
Factor VIII manufactured from plasma—the ups and downs, and the up again: a personal journey—part 1: history of the development of plasma-derived factor VIII therapies
Some personal reflections
In October 1981, after being awarded a scholarship by The British Council, I left Malta to commence a period of study in Edinburgh, aiming to generate a body of work towards a PhD. This followed the initiation of my career in blood transfusion in Malta in 1979, as a result of which, under the mentorship of Jiri Rondiak (1), I determined to involve myself in the area of haemophilia and blood products. I was fortunate in that Professor Gordon Whitby (2) provided me with an academic “home” in his Department of Clinical Chemistry in the University of Edinburgh, while Dr. Chris Prowse and Dr. Frank Boulton agreed to supervise a PhD program in the then South East Scotland Blood Transfusion Service (BTS). It was agreed that this would centre around the production of FVIII by blood banks.
Resisting too deep a plunge into nostalgia, I would simply express my lifelong appreciation at having been given this opportunity. The close association of the Edinburgh BTS with haemophilia treatment since the nascent efforts to produce FVIII (3), together with Scotland’s long-standing record in plasma fractionation which culminated with the establishment of the Protein Fractionation Centre (PFC) (4), made for a very enriching environment. Between 1981 and 1984 I was privileged to play a minor role in this landscape as the developments in the field reverberated in our own immediate environment. The extent to which the knowledge around FVIII grew during this period is illustrated in Figure 1. These advances in basic science were the prerequisites to the therapeutic advancements of the subsequent decades, dominated by the increasing availability of FVIII from recombinant sources. However, advances in the manufacture of pd-FVIII were also important, and these form the subject of this review.
The history of the development of the therapy for haemophilia A—the pre-acquired immune deficiency syndrome (AIDS) era
The modern treatment of replacing the missing component in blood dates from 1840 when the first blood transfusion for a case of haemophilic bleeding was given (5). Rather fortuitously, no incompatibility problems were recorded, but further use of this form of treatment had to await Landsteiner’s discovery of blood groups in 1901. Subsequent claims of benefit from the use of a variety of measures, ranging from the administration of lime to the use of egg white (6) must today be viewed very skeptically.
Macfarlane’s use of Russell’s viper venom (7) as a topical application on wounds was one of the first efforts based on a scientific appreciation of the defect. By 1938, however, Macfarlane (8) had realised that only blood transfusion offered effective treatment for a bleeding episode, by replacing the missing essential component. Patek’s group showed this to be present in the cell-free plasma and in Cohn’s classic fractionation scheme it was shown to be in fractions I and II of normal, but not haemophilic, plasma (9). These fractions and similar fibrinogen-rich components from ether-fractionated plasma (10) were recognised as potentially therapeutic materials. These early products were relatively crude and insoluble due to their high fibrinogen content and tended to be unstable. The Blombäck modification of Cohn fraction I (11) led to a product that was much improved in stability and purity, and was the mainstay of successful treatment in Sweden for many years (12) (Figure 2).
The discovery of cryoprecipitate as a therapeutic option is undoubtedly one of the watershed events in the history of haemophilia, and Pool’s description in 1965 (13) is justifiably a seminal publication. However, it is fair to note that this was not a discovery as much as a “re-discovery”; the little-known work of the French physician Rémigy (14) (Figure 3) preceded Pool’s work by several years. Although the gelatinous residue that remains undissolved when frozen plasma is allowed to thaw at a low temperature was known to be rich in fibrinogen and factor VIII (15,16).

Pool developed this as a clinical product which could be produced by small blood centres (Figure 4). This made the widespread adequate treatment of haemophilia feasible.
Despite the benefits of blood bank cryoprecipitate, it had several limitations: it had to be stored frozen, it had only about five times the potency of plasma, it was not standardised, making dosage difficult and it precipitated allergic reactions and other side effects (17). The technique could be used as the first step in the preparation of industrial scale FVIII concentrate, which could be assigned a potency and dose patients adequately (18). By the early 1970s, Johnson’s group’s method (19) for the bulk preparation of a factor VIII concentrate had become the basis of most available factor VIII concentrates (Figure 5).
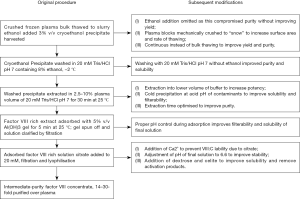
Other methods, involving treatment of plasma or plasma fractions with chemicals, were used to prepare concentrates of varying quality. These historical concentrates are summarized in Table 1.
