
Von Willebrand disease—the Nordic perspective
Introduction
The Nordic Haemophilia council has established its bylaws in 2008, and the original members include the hemophilia centers operating in Sweden, Norway, Denmark, Finland and Iceland (www.nordhemophilia.org). The members are medical doctors who treat both pediatric and adult populations, and are involved in the laboratory. In 2017, also the Baltic states of Estonia, Lithuania and Latvia have been recently offered the opportunity to take part and network in the meetings. The council has as the main aims of hosting Nordic meetings, establishing local guidelines and fostering scientific interactions in the geographical region.
The recognition and diagnosis of von Willebrand disease (VWD) originated from Finland, the Island of Åland, from the first report and innovation by Dr. Erik von Willebrand in 1926 (1). The early development in diagnostics and treatment took place in the Nordic countries, mainly Sweden, by Inga Marie Nilsson and Margareta Blombäck, the legacy of which is continued internationally. Since 2011, when the Nordic Guidelines on VWD were published (2), some new developments in the following aspects have ensued: organization, clinical practice, laboratory diagnostics, including alternative hemostatic mechanisms, and new management opportunities. The main changes have included: (I) the strategic and standardized improvement in provision of care across Europe, including the Nordic and Baltic countries, implementing the multidisciplinary approach; (II) the standardized assessment of bleeding history of the patient and the family; (III) von Willebrand factor (VWF) assessment from ristocetin cofactor activity to utilization of the platelet binding site of glycoprotein (GP) Iba, and exploration of global coagulation and thrombin generation assays; IV) focus on other, mainly platelet-derived causes of mild/moderate bleeding phenotype; and (V) pure VWF-containing concentrate has become available in the Nordic market (Wilfactin®). We will address all these issues in this short report of Nordic perspective.
Organization of care in bleeding disorders
During the same period, which has elapsed from our practical guidelines in 2011 (2), the organization of care in bleeding disorders has been upgraded at the European level (3). The activities of European Association of Haemophilia and Allied Disorders (EAHAD, year 2008) have been initiated with (I) the prospective safety surveillance register (EUHASS) with every 3-month reporting; and (II) the harmonization of the treatment centers by the common criteria of comprehensive care centers (EHCCC) and haemophilia treatment centers (EHTC) (EUHANET in year 2014). The main criteria for EHCCC include: (I) the patient numbers: at least 40 patients with severe haemophilia and/or allied disorders; (II) 24/7 access to expert care; (III) 24/7 access to coagulation laboratory; and (IV) active research initiatives (www.EAHAD.org, www.EUHASS.org, www.hclocator.org).
All Nordic centers take part in these EAHAD activities, and each country has at least one center with the EHCCC status; Sweden with its largest population has 3 EHCCC-status centers. The Nordic Haemophilia Council together with EUHASS surveyed the Nordic haemophilia centers with regard to their VWD patient numbers. The estimated numbers are as follow: 73 type 3, 409 type 2 and 725 type 1 VWD patients. Data on type 2 VWD subtypes are available from 3 centers: 163 type 2A, 68 2B, 27 2M and 5 2N (Table 1).
Table 1
| Diagnosis | Number (%) | Patients treated with annually VWF or VWF/FVIII concentrates |
|---|---|---|
| Type 3 VWD | 73 (6.0) | 46 |
| Type 2 VWD | 409 (33.8) | 58 |
| 2A | 163 (39.9) | |
| 2B | 68 (16.6) | |
| 2M | 27 (6.6) | |
| 2N | 5 (1.2) | |
| Unknown | 146 (35.7) | |
| Type 1 VWD | 725 (59.9)* | 7 |
| VWD type unknown | 3 (0.2) | 0 |
| Total | 1,210 |
Centers included were Aarhus and Copenhagen, Denmark; Helsinki, Finland; Gothenburg, Malmö and Stockholm, Sweden. The data are from years 2014–2016. *These number represents the patients who received clinical attention, not the total number of type 1 VWD. The distribution of the type 2 VWD subtypes is based on the reports by three centers (Helsinki, Gothenburg, Malmö).
