Factor VIII manufactured from plasma—the ups and downs, and the up again: a personal journey—part 2: aspects of factor VIII manufacture from plasma
The journey of Factor VIII in plasma—from donor to concentrates
Factor VIII in donors
The role of ABO blood groups
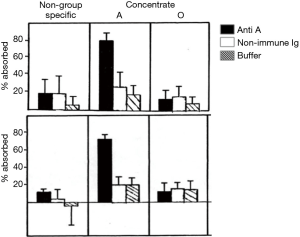
The effect of ABO blood groups on FVIII levels has been well described (1) and has been suggested to involve the secretor locus (2). The possibility of selecting plasma donors with high blood group A-associated FVIII levels was also raised in the 1980s (3,4). The practical difficulties in restricting plasma donors intended for FVIII manufacture to group A donors disallowed this approach. Concurrently, the occasional hemolytic adverse effect of contaminating isoagglutinins in early FVIII concentrates (5) led to at least one manufacturer KABI Vitrum in Sweden, now part of Octapharma) supplying blood group specific plasma derived FVIII (pd-FVIII) manufactured solely from group A or group O donors). Experiments in Chris Prowse’s laboratory in the early 1980s indicated that both FVIII:C and VWF, as measured through their respective antigens, were associated with group A substance (Figure 1).
The stimulation of donors to increase plasma FVIII levels
Mannucci’s seminal work on the use of 1-Deamino-8-d-arginine vasopressin (DDAVP) for treating mild haemophilia A and von Willebrand disease (VWD) through the release of endogenous stores (6) led to the application of this agent to increase FVIII and von Willebrand factor (VWF) levels in normal individuals (7) (Figure 2). By increasing the FVIII level in blood donors, this approach could increase FVIII yields in cryoprecipitate using a variety of protocols for treating haemophilia A (8-10) and VWD (11). Issues around the ethics of administering DDAVP led to the discontinuation of this promising development. It is unlikely that the stimulation of donors with DDAVP has had any significant adverse effects and the use of agents to elicit hyperimmune antibodies and hematopoietic stem cells (12,13).
Factor VIII in plasma
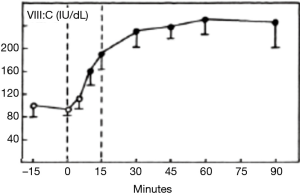
The development of the first assays of FVIII confirmed the early observations that the biological activity in plasma under blood bank conditions was extremely labile (14). Since the storage conditions of banked blood demanded refrigeration to preserve red cells and minimize bacterial growth, most early workers studied the stability of FVIII in banked blood at 4 °C, in order to assist the harvesting of FVIII from blood donations. This established the “bi-phasic” decay of FVIII, with a “golden window” within the first 6 hours after collection, when FVIII levels fall faster than during the subsequent period. We confirmed this in the early 1980s, but we also observed that refrigeration of whole blood resulted in losses of FVIII through cryoprecipitation, which were then lost into the red cell fraction when plasma was separated. These losses could be recovered through warming the blood prior to recovery of the plasma (Figure 3) (15).
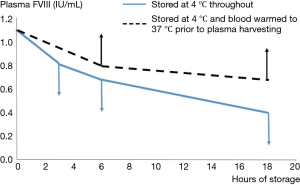
These observations demonstrate the tension which has always existed when the raw material for pd-FVIII manufacture is plasma recovered from whole blood donations. The competing needs for red cell and FVIII preservation have continued to “favour” red cells, as is to be expected in the mainstream transfusion context. It does accentuate the advantages which are accruable if plasma destined for pd-FVIII manufacture is harvested through plasmapheresis. Another aspect studied intensely has been the role of preservative, or anticoagulant, for the initial collection. This merits a section to itself.