Table 1
| Name | Production method | Purification over plasma (X)* | Comments |
|---|---|---|---|
| Cohn fraction I ( |
Precipitation of plasma proteins with 8% ethanol at −3 °C, pH 7—most of the fibrinogen and factor VIII are precipitated | 7–20 | Careful technique allows factor VIII to be harvested at high yields; however, F-I is poorly soluble, rather unstable and cannot be sterile-filtered, and so has to be prepared by elaborate sterile techniques |
| FI-0 ( |
Extraction of F-I at 0 °C with a glycine-citrate buffer and resolution of the precipitate in isotonic saline | 10–30 | Extraction procedure improves greatly the solubility and stability of F-I |
| Ether-fraction F-I ( |
Precipitation of plasma proteins with 11% ether at 0 °C | 25 | Same characteristics and drawbacks as Cohn F-I |
| FI-0-Ta ( |
Treatment of F-I-0 with tannic acid to remove fibrinogen | 40–160 | Experience with small batches; attempts to replicate initial findings produced widely varying results, probably due to wide variability in batches of tannic acid |
| Bentonite-F-I ( |
Treatment of F-I with bentonite to remove fibrinogen | 40–160 | As with FI-0-Ta |
| Glycine-precipitated fraction ( |
Addition of glycine to plasma to a concentration of 2.3 M at 0 °C to precipitate factor VIII with some | 20–30 | Use of glycine at high concentrations expensive and wasteful as it precludes the use of the supernatant plasma |
*, X indicates the number of times the purification exceeds that in plasma.
The production of animal FVIII concentrate played a role in the early years when human material was scarce (24) but had to be abandoned because of the antigenic problems and the thrombocytopenia induced by such products. Later, more purified concentrates, and recombinant versions, still play a role in the treatment of patients with inhibitors to FVIII (25).
To summarise, by the early 1980s, pd-FVIII concentrates had reached the stage of providing reliable replacement therapy in treating bleeds in the population of boys with haemophilia A in the developed economies. While some countries, notably Sweden, were already demonstrating the benefits of prophylactic treatment, the limited amount of material available precluded this option in most instances. The products could be reconstituted in volumes which, while increasingly smaller, provided limitations in patients’ abilities to infuse themselves. The classification of concentrates at this time (26) (Table 2) demonstrates the huge progress made in the generation of the concentrates today. Notably, the inverse relationship between the purity achieved and the yield of FVIII from starting plasma illustrates the key factor driving manufacture in the early 1980s. It is fair to state that the predominant paradigm driving manufacture at this time was the improvement in yield, which was low then, as it is now, relative to the amount of FVIII in the starting plasma (see below).
Table 2
| Purity assignment | Specific activity (international units FVIII/mg total protein) | Yield FVIII (international units) obtained per litre of plasma |
|---|---|---|
| Low | <0.2 | >300 |
| Intermediate | 0.2–0.5 | 200–300 |
| High | >0.5 | <200 |
Events rapidly overtook this paradigm and ushered in the developments leading to the current era. The growing awareness of the contamination of the blood and plasma supply with the infectious agent implicated in the AIDS shifted the focus from yield to safety, and we now turn to these developments.
Developments in the manufacture of pd-FVIII in the post-AIDS era
By the end of the 1970s, it was recognized that the treatment of haemophilia was associated with a high incidence of post-transfusion hepatitis in patients, and that this was not, unlike in patients transfused with mainstream blood components, ameliorated by the introduction of tests to exclude blood and plasma donations which were infected with hepatitis B virus (HBV) (27). It was clear that haemophiliacs were being infected with the agents associated with post-transfusion hepatitis. It is this author’s view that this was recognized, up to this period, as an inevitable consequence of replacement therapy, of concern, but secondary to the morbidity and mortality still ensuing from inadequate treatment. This attitude on the part of all members of the community, whether patients, treaters or industry, has been often described as “complacent”. As a participant in the landscape during this time, I would suggest that the predominant paradigm in haemophilia care, emerging after decades of hopelessness in the face of lack of therapeutic options, was supply, first and foremost. The early recognition of the extraordinary lability of plasma FVIII, with the consequent low yields obtained from a limited plasma supply, focused attention on improving FVIII yield and supply, while avoiding measures to decrease the former. By the late 1970s, a small number of manufacturers had developed processes which allowed FVIII concentrates to be heated in solution (28), but the yields obtained were low, such as to allow treatment for only a small minority of patients. This difficult, controversial and emotional issue has underpinned the considerable number of judicial processes, some still ongoing, of the past 30 years.