Clinical standardized assessment
Along the EUHANET project, the comprehensive and treatment centers have been launched in Europe based on the defined criteria of EAHAD (www.EUHANET.org). The active use of bleeding score (BS) assessment (Bleeding Assessment Tool of International Society of Thrombosis and Haemostasis, BAT-ISTH, Tosetto BS, and recent BS to be used among pediatric population) assists in grading the severity of the bleeding tendency, and excluding any bleeding tendency, which is not of clinical or diagnostic importance (4,5). However, the BS fails to differentiate VWD from other bleeding disorders. To conclude, females gaining more than 5 points (beyond the weakened hemostasis related to menorrhagia and bleeds during delivery, which can also occur without VWD, typically in platelet function defects) and males gaining more than 3 points (several sites of bleeds) fulfill the criteria of VWD, assuming their laboratory assessment is compatible with this diagnosis (1). The family history can be subjective in uncertain cases, and may need the objectivity of patient files. As an example, sometimes in deviation from true mucocutaneous and spontaneous bleeds, bleeds that do not associate with a bleeding disorder, need further assessment. These include local, such as surgical or postpartum bleeds, which due to arising complications may achieve the misconception of a bleeding phenotype among the family members. The role of a coagulation expert in providing genetic and diagnostic counseling and clinical guidance, for the future clinical approach is very important (6).
Upon appropriate bleeding phenotype, the clinical evaluation should be compared with the laboratory assessment. When there is concordance in both assessments, the diagnosis of VWD as the underlying bleeding disorder is clear. The VWD subtyping needs more commitment (Table 2). Sub-typing will rarely have an impact on the patient management, excluding type 2B and 2N, but family issues associated with genetic counseling should be the main motivation for the subtyping, unless this is performed within the setting of a research project. In the subtype 2B the use of desmopressin is not recommended due to the risk of inefficacy and deepening thrombocytopenia by the release of functionally impaired VWF. One differential diagnostic issue is the recognition of mild hemophilia A versus type 2N VWD with the deficient FVIII binding, wherein the genotypic diagnosis will usually provide the definite answer.
Table 2
| VWD type | Frequency (% of VWD) | Bleeding phenotype | Genetic inheritance | Response to DDAVP | First line VWF assays | Second line VWF assays | Genotyping |
|---|---|---|---|---|---|---|---|
| 1 | 70 | Mild to moderately severe | Autosomal dominant | Good | VWF activity <0.35 IU/dL; Activity to Ag ratio ≥0.7; VWF:CB normal; VWF:CB to Ag ratio ≥0.7 | High RIPA variable decreased or normal; VWF multimers for equivocal cases, all MWM present | Not indicated |
| 2A | 10–15 | Moderate to moderately severe | Autosomal dominant or recessive | Mild to moderate | Activity to Ag ratio <0.7; VWF:CB to Ag ratio <0.7 | High RIPA decreased; high & intermediate MWM missing | Exons 20–27 |
| 2B | <5 | Moderate to moderately severe | Autosomal dominant | Not indicated | Activity to Ag ratio <0.7; VWF:CB to Ag ratio <0.7 | Low RIPA increased, high RIPA decreased; high MWM usually missing | Exon 28 |
| 2M | <10 | Significant | Autosomal dominant | Mild to moderate | Activity to Ag ratio <0.7; VWF:CB normal; VWF:CB to Ag ratio ≥0.7 | High RIPA decreased; all MWM present | Exons 29–52 |
| 2N | Uncommon | Mild to moderate | Autosomal recessive | Suboptimal | Activity to Ag ratio <0.7; VWF:CB normal; VWF:CB to Ag ratio ≥0.7 | VWF:FVIIIB decreased; all MWM present | Promoter |
| 3 | Rare, but high in Scandinavia | Severe | Autosomal recessive | No response | Virtually absent VWF | Virtually absent VWF | Whole gene |
CB, collagen binding; DDAVP, desamino-8-arginine vasopressin; MWM, molecular weight multimers; RIPA, ristocetin-induced platelet aggregation; VWF, von Willebrand factor; VWF:FVIIIB, VWF-factor VIII binding.