Factor VIII and “M2+”
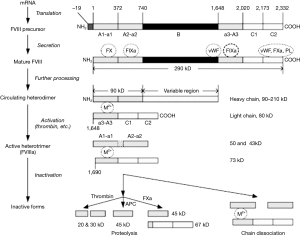
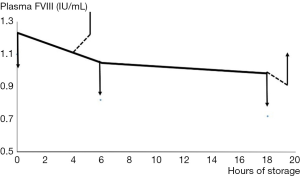
Weiss’ work from over fifty years ago demonstrated that the stability of FVIII was dependable on divalent metal ions, and that increasing levels of chelating anticoagulant affected the FVIII in plasma substantially (16). For many years it was thought that this was due to calcium ions, but recent developments involving our current knowledge of the molecular structure of FVIII (Figure 4) suggests that the divalent metal ion mediating the association between the two chains of FVII may also include copper (17). Our own studies suggested that adding Ca2+ with heparin cover to prevent clotting allowed the recovery of FVIII activity in stored blood after 3 hours but not after 18 hours, when the lost activity was irrecoverable (Figure 5).
Studies by Rock et al. (18) attempted to exploit this dependence through collection of blood in heparin, in order to retain physiological M2+ levels, but this would affect the capacity to harvest additional proteins. Weiss’ work (16) suggested that the citrate concentrations achieved in standard anticoagulants were in excess of what was needed to prevent gross coagulation. We therefore approached this issue by a number of alternative ways [Farrugia, 1984 (15)], finally settling on the collection of blood into citrate anticoagulants with half the standard citrate concentration—half-strength citrate [½ citrate-phosphate-dextrose (CPD)]. This stabilized FVIII in plasma significantly (Table 1) (19).
Table 1
| Estimated plasma citrate (mM) | Residual percentage FVIII:C | Median FpA (ng/mL) | |
|---|---|---|---|
| 3 hours | 23 hours | ||
| 20 | 100 | 71±9 | 40 |
| 16 | 82±10 | 76±9 | 30 |
| 12 | 105±13 | 84±9 | 28 |
| 10 | 112±22 | 80±13 | 25 |
| 8 | 118±15 | 92±18 | 17 |
FpA, fibrinopeptide A.
But…does it matter really?
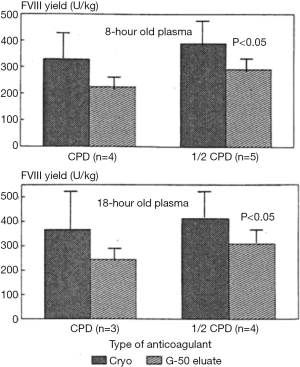
Preserving FVIII in plasma to ensure high levels in frozen raw material would be anticipated to result in final FVIII yields which are commensurately improved. Similarly, the modifications in formulation of plasma and intermediate fractions to preserve Ca2+ levels which are compatible with FVIII would have little practical use if they were not able to achieve higher yields in FVIII purification to products. As an example, the logistical efforts (and expense) involved in ensuring rapid separation and freezing of recovered plasma within the “golden window” to ensure that the FVIII levels are high would be of little use if the higher plasma FVIII are not recovered downstream, compared to plasma frozen outside the “golden window”. Yes, there is little evidence to suggest that this may be the case; plasma frozen within the “golden window” does yield higher FVIII levels in the cryoprecipitate, but this is not always reflected into final concentrate (20-22) (Table 2).
Table 2
| Production stage | 6-hour plasma (FVIII yield IU/kg plasma) | 18-hour plasma(FVIII yield IU/kg plasma) |
|---|---|---|
| Cryoprecipitate | 630±195 | 428±140 |
| Intermediate purity concentrate | 203±19 | 192±16 |
One well known authority has addressed this issue with some well-justified skepticism (23), describing manufacturing problems encountered with the use of ½ CPD. Experience with blood bank cryoprecipitate (Table 3) (19) and small-scale models (Figure 6) (24) indicates yield improvements which are reflected downstream of the initial plasma. In large scale manufacture, formulating in-process intermediates to higher Ca2+ levels resulted in improved FVIII stability (25), but limited data using ½ CPD did not confirm higher yields further downstream (26). It is probable that the two factors assessed in this section—rapid freezing of uncooled plasma and low citrate levels in machine-delivered anticoagulants (27-29)—are converging to result in the higher FVIII yields which current era fractionators know (but seldom publish) are derived with plasmapheresis, as compared to recovered, plasma.