The paradigm shifted, as paradigms do, in crisis (29), in the form of another infection entering the blood supply. The ensuing tragedy in the haemophilia population has been well described and I draw the readers’ attention to Evatt’s reviews (30,31), which objectively assess the progression of events through the perspective of a major player. In terms of the manufacturing paradigm, the shift from supply to safety was rapid and driven by the need to introduce viral inactivation steps in the manufacture of concentrates. The earliest efforts employing heat treatment in solution—known somewhat imprecisely as “pasteurization”—had been under development for some years and led to the transient appearance of an additional number of concentrates. The chemistries employed required the addition, and subsequent removal, of considerable amounts of thermo-stabilising agents which had to be removed after the heat treatment, a cumbersome process with an associated yield penalty (32,33), and which occasioned neo-antigenicity in at least one concentrate subjected to this process (34,35). One such concentrate has withstood these challenges for the nearly 40 years it has been in use (36), but an alternative form of heat treatment of the lyophilized product in the final container quickly gained ground. It was rapidly appreciated that the conditions under which was done were crucial in the inactivation of the relevant pathogens; somewhat sadly, the virus associated with AIDS—the human immunodeficiency virus HIV—was found to be susceptible to relatively low temperatures, with sufficient viral kill achievable at 60 °C, while the agent associated with non-A non-B (NANB) hepatitis, later identified as hepatitis C virus (HCV) in most instances, required higher temperatures (28). These developments led to a number of products which had to have a higher purity than the previous generation of non-virally inactivated products, mostly because the heat lability of the major contaminant fibrinogen required its removal, but which were still in the range of 5 to 10 international units of FVIII per mg of total protein. Some of these products are still on the international market.
Concurrently with these developments, two new classes of pd-FVIII evolved, as a result of the viral infection threat. One class employed the use of immuno-affinity chromatography in the purification of FVIII. Developments in the production of monoclonal antibodies to different parts of the FVIII-von Willebrand Factor (VWF) complex in the early 1980s led to two products from two different companies, at that stage in corporate evolution Armour and Baxter. The Armour product Monoclate-purified FVIII using a solid-phased monoclonal antibody to VWF, which captured the FVIII-VWF complex, following which FVIII was dissociated using ionized calcium, further purified on other resins and formulated (37,38). The other product Hemophil-M from Baxter employed a solid-phased monoclonal antibody to FVIII to capture the complex, after which FVIII was selectively purified from VWF on other resins. Initial preparations of these products were manufactured without specific viral inactivation measures, but were quickly superseded by versions which incorporated first dry heat and subsequently pasteurization for Monoclate, while the solvent-detergent (S-D) method was incorporated into Hemophil M (Figure 6) (39). Analysis of these products indicated that they included biochemically pure FVIII, absent from the impurities of then current pd-FVIII concentrates, such as fibrinogen and fibronectin, but the products has to be stabilized with the addition of human albumin. The manufacturing techniques also removed VWF, the major component of the FVIII-VWF complex, making the products unsuitable for the treatment of von Willebrand’s Disease (VWD).
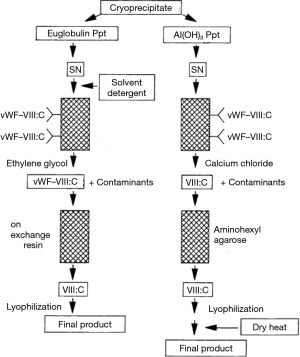
Practically concurrently, another class of concentrates was developed by a number of companies which employed ion-exchange chromatography to purify the FVIII-VWF complex and incorporate viral inactivation techniques. These concentrates contained VWF to different extents, depending on the elution conditions chosen to concentrate the complex from ion-exchange resins (40,41). This form of chromatography was used to remove the S-D reagents introduced to inactivate lipid-enveloped viruses in the starting cryoprecipitate. By the late 1980s, this S-D technique developed by Horowitz (42,43) was recognized as the most effective technique to eliminate the highly pathogenic lipid-enveloped transfusion transmitted viruses—and it still is. The subsequent chromatographic elution of FVIII also resulted in a substantial purification, allowing the removal, among other impurities, of immunoglobulin, including anti-A and anti-B immunoglobulin implicated in hemolytic reactions (17). It should be mentioned that the level of purity achieved was not equivalent to what was achieved by the more specific immune-affinity purified products.
The primary driver behind the development of these products was safety, and the higher number of purification and viral inactivation steps had a penalty in terms of the yield of factor VIII, which settled at a maximum of around 200 IU per litre of plasma processed. It is ironic that this drive towards higher purity and safety had an unintended and temporary consequence in decreasing the level of safety relative to a particular pathogen, which had previously not been relevant to haemophilia. In the early 1990s, a spate of independent reports (44-46) described outbreaks of hepatitis A in their respective haemophilia populations following administration of a S-D treated FVIII concentrate, subsequently determined to contain hepatitis A virus (HAV) RNA (47). It is possible that these processes lost their content of protective antibodies to HAV as a result of the purification process, which, unaccompanied by a viral inactivation step which could eliminate the non-enveloped HAV, caused infection in the patients (48). It was at this time that I joined the Australian regulatory authority, the Therapeutic Goods Administration, with the early charge to implement the newly emerging European requirements for two viral inactivation steps, one of which was to address non-enveloped viruses (49). While companies moved rapidly to implement this requirement, it was not a fully uneventful process: similar to previous outbreaks involving pasteurized FVIII, the introduction of heating at 63 °C, intended to inactivate HAV (50), led to an outbreak of inhibitors in patients, characterized, unlike the usual inhibitors to treatment, but similarly to the previous outbreaks involving pasteurized FVIII (34,35), resolvable by changing the implicated concentrate (51). These outbreaks seemed to confirm the initial reluctance of some treaters to switch patients to heated products in the early years of the AIDS crisis, and have contributed to the awareness around the possibility of manufacturing changes inducing neo-antigenicity in FVIII products.