One clinical aim should also be the re-evaluation of historical diagnoses, to focus on the bleeding history, and remove any unnecessary concern of a bleeding disorder in the absence of significant bleeding tendency within a family and individuals. The modestly low VWF levels alone may get improved during aging, and blood group O may in fact offer protection from thromboembolic diseases (7,8). The re-evaluation will assist to reach management decisions concerning the thrombotic risk or disorders, which increase in prevalence among the aging population or populations with many thrombotic risk factors and co-morbidities, including atrial fibrillation (7). The priority of pure VWF containing concentrate can be motivated to avoid elevated (>190 IU/dL) FVIII concentrations, which in the non-bleeder population associate with enhanced thrombotic risks and is sustained at least in the recovery period after major surgery with the use of FVIII containing VWF concentrates, e.g., at least up to 30 days after hip replacement, even under effective thromboprophylaxis (9,10).
New laboratory perspectives
The proper identification of VWD and differentiation of its major types are important for optimal treatment and require a panel of different VWF assays, including VWF activity. The Nordic centers collaborate because some tests, i.e., VWF multimers and VWF:FVIII binding assay are not available at all laboratories. We present the diagnostic algorithm followed by the Nordic centers in association with clinical suspicion for VWD in Figure 1.
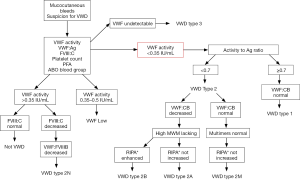
The clinical utility of traditional ristocetin cofactor (VWF:RCo) assay is compromised by the large assay variability, suboptimal sensitivity and high VWF detection limit (11). The new VWF activity assays, being independent of ristocetin and lyophilized platelets, may overcome these disadvantages, and have become available also in the Nordic countries (12,13). These VWF activity assays rely either on the gain-of-function GPIb mutants that bind VWF without the site-specific change of the charge caused by ristocetin (general name: VWF:GPIbM) or on a monoclonal antibody that recognizes the functional GPIb-binding epitope on VWF (general name: VWF:Ab). External quality assessment (EQA) programs in Europe, involving Nordic treatment centers, report an increasing trend for laboratories to incorporate the new VWF activity assays into the routine practice. The VWF:GPIbM test benefits from its simplicity, comparability with VWF:RCo, but improved diagnostic accuracy at the very low VWF levels (14). Although the VWF:RCo activity method is not yet replaced in official recommendations for VWD testing, the number of correlation studies between VWF:RCo and VWF:GPIbM is increasing, and it will probably reach the guidelines as the data are quite convincing (15).
Platelet function tests in whole blood
Evaluation of the severity of VWD types based on traditional plasma assays remains difficult. No blood flow and shear-forced based clinical assays are available for routine use. Evaluation of VWF-dependent platelet function in whole blood can add to screening and provide support for the challenging diagnostics. PFA-100 and PFA-200 (Platelet Function Analyzer) indeed show high sensitivity for severe VWD, but have been questioned for the screening of mild VWD and 2N VWD (16). While some Nordic centers have demonstrated an added value of PFA in all mild VWD types (e.g., Nummi et al., in press), others have removed it from their otherwise complete laboratory test battery of VWD. It is to be noted that the complete panel of plasma based VWD tests and additional global coagulation assay do not explore VWF function under physiologic blood cells and flow conditions, which the newest microfluidic assays do and are suggested for the future evaluation of primary hemostasis (17).
Multiplate® is the new generation of whole blood aggregometry with potential diagnostic value in VWD (18). Recent studies on Multiplate have demonstrated both (I) agreement with Born aggregometry and (II) diagnostic accuracy of ristocetin-induced platelet aggregation (WB-RIPA) in VWD (19,20). Further studies are required to improve diagnostic capability and demonstrate correlation of Multiplate with patient clinical features. So far, some of the treating centers have used Multiplate either as a rule out test (19), or as a diagnostic test by means of validated, in-house WB-RIPA (e.g., Nummi et al., in press). It is likely that the simplicity of Multiplate encourages other centers to include WB-RIPA in their diagnostic test repertoire for VWD in the future. However, lately there have been problems associated with the Multiplate collagen reagent used for differential diagnostics. The liquid collagen reagent from another source can be used in Multiplate to assess platelet receptors for collagen.