Table 3
| Production phase | Standard citrate | Half-strength citrate | |||
|---|---|---|---|---|---|
| Plasma frozen 3 hours post donation | Plasma frozen 18 hours post donation | Plasma frozen 3 hours post donation | Plasma frozen 18 hours post donation | ||
| Plasma | |||||
| FVIII:C (IU/mL) | 0.86±0.06 | 0.56±0.11 | 1.01±0.13 | 0.89±0.05 | |
| Ca2+ (µM) | 50±5 | 48±6 | 95±2 | 95±5 | |
| Cryoprecipitate FVIII:C (IU from 100 mL of plasma) | |||||
| Fast-thaw method | 54±7 | 44±8* | 55±5 | 53±8 | |
| Thaw-siphon method | 67±20 | 41±11 | 69±10 | 75±7 | |
| All methods | 60±15 | 42±9** | 62±11 | 64±14 | |
*, P<0.05 for difference between 3-hour and corresponding 18-hour units; **, P<0.01 for difference between 3-hour and corresponding 18-hour units.
From plasma to cryoprecipitate
What makes cryoprecipitate?
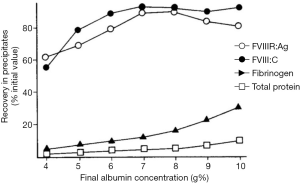
The production of cryoprecipitate has produced a voluminous, literature, although not well maintained. An area of interest in attempting to optimize cryoprecipitate production is to understand the actual mechanism of this phenomenon. A hypothesis based on conventional eutectics led Polson (30) to propose that a simple “salting out” of proteins through the increasing salt concentrations generated by the freezing of plasma was involved. Mackenzie’s work (31) cited by McIntosh et al. (32), based on electric resistivity measurements in plasma and other colloid solutions, indicated that there was no simple eutectic transition, and was confirmed by McIntosh’s measurements showing no evidence of any eutectic freezing (Figure 7) (32). Our own work increasing the protein concentration of plasma through the addition of albumin suggested that a similar concentration effect of the hydrophilic protein albumin might contribute to a differential precipitation of the least soluble plasma proteins in the cold (33) (Figure 8). Additional studies indicated the crucial role of the cold insoluble proteins themselves, in the form of fibrinogen and fibronectin, in the cryoprecipitation of FVIII (Table 4); or rather FVIII complexed to VWF, as Over’s earlier work had shown that cryoprecipitate from VWD plasma was deficient in FVIII (35).
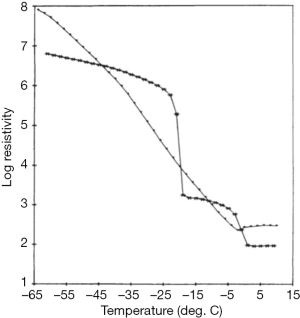
Table 4
| Plasma composition | Yield in cryoprecipitate (% of plasma) (mean ± SD for six experiments) | |||
|---|---|---|---|---|
| FVIII:C | VWF Ag | Fibrinogen | Fibronectin | |
| Physiologic ionic strength | 47±4 | 64±12 | 34±17 | 56±22 |
| Low ionic strength | 60±13 | 51±6 | 42±14 | 34±12 |
| High ionic strength | 5±2 | 31±19 | 19±7 | 89±47 |
| Physiologic protein content | 53 | 84 | 50 | 94 |
| Low protein content | Unmeasurable | 10 | 2 | 2 |
| Physiologic fibrinogen level | 47±8 | 73±24 | 55±28 | 68±26 |
| Low fibrinogen | 11±6 | 11±6 | 92±33 | 13±26 |
| Physiologic fibronectin level | 42±4 | 48±20 | 32±6 | 88±23 |
| Low fibronectin | 3±1 | 5±3 | 2±1 | 25 |
Cryoprecipitate—the processing of plasma
Plasma destined for FVIII production needs to be frozen within a timeframe which preserves recoverable FVIII. Currently available blast freezers were not available in the early years of development, but a variety of methods were shown to be adequate as far as cryoprecipitate yields were concerned (36). In our work in the early 1980s, freezing in −40 °C cabinet freezers gave inferior results to faster freezing achieved in ethanol/dry ice baths (37) (Figure 9).