This awareness has influenced issues, which have arisen over the past 35 years. The introduction of the monoclonal products, bereft of the traditional impurities and containing FVIII without its physiological carrier protein, was shown to result in a lesser degree of certain immunological abnormalities in patients than the use of the old intermediate purity products [reviewed by Schulman (52)]. Compelling data from the National Institute of Biological Standards and Control (now absorbed into the British regulatory agency) suggest that transforming growth factor-beta (TGF-beta) contamination is a major contributory factor to the inhibitory activity of some factor VIII concentrates on cytokine secretion or activity, and may partially explain the reported immunosuppressive effects in recipients of these blood products (53). These observations were augmented by the publication of studies in HIV positive haemophiliacs which indicated a slower deterioration in CD4 lymphocytes in such patients given monoclonal concentrates [reviewed by Mannucci (54)]. I have always been puzzled by this concept, as it seemed to imply that monoclonal purified FVIII had a protective effect against HIV. Subsequent in vitro studies (55), large prospective surveys (56), and randomized studies (57) negated the initial observations, finding no difference in the capacity of concentrates of different types to influence the CD4 count, and the introduction of increasingly effective AIDS drugs made further study of this issue impossible.
This issue did, however, focus attention on the unquestionable immunosuppressive effects of low and intermediate purity FVIII in vitro, contributing to the controversy which has raged over the past quarter century on the relationship of the type of concentrate on the risk of haemophiliacs developing inhibitor to FVIII after first exposure. Early studies summarized by Aledort (58) indicated that the type of plasma derived FVIII did not appear to influence the range of prevalence observed for FVIII inhibitors in previously untreated patients (PUPs) with haemophilia A. This comforting status was not reflected with the introduction of recombinant FVIII (rFVIII) and its increasing dominance in the established economies over the past 25 years.
The development of recombinant products for the treatment of haemophilia is beyond the scope of this review, and I will limit my comments to observing that the development of such products has enabled two potential advances in the treatment of haemophilia:
- By liberating provision from the necessarily constantly constrained plasma supply, it has generated the hypothetical scenario of unlimited access to product for the global haemophilia population—if you can pay for it!
- By allowing the production of molecular forms of coagulation factor modified to “improve upon nature”, it has widened considerably the therapeutic options available and the possibility for personalized treatment—if you can pay for it (59)!
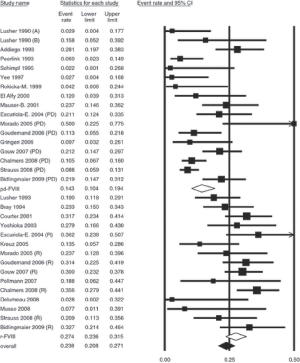
These benefits have been countered by the controversy over the issue of inhibitors, which has been visible in the haemophilia therapeutic landscape since the first reasonably sized studies of rFVIII in PUPs (54), showed a higher inhibitor incidence than previously observed with pd-FVIII. The uncertainty dogging this issue has been summarized by Burnouf and Strengers (60), and has led to repeated analyses of the available studies in an attempt to reach a conclusion. Most of these exercises analyzing the range of available studies concluded that a higher inhibitor incidence is associated with rFVIII compared to pd-FVIII (61,62), but the differences observed, although substantial, were statistically insignificant (Figure 7). It seemed that the genuine uncertainty on this important issue justified, according to the tenets of evidence-based medicine, a randomized clinical trial. Such a trial was carried out over 2001–2015, under the auspices of the Survey of Inhibitors in Plasma-Product Exposed Toddlers, now known as the SIPPET study.
The aftermath of SIPPET—pd-FVIII up again!
