apher Sci 2013;49:522-7. [Crossref] [PubMed]
- Joubert J, Joubert S, Raubenheimer J, et al. The long-term effects of training interventions on transfusion practice: A follow-up audit of red cell concentrate utilisation at Kimberley Hospital, South Africa. Transfus Apher Sci 2014;51:25-32. [Crossref] [PubMed]
Cite this article as: Meiring M, Haupt L, Conradie C, Joubert J. Challenges in the laboratory diagnosis and management of von Willebrand disease in South Africa. Ann Blood 2017;2:19.