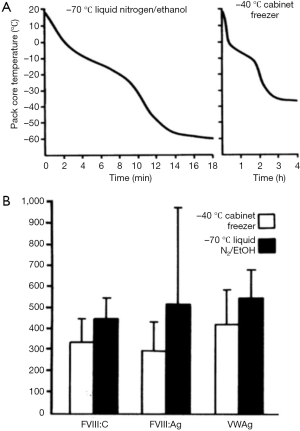
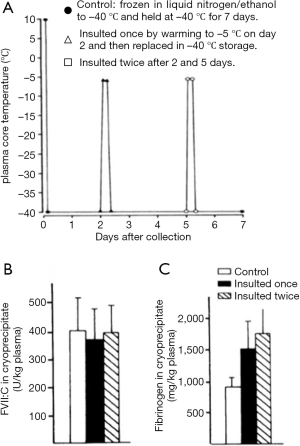
Once frozen, the storage conditions of plasma require the optimization of FVIII yield and cryoprecipitate quality, compatible with its further purification. Storage temperatures of −20 °C appear satisfactory (37), but the maintenance of steady temperatures during frozen storage are important. Temperature insults of the frozen plasma, such as may be encountered during power cuts have some effect on cryoprecipitate FVIII yield but have a much more marked effect on the fibrinogen content of the cryoprecipitate (37) (Figure 10).
The generation of cryoprecipitate on an industrial scale requires the frozen plasma packs to be conditioned to a state which will allow the plasma to be thawed under controlled conditions. The frozen plasma has to be softened to a higher temperature to allow detachment of the plasma from the plastic pack. The softened but still frozen plasma is then thawed. Extending the observations made when studying the conditions for storing frozen plasma for blood bank cryoprecipitate, we and others (38,39) developed conditions for conditioning plasma which minimized the fibrinogen content of cryoprecipitate, thus facilitating its extraction and purification to concentrate (40) (Table 5). This approach allows the fibrinogen content of cryoprecipitate to be adjusted to low levels as required in fractionation to pd-FVIII, and increased to heighten the efficacy of blood bank cryoprecipitate as a source of therapeutic fibrinogen.
Table 5
| Softening method [n] | Cryoprecipitate weight (g/kg plasma) | Fibrinogen (mg/kg plasma) | FVIII (IU/kg plasma) | |||
|---|---|---|---|---|---|---|
| Cryoprecipitate | Final eluate | Cryoprecipitate | Final eluate | |||
| Warm [10] | 10.9±0.61 | 1,200±247 | 90±33 | 435±51 | 276±42 | |
| Cold [9] | 8.8±0.12 | 800±148 | 30±6 | 411±68 | 264±29 | |
| None [7] | 7.9±0.07 | 480±140 | 28±3 | 452±77 | 250±26 | |
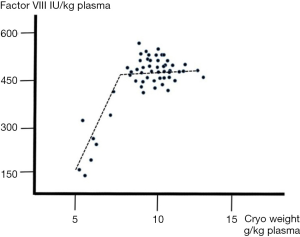
The effect of plasma conditioning on cryoprecipitate weight has also been described for large-scale manufacture [table 3 in (32) confirming the independence of FVIII yield from the weight of solid cryoprecipitate]. Studying this relationship some years ago, I concluded that this independence held as long as cryoprecipitate weights did not go below 7 g/kg of plasma. Once weights became lower than this level, losses of FVIII were increasingly observed, indicating that the structure of the solid cryoprecipitate was insufficiently robust to retain the FVIII (unpublished observations, Figure 11). It is important to note that FVIII is an incidental “contaminant” in cryoprecipitate, whose bulk composition is other proteins. I continue to be surprised at fractionation chemists in today’s modern plants who express astonishment when told that cryoprecipitate from people with haemophilia forms as easily as from normal donors!