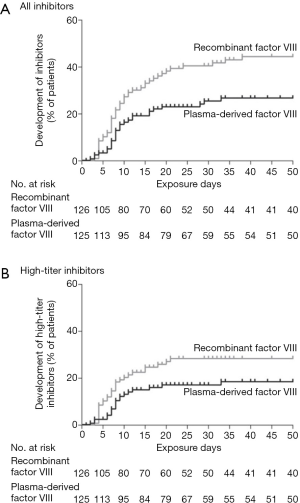
The SIPPET study published in 2016 included patients either never or minimally exposed to FVIII replacement. The patients were randomized to receive either one of a number of pd-FVIII-VWF concentrates or one of a number of rFVIII concentrates (63). Patients receiving rFVIII showed significantly higher risk of developing inhibitors, although the risk of developing high-titre inhibitors, although higher than with pd-FVIII, was not significant (Figure 8). The study resulted in considerable interest and discussion, and a literature has augmented the initial publication, extending and explaining the study further (64). Prospectively gathered data from at least one VWF-containing plasma concentrate has augmented the SIPPET findings in the “real world” (65). These studies have not resolved the polarization in the treater community regarding this issue; I suspect that this will never be the case (66). It has focused the attention of regulators more closely on the problem of inhibitors and especially in PUPs, where the European Medicines Agency (EMA), despite rejecting the concept of class differences between different FVIII products, now considers this a “major” adverse effect. I would offer the following thoughts as part of this debate:
- Irrespective of the arguments are the SIPPET study, the issue which precipitated the study, i.e., whether pd-FVIII or rFVIII is associated with a higher incidence of inhibitors in PUPs still exists. If treaters remain in a state of uncertainty on this issue, given the onus of evidence that indicates that rFVIII is associated with a higher prevalence, then action is required to minimize this major hazard;
- This situation is therefore, for those unconvinced by the SIPPET, evocative of the precautionary principle which requires action in circumstances of scientific uncertainty. In other words, in this particular situation, the higher incidence of rFVIII when given to PUPS is a “harm which should be assumed will occur”, to paraphrase Krever [p1049 in (67)]: “Preventive action should be taken when there is evidence [of inhibitor formation] even when there is no evidence that recipients have been affected. If harm can occur, it should be assumed that it will occur. If there are no measures that will entirely prevent the harm, measures that may only partially prevent [inhibitor formation] should be taken”;
- In the circumstances that, while unquestionably being insufficient to treat the world population of haemophilia throughout life, there is a sufficiency of pd-FVIII for the treatment of PUPs for the first 50 days of treatment [50 exposure days (EDs)], then this treatment should be preferably with a pd-FVIII;
- The question remains, which pd-FVIII? The PRAC decision to not endorse the SIPPET conclusion was based on a rejection of the concept of a “class” of products, in this instance defined by pd-FVIII complexed with VWF. This rejection of a degree of “biosimilarity” between different FVIII-VWF concentrates is conformant to mainstream regulatory—and plasma industry—opinion, which maintains that individual products differ too much from each other in a multitude of ways, to allow effects to be ascribed to one common feature, in this instance the presence of VWF;
- The role of VWF in modulating the immunogenicity of FVIII has been studied extensively (68). I would propose, however, that product to product differences in this regard, which certainly exist, may be also related to the immunosuppressive properties of impurities still present in the current generation of pd-FVIII concentrates (69-71). More work is required in assessing product to product differences between FVIII concentrates, including the assessment of manufacturing steps such as viral inactivation. It is worth noting that the relatively “gentle” S-D and nanofiltration processes have never been implicated in neo-antigenicity, unlike the heat-treatment methods implicated in the incidents noted above.
Hence, a role for pd-FVIII still exists, not only in the emerging markets where the vagaries of cost allocation in plasma fractionation allows some manufacturers to sell it for lower prices than rFVIII, despite its cost of manufacture being unquestionably higher. In this context, it is worth assessing the current global supply situation regarding FVIII concentrate. In 2014, 45 million litres of plasma were collected, which, if fractionated with current technology yielding 200 IU per litre, would have resulted in 9 billion IU. This, incidentally, is the current total amount of FVIII provided globally. Instead, 4 billion IU of pd-VIII were actually provided (72). It is clear that around half the possible amount of FVIII is not being converted into concentrate. In contrast, similar estimates for other products indicate that approximately 85% of the available IG (probably an underestimate) and albumin are being extracted. The provision of FVIII from plasma would be greatly facilitated if the low yield of 200 IU out of a theoretical 1,000 IU per litre of plasma were to be improved. The factors affecting this yield are the subject of an accompanying paper, through particular aspects in which I was also a contributor.
Acknowledgments
This work is dedicated to the memory of Duncan Stephen Pepper, whose wisdom and friendship contributed to my lifelong interest in the perpetual puzzle which is factor VIII.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Thierry Burnouf) for the series “Plasma Fractionation” published in Annals of Blood. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob.2018.02.04). The series “Plasma Fractionation” was commissioned by the editorial office without any funding or sponsorship. Albert Farrugia serves as an unpaid editorial board member of Annals of Blood from Feb 2017 to Feb 2020. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the manuscript and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Farrugia A. Vale Jirí Rondiak - 1934 – 2013. Malta Med J 2014;26:55-6.