VWF multimer analysis
The size distribution of VWF multimers, determined by gel electrophoresis, followed by Western blotting, is performed for subtyping of VWD, and can differentiate types 2A and 2B, from 2M and type 1 VWD (20). In addition, some centers prefer routine testing of VWF multimers in all type 3 patients as part of their comprehensive laboratory evaluation because of the concern of residual replacement therapy and the sensitivity limits of the VWF:Ag assay. Acquired VWD is associated with variable multimeric patterns, depending on the underlying etiology, and such, testing may add to the optimal management of these patients, including patients with the aortic valve stenosis and impaired hemostasis.
VWF multimer analysis is not available in all Nordic countries. The assay is complex, time consuming, requires specialized equipment and technical expertise, and is not standardized. New rapid and semi-automated assays, providing quantitative determination of VWF multimers have been developed to standardize the method and overcome technical difficulties (21,22). EQA programs need to assess their diagnostic accuracy before implementation into routine diagnostics. This initial testing of the semi-automated method has now reached the Nordic and Baltic countries, and data are soon expected to be published.
Global coagulation tests
Global hemostatic assays, i.e., viscoelastic coagulation analyses and thrombin generation (TG), are increasingly used in perioperative, emergency and elective assessment of bleeding tendency and show also potential in evaluating clotting capacity beyond factor levels in inherited bleeding disorders (23,24). Most data are available in hemophilia patients with inhibitors and with modified reagents (i.e., kaolin, diluted Innovin), but deviations of being able or unable to correlate the observations with the clinical phenotype are reported (25,26). One should consider the contribution of platelets in trauma and surgery settings, as they may better reflect the hemostatic capacity of VWD patients. However, the requirement of platelets in the TG assay restricts evaluation in multicentre settings. In contrast, standard rotational thromboelastometry is of low diagnostic value in VWD (27).
Indeed, TG assays are not in routine clinical use, and little is known about TG in VWD. Moreover, laboratories and clinicians also need to deal with many pre-analytical variables affecting TG test results, as well as significant both intra-patient and inter-laboratory variability of the data due to lack of standardization. There is an on-going multicenter effort in the Nordic countries for standardization of TG by Calibrated Automated Thromboelastography (Zetterberg et al., under preparation). Recent data have demonstrated a clear discriminative power for TG in VWD, with seriously impaired TG and potential use in clinical practice (27,28). Although FVIII has a clear role in TG capacity in VWD, this does not seem to be the main cause for the low TG capacity in type 3 VWD (Szanto et al., ISTH-SSC meeting 2016).
Genotyping
The genotyping of VWD needs centralized analysis with specialized expertise. A cohort of 10 type 3 patients from Finland have been genotyped, indicating little genetic heterogeneity with mainly two mutations of common genetic origin among these type 3 cases (29). The same patients have been later included also in the prospective study, entitled ‘Type 3 von Willebrand Disease International Registries and Inhibitor Prospective Study (3WINTERS-IPS)’ organized on behalf of the European Group on VWD3 and approved by the VWF ISTH Scientific and Standardization Committee and EAHAD.
Genotyping is available in some laboratories of the Nordic countries or the service is outsourced. The impact of genotyping is in the differential diagnosis, i.e., VWD types 2N and 2B for differentiating from platelet-type VWD and mild to moderate hemophilia. In addition, genetic testing should be undertaken in VWD patients having inconsistent clinical/laboratory findings, and in all type 3 cases in connection with genetic counseling. The multidisciplinary approach is recommended, involving coagulation experts from the clinic, both coagulation and genetic laboratories and genetic counselors. They should together provide information on the personal and familial risks associated with type 3 VWD and on management strategies, and for identification of relatives who are carriers of the disease-causing mutation. Also, in uncertain mild cases the genotyping is helpful, but the multimer analysis together with VWF antigen gave compatible data in exclusion of type 1 disease in the European VWD study (30). The broader next generation sequencing will likely spread and provide new insights with a comprehensive package analysis covering also differential diagnostics.