The thawing of industrial scale lots of frozen plasma to cryoprecipitate was shown by the group under Peter Foster at the Protein Fractionation Centre to result in optimized FVIII yields when using continuous thawing (41), analogous to the “thaw-siphon” technique for blood bank cryoprecipitate developed in Brisbane and Edinburgh (42,43). I reflect that these principles, both at blood bank scale and at industrial scale, achieved FVIII yields in cryoprecipitate approaching 600 IU/kg. This indicates that, if progress had been made at commensurate levels in the further purification of FVIII, yields would exceed the apparently immutable 200 IU/kg. But, events, particularly the need to develop viral inactivation, overtook these developments, and it appears that the dominance of recombinant products has dissuaded fractionators from continuing to study the optimization of FVIII.
Final reflections
For the generation of patients born after the 1980s, pd-FVIII, at least in the developed economies, has little relevance. And for the scientists engaged in the rapidly consolidating Western plasma industry, dominated by companies which generate most of their revenue from recombinant products, pd-FVIII is rapidly assuming the status of a quaint anachronism.
Balancing this perspective is the importance of pd-FVIII in delivering care to haemophiliacs in the developing world. Efforts are continuing to attempt to divert the surplus of pd-FVIII in publicly funded blood systems to areas which need them and cannot afford the sophisticated, but hugely expensive, biotechnology products (44). Hence, pd-FVIII is still important and life-saving.
As discussed in the accompanying paper, the SIPPET study (ref) has only been the most recent addition to an impressive body of evidence that pd-FVIII results in a lower incidence of inhibitors in previously untreated patients (PUPs) than recombinant FVIII (rFVIII). This evidence cannot continue to be ignored. Until a final cure is achieved for haemophilia A, the problem of inhibitors demands the implementation of any measure which can lessen it. The patients deserve nothing less.
As for this author, I am happy with my career with factor VIII. The fresh-faced researcher who thawed plasma in Edinburgh 35 years ago (Figure 12) has seen much in the field since then. And pd-FVIII was up at that time, then it went down…and now, happily, it is up again.

Acknowledgments
I thank Dr. Chris Prowse, in whose laboratory at the Scottish National Blood Transfusion Service in Edinburgh I performed most of the studies in the 1980s cited in this paper. Dr. Peter Foster, the then head of Research and Development at the Protein Fractionation Centre (now closed, alas!) and Dr. James Smith, then head of Research and Development in the Plasma Fractionation Laboratory in Oxford (also no longer existing) taught me most of what I know about the practical aspects of manufacturing FVIII. All these colleagues are now enjoying a well-earned retirement, and it is perhaps time to join them. In the meantime, I hope that their wisdom is somewhat reflected in this paper, and that the new generation of fractionators may benefit from it.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Thierry Burnouf) for the series “Plasma Fractionation” published in Annals of Blood. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob.2018.02.05). The series “Plasma Fractionation” was commissioned by the editorial office without any funding or sponsorship. Albert Farrugia serves as an unpaid editorial board member of Annals of Blood from Feb 2017 to Feb 2020. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the manuscript and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jeremic M, Weisert O, Gedde-Dahl TW. Factor VIII (AHG) levels in 1016 regular blood donors. The effects of age, sex, and ABO blood groups. Scand J Clin Lab Invest 1976;36:461-6. [Crossref] [PubMed]
- Orstavik KH, Kornstad L, Reisner H, et al. Possible effect of secretor locus on plasma concentration of factor VIII and von Willebrand factor. Blood 1989;73:990-3. [PubMed]
- Tomasulo PA, Richards W, Bailey M, et al. Preselection of donors to improve the quality of cryoprecipitate. Am J Hematol 1980;8:191-6. [Crossref]
- Carlebjörk G, Blombäck M, Blomstedt M, et al. Screening of factor VIII:C levels in blood donors. Vox Sang 1986;51:306-9. [PubMed]
- Soni NS, Patel AR, Vohra RM, et al. Hemophiliac with hemolytic anemia resulting from factor VIII concentrate. Acta Haematol 1977;58:294-7. [Crossref] [PubMed]
- Mannucci PM, Ruggeri ZM, Pareti FI, et al. 1-Deamino-8-d-arginine vasopressin: a new pharmacological approach to the management of haemophilia and von Willebrands' diseases. Lancet 1977;1:869-72. [Crossref] [PubMed]