- Cash J. Lionel Gordon Whitby. 2000. Available online: https://www.rse.org.uk/cms/files/fellows/obits_alpha/whitby_lionel.pdf
- Cumming RA, Davies SH, Ellis D, et al. Red Cell Banking and the Production of a Factor VIII Concentrate. Vox Sang 1965;10:687-99. [Crossref] [PubMed]
- Foster PR. The manufacture of blood plasma products in Scotland: a brief history. Scott Med J 2016;61:34-41. [Crossref] [PubMed]
- Farr AD. Treatment of haemophilia by transfusion: the first recorded case. J R Soc Med 1981;74:301-5. [PubMed]
- Ingram GI. The history of haemophilia. J Clin Pathol 1976;29:469-79. [Crossref] [PubMed]
- Macfarlane RG, Barnett B. The Hæmostatic Possibilities of Snake-Venom. Lancet 1934;224:985-7. [Crossref]
- Macfarlane RG. The normal haemostatic mechanism and its failure in the haemorrhagic states. University of London, 1938.
- Minot GR, Taylor FH. Hemophilia; the clinical use of antihemophilic globulin. Ann Intern Med 1947;26:363-7. [Crossref] [PubMed]
- Kekwick RA, Wolf P. A concentrate of human antihaemophilic factor; its use in six cases of haemophilia. Lancet 1957;272:647-50. [Crossref] [PubMed]
- Blombäck M. Studies on antihemophilic globulin. Acta Paediatr Suppl 1958;47:1-32. [Crossref] [PubMed]
- Nilsson IM, Blomback M, Blomback B, et al. The use of human AHF (Fraction I-0) in haemophilia A. Blut 1962;8:92-101. [Crossref] [PubMed]
- Pool JG, Shannon AE. Production of high-potency concentrates of antihemophilic globulin in a closed-bag system. N Engl J Med 1965;273:1443-7. [Crossref] [PubMed]
- Sibinga CT. Emile Rémigy and the discovery of anti-haemophilic activity in cryoprecipitate. Haemophilia 1996;2:56-60. [Crossref] [PubMed]
- Ware AG, Guest MM, Seegers WH. Fibrinogen; with special reference to its preparation and certain properties of the product. Arch Biochem 1947;13:231-6. [PubMed]
- Brinkhous KM. Plasma antihemophilic factor biological and clinical aspects. Le Sang 1954;25:738-41. [PubMed]
- McVerry BA, Machin SJ. Incidence of allo-immunization and allergic reactions to cryoprecipitate in haemophilia. Vox Sang 1979;36:77-80. [Crossref] [PubMed]
- Brinkhous KM. A New High-Potency Glycine-Precipitated Antihemophilic Factor (AHF) Concentrate: Treatment of Classical Hemophilia and Hemophilia With Inhibitors. JAMA 1968;205:613. [Crossref] [PubMed]
- Newman J, Johnson AJ, Karpatkin MH, et al. Methods for the production of clinically effective intermediate- and high-purity factor-VIII concentrates. Br J Haematol 1971;21:1-20. [Crossref] [PubMed]
- Cohn EJ, Strong LE. Preparation and properties of serum and plasma proteins; a system for the separation into fractions of the protein and lipoprotein components of biological tissues and fluids. J Am Chem Soc 1946;68:459-75. [Crossref] [PubMed]
- Simonetti C, Casillas G, Pavlovsky A, et al. Concentrate of factor VIII (FI-O-Ta) for clinical use. Thromb Diath Haemorrh Suppl 1968;35:245-52. [PubMed]
- Soulier JP. Separation of fibrinogen and of antihemophilic factor A. II. With the aid of bentonite. Pathol Biol 1959;7:2451-4. [PubMed]
- Wagner BH, McLester WD, Smith M, et al. Purification of antihemophilic factor (factor VIII) by amino-acid precipitation. Thromb Diath Haemorrh 1964;11:64-74. [PubMed]
- Bidwell E. The purification of antihaemophilic globulin from animal plasma. Br J Haematol 1955;1:386-9. [Crossref] [PubMed]
- Fosbury E, Drebes A, Riddell A, et al. Review of recombinant anti-haemophilic porcine sequence factor VIII in adults with acquired haemophilia A. Ther Adv Hematol 2017;8:263-72. [Crossref] [PubMed]
- Smith JK, Bidwell E. Therapeutic materials used in the treatment of coagulation defects. Clin Haematol 1979;8:183-206. [PubMed]
- Mannucci PM. Problems in hemophilia therapy. Ric Clin Lab 1981;11:301-11. [PubMed]
- Foster PR, Bienek C. Fractionated products. In: Barbara JA, Regan FA, Contreras M. editors. Transfusion Microbiology. Cambridge: Cambridge University Press, 2008:259-304.