New management opportunities
The management algorithm followed by the Nordic treatment centers is presented in Figure 2. The upgraded European view on the management of bleeding disorders includes the comprehensive and multidisciplinary approach, also in patients with VWD. The physiotherapeutic and rehabilitation programs, vascular access, and comorbid conditions, including arthropathy and hepatitis causing specific VWD-associated demands need focus. Women’s issues, pregnancy planning with the obstetric team and delivery and its recovery call upon hemostatic personalized planning. Moreover, dietary and psychological consulting and avoidance of drugs (i.e., melatonin, serotonin receptor uptake inhibitors, omega 3 fatty acids) benefit certain patients. One concern of VWD is the repetitive bleeds to the gastrointestinal track, which create a viscous circle of anemia due to inefficient iron metabolism, which obviously should be based on the laboratory assessment. Because of limited absorption and slow response of the oral iron, these patients acutely benefit from intravenous iron. The every other day dosing has been recently recommended to enhance the outcome of the therapy (Schrier SL, Auerbach MA. Treatment of iron deficiency anemia in adults. Up to Date, Sep 2017). In addition, the loss of other coagulation factors, including FXIII needs to be excluded, at least if the major bleeding episodes have been reoccurring. Sometimes acute platelet infusions and modifiers of angiogenesis are of value.

The international study group on VWD prophylaxis network has confirmed the role of prophylaxis for the patients in need (31). These patients had a clear bleeding phenotype, and represented mainly type 3, around 60% of them on regular prophylaxis. In the Nordic region, regular prophylaxis is available for all patients with VWD, who have a clinically significant bleeding phenotype and do not benefit from desmopressin. It is interesting, however, that only around 50% of type 3 patients need prophylaxis due to their bleeding manifestations of gastrointestinal, joint bleeds and severe nose or menstrual bleeds, whereas in the other 50% the bleeding phenotype is mild and the patients are not motivated to use prophylaxis (29). This illustrates that new phenotypic and laboratory assessments are needed to gain better understanding of the compensatory role of platelets and other regulators of coagulation (32).
The VWD-indicated concentrates in their historical order include (I) FVIII and VWF (1:2 ratio); (II) FVIII and VWF (1:1); and (III) VWF alone (Figure 2). There is good evidence on all of them to manage prophylaxis (33). The choice of concentrate under major bleed or urgent surgery in a patient without regular prophylaxis in type 3 or some type 2 patients with a severe clinical bleeding phenotype differs. The distinction is that the pure VWF concentrate may need additional (initial) support by FVIII, before it rises to the hemostatic levels which the supplemented VWF will carry, this usually takes 6–12 h. In contrast, in the patients who are at risk of thrombosis, FVIII levels should be followed up and the dosing of the replacement therapy should be tailored accordingly. The use of pure VWF concentrate provides the way to control the natural FVIII levels. The novel recombinant VWF is also devoid of FVIII and it has entered the phase III extension studies, and the data of this platelet VWF—like ultra-large molecule (Vonvendi®) will be of interest in the future (34).
Acknowledgments
The authors would like to acknowledge the members of the Nordic Haemophilia Council and the personnel from all Nordic Centers. We thank Dr. Estelle Gillman and Professor Mike Makris for providing the VWD patient numbers reported to EUHASS activities.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Emmanuel J. Favaloro) for the series “Diagnosis and Management of von Willebrand Disease: Diverse Approaches to a Global and Common Bleeding Disorder” published in Annals of Blood. The article has undergone external peer review.