- Prowse CV, Farrugia A, Boulton FE, et al. A comparative study using immunological and biological assay of the haemostatic responses to DDAVP infusion venous occlusion and exercise in normal men. Thromb Haemost 1984;51:110-4. [PubMed]
- Mikaelsson M, Nilsson IM, Vilhardt H, et al. Factor VIII concentrate prepared from blood donors stimulated by intranasal administration of a vasopressin analogue. Transfusion 1982;22:229-33. [Crossref] [PubMed]
- Noel B, Coudurier E, Brochier G. Donation of factor VIII by plasma exchange after DDAVP administration. Transfus Sci 1989;10:253-6. [Crossref]
- Konecka G, Bykowska K, Ludwicka A, et al. Cryoprecipitate of intermediate purity produced in a closed thaw-siphon system from DDAVP stimulated blood donor plasma. Folia Haematol Int Mag Klin Morphol Blutforsch 1990;117:565-70. [PubMed]
- Pomper GJ, Rick ME, Epstein JS, et al. Management of severe VWD with cryoprecipitate collected by repeated apheresis of a single dedicated donor. Transfusion 2003;43:1514-21. [Crossref] [PubMed]
- Australia Red Cross Blood Service. Australia’s pioneering Rh Program turns 50. Available online: https://transfusion.com.au/bsib_july2017_2
- Stroncek D, McCullough J. Safeguarding the long-term health of hematopoietic stem cell donors: a continuous and evolving process to maintain donor safety and trust. Expert Rev Hematol 2012;5:1-3. [Crossref] [PubMed]
- Brinkhous KM, Penick GD. Relative stability of plasma antihemophilic factor (AHF)under different conditions of storage. Am J Med Sci 1956;232:434-42. [Crossref] [PubMed]
- Farrugia A. Albert Farrugia PhD CH III- FVIII in Blood Donations. University of Edinburgh, 1984. Available online: https://onedrive.live.com/?cid=393430B0FC3610B5&id=393430B0FC3610B5%2136713&parId=393430B0FC3610B5%2136694&o=OneUp
- Weiss HJ. A study of the cation- and pH-dependent stability of factors V and VIII in plasma. Thromb Diath Haemorrh 1965;14:32-51. [PubMed]
- Wang W, Wang YJ, Kelner DN. Coagulation factor VIII: structure and stability. Int J Pharm 2003;259:1-15. [Crossref] [PubMed]
- Rock GA, Cruickshank WH, Tackaberry ES, et al. Improved yields of factor VIII from heparinized plasma. Vox Sang 1979;36:294-300. [Crossref] [PubMed]
- Prowse C, Waterston YG, Dawes J, et al. Studies on the procurement of blood coagulation factor VIII in vitro studies on blood components prepared in half-strength citrate anticoagulant. Vox Sang 1987;52:257-64. [Crossref] [PubMed]
- Pepper MD, Learoyd PA, Rajah SM. Plasma factor VIII, variables affecting stability under standard blood bank conditions and correlation with recovery in concentrates. Transfusion 1978;18:756-60. [Crossref] [PubMed]
- Smith JK, Evans DR, Stone V, et al. A factor VIII concentrate of intermediate purity and higher potency. Transfusion 1979;19:299-306. [Crossref] [PubMed]
- Hughes C, Thomas KB, Schiff P, et al. Effect of delayed blood processing on the yield of factor VIII in cryoprecipitate and factor VIII concentrate. Transfusion 1988;28:566-70. [Crossref] [PubMed]
- Smith JK. Quality of plasma for fractionation--does it matter? Transfus Sci 1994;15:343-50. [Crossref] [PubMed]
- Farrugia A, Douglas S, James J, et al. Use of plasma with high levels of ionised calcium in the production of model scale coagulation factor concentrates. Thromb Haemost 1990;64:374-8. [PubMed]
- Foster PR, Dickson IH, McQuillan TA, et al. Studies on the Stability of VIII:C during the Manufacture of a Factor VIII Concentrate for Clinical Use1. Vox Sang 1988;55:81-9. [Crossref] [PubMed]
- McIntosh RV, Foster PR. The effect of solution formulation on the stability and surface interactions of factor VIII during plasma fractionation. Transfus Sci 1990;11:55-66. [Crossref]
- Beeck H, Becker T, Kiessig ST, et al. The influence of citrate concentration on the quality of plasma obtained by automated plasmapheresis: a prospective study. Transfusion 1999;39:1266-70. [Crossref] [PubMed]
- Rock G, Tittley P, Fuller V. Effect of citrate anticoagulants on factor VIII levels in plasma. Transfusion 1988;28:248-52. [Crossref] [PubMed]
- Robinson AE, Penny AF, Smith J, et al. Pilot study for large-scale plasma procurement using automated plasmapheresis. Vox Sang 1983;44:143-50. [Crossref] [PubMed]
- Polson A. Mechanism of cryoprecipitation. Prep Biochem 1972;2:53-9. [Crossref] [PubMed]
- MacKenzie AP. First and second order transitions during the freezing and thawing of source plasma (human). American Institute of Chemical Engineers (New York) Symposium on Processing and Fractionation of Blood Plasma, Philadelphia, June 8-12, 1980.