- Forster M. Guide to Kuhn’s Structure of Scientific Revolutions. 1998. Available online: http://philosophy.wisc.edu/forster/220/kuhn.htm
- Evatt BL. The tragic history of AIDS in the hemophilia population, 1982-1984. J Thromb Haemost 2006;4:2295-301. [Crossref] [PubMed]
- Evatt BL. The AIDS epidemic in haemophilia patients II: pursuing absolute viral safety of clotting factor concentrates 1985–1988. Haemophilia 2012;18:649-54. [Crossref] [PubMed]
- Stabilisation of proteins to heat - Extract from The Penrose Inquiry. 2015. Available online: http://www.penroseinquiry.org.uk/pdf/SNB0074941.PDF
- Farrugia A. Albert Farrugia PhD Heat Treatment, Donor Selection and Stimulation. University of Edinburgh, 1984. Available online: https://onedrive.live.com/?cid=393430B0FC3610B5&id=393430B0FC3610B5%2136710&parId=393430B0FC3610B5%2136694&o=OneUp
- Rosendaal FR, Nieuwenhuis HK, van den Berg HM, et al. A sudden increase in factor VIII inhibitor development in multitransfused hemophilia A patients in The Netherlands. Dutch Hemophilia Study Group. Blood 1993;81:2180-6. [PubMed]
- Peerlinck K, Arnout J, Gilles JG, et al. A higher than expected incidence of factor VIII inhibitors in multitransfused haemophilia A patients treated with an intermediate purity pasteurized factor VIII concentrate. Thromb Haemost 1993;69:115-8. [PubMed]
- Kouides P, Wawra-Hehenberger K, Sajan A, et al. Safety of a pasteurized plasma-derived Factor VIII and von Willebrand factor concentrate: analysis of 33 years of pharmacovigilance data. Transfusion 2017;57:2390-403. [Crossref] [PubMed]
- Zimmerman TS, Fulcher CA. Purification of factor VIII by monoclonal antibody affinity chromatography. Thromb Res 1987;45:58. [Crossref]
- Brettler DB, Forsberg AD, Levine PH, et al. Factor VIII:C concentrate purified from plasma using monoclonal antibodies: human studies. Blood 1989;73:1859-63. [PubMed]
- Weinstein RE. Immunoaffinity purification of factor VIII. Ann Clin Lab Sci 1989;19:84-91. [PubMed]
- Burnouf T, Radosevich M. Affinity chromatography in the industrial purification of plasma proteins for therapeutic use. J Biochem Biophys Methods 2001;49:575-86. [Crossref] [PubMed]
- Burnouf T, Burnouf-Radosevich M, Huart JJ, et al. A Highly Purified Factor VIII:c Concentrate Prepared from Cryoprecipitate by Ion-Exchange Chromatography. Vox Sang 1991;60:8-15. [Crossref] [PubMed]
- Horowitz B, Prince AM, Hamman J, et al. Viral safety of solvent/detergent-treated blood products. Blood Coagul Fibrinolysis 1994;5 Suppl 3:S21-8; discussion S29-30.
- Horowitz MS, Rooks C, Horowitz B, et al. Virus safety of solvent/detergent-treated antihaemophilic factor concentrate. Lancet 1988;2:186-9. [Crossref] [PubMed]
- Mannucci PM. Outbreak of hepatitis A among Italian patients with haemophilia. Lancet 1992;339:819. [Crossref] [PubMed]
- Gerritzen A, Schneweis KE, Brackmann HH, et al. Acute hepatitis A in haemophiliacs. Lancet 1992;340:1231-2. [Crossref] [PubMed]
- Peerlinck K, Vermylen J. Acute hepatitis A in patients with haemophilia A. Lancet 1993;341:179. [Crossref] [PubMed]
- Normann A, Graff J, Gerritzen A, et al. Detection of hepatitis A virus RNA in commercially available factor VIII preparation. Lancet 1992;340:1232-3. [Crossref] [PubMed]
- Peerlinck K, Goubau P, Coppens G, et al. Is the apparent outbreak of hepatitis A in Belgian hemophiliacs due to a loss of previous passive immunity? Vox Sang 1994;67:14-6; discussion 17. [Crossref] [PubMed]
- Willkommen H, Löwer J. Theoretical considerations on viral inactivation or elimination. Dev Biol Stand 1993;81:109-16. [PubMed]
- Schwinn H, Stadler M, Josic D, et al. A solvent I detergent treated, pasteurised and highly purified factor VIII concentrate. Arzneimittelforschung 1994;44:188-91. [PubMed]
- Peerlinck K, Arnout J, Di Giambattista M, et al. Factor VIII inhibitors in previously treated haemophilia A patients with a double virus-inactivated plasma derived factor VIII concentrate. Thromb Haemost 1997;77:80-6. [PubMed]
- Schulman S. Effects of factor VIII concentrates on the immune system in hemophilic patients. Ann Hematol 1991;63:145-51. [Crossref] [PubMed]