Conflicts of Interest: The series “Diagnosis and Management of von Willebrand Disease: Diverse Approaches to a Global and Common Bleeding Disorder” was commissioned by the editorial office without any funding or sponsorship. R Lassila is participating to the Wings study by LFB and has been a member in the advisory board of Shire and CSL Behring; the other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lassila R, Lindberg O. Erik von Willebrand. Haemophilia 2013;19:643-7. [Crossref] [PubMed]
- Lassila R, Holme PA, Landorph A, et al. Nordic Haemophilia Council's practical guidelines on diagnosis and management of von Willebrand disease. Semin Thromb Hemost 2011;37:495-502. [Crossref] [PubMed]
- Makris M, Calizzani G, Fischer K, et al. EUHASS: The European Haemophilia Safety Surveillance system. Thromb Res 2011;127:S22-5. [Crossref] [PubMed]
- Tosetto A, Rodeghiero F, Castaman G, et al. A quantitative analysis of bleeding symptoms in type 1 von Willebrand disease: results from a multicenter European study (MCMDM-1 VWD). J Thromb Haemost 2006;4:766-73. [Crossref] [PubMed]
- Mittal N, Naridze R, James P, et al. Utility of a Paediatric Bleeding Questionnaire as a screening tool for von Willebrand disease in apparently healthy children. Haemophilia 2015;21:806-11. [Crossref] [PubMed]
- Castaman G, Linari S. Diagnosis and Treatment of von Willebrand Disease and Rare Bleeding Disorders. J Clin Med 2017;6:E45 [Crossref] [PubMed]
- Rydz N, Grabell J, Lillicrap D, et al. Changes in von Willebrand factor level and von Willebrand activity with age in type 1 von Willebrand disease. Haemophilia 2015;21:636-41. [Crossref] [PubMed]
- Albanez S, Ogiwara K, Michels A, et al. Aging and ABO blood type influence von Willebrand factor and factor VIII levels through interrelated mechanisms. J Thromb Haemost 2016;14:953-63. [Crossref] [PubMed]
- Jenkins PV, Rawley O, Smith OP, et al. Elevated factor VIII levels and risk of venous thrombosis. Br J Haematol 2012;157:653-63. [Crossref] [PubMed]
- Virtanen L, Salmela B, Leinonen J, et al. Laboratory-monitored fondaparinux and coagulation activity in association with total hip replacement. Blood Coagul Fibrinolysis 2014;25:597-603. [PubMed]
- Favaloro EJ, Bonar R, Marsden K. Lower limit of assay sensitivity: an under-recognised and significant problem in von Willebrand disease identification and classification. Clin Lab Sci 2008;21:178-83. [PubMed]
- Favaloro EJ, Mohammed S. Towards improved diagnosis of von Willebrand disease: comparative evaluations of several automated von Willebrand factor antigen and activity assays. Thromb Res 2014;134:1292-300. [Crossref] [PubMed]
- Timm A, Hillarp A, Philips M, et al. Comparison of automated von Willebrand factor activity assays. Thromb Res 2015;135:684-91. [Crossref] [PubMed]
- Castaman G, Hillarp A, Goodeve A. Laboratory aspects of von Willebrand disease: test repertoire and options for activity assays and genetic analysis. Haemophilia 2014;20:65-70. [Crossref] [PubMed]
- Bodo I, Eikenboom J, Montgomery R, et al. Platelet-dependent von Willebrand factor activity. Nomenclature and methodology: communication from the SSC of the ISTH. J Thromb Haemost 2015;13:1345-50. [Crossref] [PubMed]
- Favaloro EJ. The utility of the PFA-100 in the identification of von Willebrand disease: a concise review. Semin Thromb Hemost 2006;32:537-45. [Crossref] [PubMed]
- Colace TV, Fogarty PF, Panckeri KA, et al. Microfluidic assay of hemophilic blood clotting: distinct deficits in platelet and fibrin deposition at low factor levels. J Thromb Haemost 2014;12:147-58. [Crossref] [PubMed]