- McIntosh RV, Dickson AJ, Smith D, et al. Freezing and Thawing Plasma. In: Smit Sibinga CT, Das PC, Meryman HT. editors. Cryopreservation and low temperature biology in blood transfusion. Developments in Hematology and Immunology, vol 24. MA: Springer, Boston, 1990:11-24.
- Farrugia A, Griffin B, Pepper D, et al. Studies on the procurement of coagulation factor VIII: selective precipitation of factor VIII with hydrophilic polymers. Thromb Haemost 1984;51:338-42. [PubMed]
- Farrugia A. Cryoprecipitate - a quarter of a century on. Pathology (Phila) 1992;24:17.
- Over J, Bouma BN, van Mourik JA, et al. Heterogeneity of human factor VIII. I. Characterization of factor VIII present in the supernatant of cryoprecipitate. J Lab Clin Med 1978;91:32-46. [PubMed]
- Vermeer C, Soute BA, Ates G, et al. Contribution to the optimal use of human blood. VII. Increase of the yield of factor VIII in four-donor cryoprecipitate by an improved processing of blood and plasma. Vox Sang 1976;30:1-22. [Crossref] [PubMed]
- Farrugia A, Prowse C. Studies on the procurement of blood coagulation factor VIII: effects of plasma freezing rate and storage conditions on cryoprecipitate quality. J Clin Pathol 1985;38:433-7. [Crossref] [PubMed]
- Farrugia A, Spiers D, Conway R. Temperature manipulation during plasma processing; a route to improved purity of Factor VIII concentrates. In: The XX Congress of the International Society of Blood Transfusion in association with the British Blood Transfusion Society. Book of Abstracts. Manchester: British Blood Transfusion Centre, 1988:68.
- Winkelman L, Pinnel M. Cryoprecipitate composition as a function of plasma softening rate. Thromb Haemostas 1987;58:338.
- Farrugia A, Grasso S, Douglas S, et al. Modulation of fibrinogen content in cryoprecipitate by temperature manipulation during plasma processing. Transfusion 1992;32:755-9. [Crossref] [PubMed]
- Foster PR, Dickson AJ, McQuillan TA, et al. Control of large-scale plasma thawing for recovery of cryoprecipitate factor VIII. Vox Sang 1982;42:180-9. [Crossref] [PubMed]
- Mason EC. Thaw-siphon technique for production of cryoprecipitate concentrate of factor VIII. Lancet 1978;2:15-7. [Crossref] [PubMed]
- Mason EC, Pepper DS, Griffin B. Production of cryoprecipitate of intermediate purity in a closed system thaw-siphon process. Thromb Haemost 1981;46:543-6. [PubMed]
- Aledort LM, Luca MD, Pinilla MJ, et al. Hemophilia Lend-Lease Program in Italy Successfully Meets Albania Factor Needs. Blood 2016;128:5916.
Cite this article as: Farrugia A. Factor VIII manufactured from plasma—the ups and downs, and the up again: a personal journey—part 2: aspects of factor VIII manufacture from plasma. Ann Blood 2018;3:20.