- Wadhwa M, Barrowcliffe TW, Mire-Sluis AR, et al. Factor VIII concentrates and the immune system--laboratory investigations. Blood Coagul Fibrinolysis 1995;6:S65-79. [Crossref] [PubMed]
- Mannucci PM. The choice of plasma-derived clotting factor concentrates. Baillieres Clin Haematol 1996;9:273-90. [Crossref] [PubMed]
- Schögl D, Zimmermann K, Turecek PL, et al. Effect of high- versus intermediate-purity blood coagulation factor concentrates on HIV-1 replication. Vox Sang 1996;70:195-7. [PubMed]
- Gjerset GF, Pike MC, Mosley JW, et al. Effect of low- and intermediate-purity clotting factor therapy on progression of human immunodeficiency virus infection in congenital clotting disorders. Transfusion Safety Study Group. Blood 1994;84:1666-71. [PubMed]
- de Biasi R, Rocino A, Quirino AA, et al. The impact of a very high-purity factor VIII concentrate on the immune system of HIV-infected haemophiliacs: a randomized, two-year comparison with a high-purity concentrate. Haemophilia 1996;2:82-7. [Crossref] [PubMed]
- Aledort L. Inhibitors in hemophilia patients: Current status and management. Am J Hematol 1994;47:208-17. [Crossref] [PubMed]
- Lieuw K. Many factor VIII products available in the treatment of hemophilia A: an embarrassment of riches? J Blood Med 2017;8:67-73. [Crossref] [PubMed]
- Burnouf T, Strengers PF. Risks of inhibitors from recombinant factor VIII: a quarter of a century to reach the conclusion. J Thromb Haemost 2016;14:2073-4. [Crossref] [PubMed]
- Iorio A, Halimeh S, Holzhauer S, et al. Rate of inhibitor development in previously untreated hemophilia A patients treated with plasma-derived or recombinant factor VIII concentrates: a systematic review. J Thromb Haemost 2010;8:1256-65. [Crossref] [PubMed]
- Franchini M, Coppola A, Rocino A, et al. Systematic review of the role of FVIII concentrates in inhibitor development in previously untreated patients with severe hemophilia a: a 2013 update. Semin Thromb Hemost 2013;39:752-66. [Crossref] [PubMed]
- Peyvandi F, Mannucci PM, Garagiola I, et al. A Randomized Trial of Factor VIII and Neutralizing Antibodies in Hemophilia A. N Engl J Med 2016;374:2054-64. [Crossref] [PubMed]
- Peyvandi F, Mannucci PM, Palla R, et al. SIPPET: methodology, analysis and generalizability. Haemophilia 2017;23:353-61. [Crossref] [PubMed]
- Calvez T, Chambost H, d'Oiron R, et al. Analyses of the FranceCoag cohort support differences in immunogenicity among one plasma-derived and two recombinant factor VIII brands in boys with severe hemophilia A. Haematologica 2018;103:179-89. [Crossref] [PubMed]
- Berntorp E. Plasma-derived versus recombinant factor concentrates in PUPs: a never ending debate? Hamostaseologie 2017;37:53-7. [Crossref] [PubMed]
-
Commission of Inquiry on the Blood System in Canada 1997 . Available online: http://publications.gc.ca/collections/Collection/CP32-62-3-1997-1E.pdf</eref> - Hartholt RB, van Velzen AS, Peyron I, et al. To serve and protect: The modulatory role of von Willebrand factor on factor VIII immunogenicity. Blood Rev 2017;31:339-47. [Crossref] [PubMed]
- Hodge G, Han P. Factor VIII concentrate inhibits T helper type 2 cytokine production in vitro: relevance to inhibitor antibody formation. Haemophilia 2001;7:490-6. [Crossref] [PubMed]
- Hodge G, Han P. Effect of factor VIII concentrate on antigen-presenting cell (APC)/T-cell interactions in vitro: relevance to inhibitor formation and tolerance induction. Br J Haematol 2000;109:195-200. [Crossref] [PubMed]
- Hodge G, Saxon B, Revesz T. Effect of factor VIII concentrate on leucocyte cytokine receptor expression in vitro: relevance to inhibitor formation and tolerance induction. Haemophilia 2006;12:133-9. [Crossref] [PubMed]
- Farrugia A. Humanitarian aid and access to haemophilia care. Available online: https://www.slideshare.net/albfar/humanitarian-aid-and-access-to-haemophilia-care
Cite this article as: Farrugia A. Factor VIII manufactured from plasma—the ups and downs, and the up again: a personal journey—part 1: history of the development of plasma-derived factor VIII therapies. Ann Blood 2018;3:17.