- Valarche V, Desconclois C, Boutekedjiret T, et al. Multiplate whole blood impedance aggregometry: a new tool for von Willebrand disease. J Thromb Haemost 2011;9:1645-7. [Crossref] [PubMed]
- Schmidt DE, Bruzelius M, Majeed A, et al. Whole blood ristocetin-activated platelet impedance aggregometry (Multiplate) for the rapid detection of Von Willebrand disease. Thromb Haemost 2017;117:1528-33. [Crossref] [PubMed]
- Michiels JJ, Smejkal P, Penka M, et al. Diagnostic differentiation of von Willebrand disease types 1 and 2 by von Willebrand factor multimer analysis and DDAVP challenge test. Clin Appl Thromb Hemost 2017;23:518-31. [Crossref] [PubMed]
- Favaloro EJ, Oliver S. Evaluation of a new commercial von Willebrand factor multimer assay. Haemophilia 2017;23:e373-e7. [Crossref] [PubMed]
- Oliver S, Lau KKE, Chapman K, et al. Laboratory testing for von Willebrand factor multimers. Methods Mol Biol 2017;1646:495-511. [Crossref] [PubMed]
- Chitlur M, Rivard GE, Lillicrap D, et al. Recommendations for performing thromboelastography/thromboelastometry in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost 2014;12:103-6. [Crossref] [PubMed]
- Dargaud Y, Wolberg AS, Gray E, et al. Proposal for standardized preanalytical and analytical conditions for measuring thrombin generation in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost 2017;15:1704-7. [Crossref] [PubMed]
- Tran HT, Tjonnfjord GE, Holme PA. Use of thromboelastography and thrombin generation assay to predict clinical phenotype in patients with severe FVII deficiency. Haemophilia 2014;20:141-6. [Crossref] [PubMed]
- Olsson A, Hellgren M, Berntorp E, et al. Bleeding phenotype in carriers of haemophilia A does not correlate with thrombin generation. Haemophilia 2015;21:e111-3. [Crossref] [PubMed]
- Schmidt DE, Majeed A, Bruzelius M, et al. A prospective diagnostic accuracy study evaluating rotational thromboelastometry and thromboelastography in 100 patients with von Willebrand disease. Haemophilia 2017;23:309-18. [Crossref] [PubMed]
- Rugeri L, Beguin S, Hemker C, et al. Thrombin-generating capacity in patients with von Willebrand's disease. Haematologica 2007;92:1639-46. [Crossref] [PubMed]
- Jokela V, Lassila R, Szanto T, et al. Phenotypic and genotypic characterization of 10 Finnish patients with von Willebrand disease type 3: discovery of two main mutations. Haemophilia 2013;19:e344-8. [Crossref] [PubMed]
- Budde U, Schneppenheim R, Eikenboom J, et al. Detailed von Willebrand factor multimer analysis in patients with von Willebrand disease in the European study, molecular and clinical markers for the diagnosis and management of type 1 von Willebrand disease (MCMDM-1VWD). J Thromb Haemost 2008;6:762-71. [Crossref] [PubMed]
- Abshire TC, Federici AB, Alvarez MT, et al. Prophylaxis in severe forms of von Willebrand's disease: results from the von Willebrand Disease Prophylaxis Network (VWD PN). Haemophilia 2013;19:76-81. [Crossref] [PubMed]
- Szanto T, Joutsi-Korhonen L, Deckmyn H, et al. New insights into von Willebrand disease and platelet function. Semin Thromb Hemost 2012;38:55-63. [Crossref] [PubMed]
- Goudemand J, Scharrer I, Berntorp E, et al. Pharmacokinetic studies on Wilfactin, a von Willebrand factor concentrate with a low factor VIII content treated with three virus-inactivation/removal methods. J Thromb Haemost 2005;3:2219-27. [Crossref] [PubMed]
- Singal M, Kouides PA. Recombinant von Willebrand factor: a first-of-its-kind product for von Willebrand disease. Drugs Today (Barc) 2016;52:653-64. [Crossref] [PubMed]
Cite this article as: Szanto T, Lassila R, Funding E, Onundarson PT, Strandberg K, Baghaei F; the Nordic Haemophilia Council. Von Willebrand disease—the Nordic perspective. Ann Blood 2018;3:2.


