
Role of the quality assurance person in the production of recovered plasma for fractionation
Introduction
In 2013, World Health Organization (WHO) added the “Blood and Blood Products” to the Model List of Essential Medicines (EML) (1). EML would boost the government awareness to the importance of appropriate regulation oversight of blood collection, processing, testing and distribution to ensure safety and quality of blood products in blood establishments (BEs). Like other medicines, all blood-component regulations are required to assure blood products meet product standards through controls on manufacturing. According to the latest guidelines of the PIC/S Good Manufacturing Practices (GMPs) published in 2015, medicinal products are fit for their intended use and comply with the requirements of the local marketing authorization and must not place patients at risk due to inadequate safety, quality or efficacy. Therefore, the growing implementation of quality system in BEs has entailed the need of quality assurance. To improve blood safety, blood donations and the components derived from whole blood need to be traced from donor to the recipient or plasma fractionation applying a GMP-based quality system.
Recently, blood and plasma have been considered as strategic resources comparable to energy and water. It has been deemed important for national independence (2). However, WHO had estimated about 9.3 million liters of recovered plasma in the world is wasted each year due to non-compliance with the standards for fractionation (3). Producing quality plasma that can be used for fractionation is priority for BEs. It requires a regulatory framework responsible for setting standards that could be applied to blood component processing, cold chain (including rapid blast freezing), blood storage, donation testing, and a comprehensive QA system. To comply with the national self-sufficiency policy in the supply of safe blood components and plasma-derived medicinal products (PDMPs), Taiwan had set-up a validated QA system in 2001 and began to collect more recovered plasma for PDMPs processing through contract plasma fractionation program since 2006. The aim of this article is to share our experience in the activities and the role of the quality assurance person (QAP) in supplying recovered plasma for fractionation. Here the QAP does not address only one person but all professionals in QA department or unit covering the quality-related tasks in BEs.
Role of QAP in BEs
PIC/S GMP guide defined QA as the overall organized arrangements made with the objective of ensuring that medicinal products are of the quality required for their intended use. Many international standards for manufacture of blood and blood products underscore the need for all critical procedures, such as the purchase of raw and starting materials, selection of donors, collection of blood, preparation of blood components, storage, laboratory testing, dispatch and associated quality control measures. All these standards are performed in accordance with the principles of GMP and comply with the regulations established by the National Regulatory Authority (NRA).
Figure 1 shows the overview of the QA function in BEs. Each BE estimates the amount of blood products that are needed by hospitals and plasma fractionation program. Based on these estimates, the BE starts out with donor recruitment, then donor selection, medical procedures of blood collection, donation testing, component processing and storage, and checking the testing results. If any of the units fails to pass the testing results or QC, they should be quarantined and rejected. If the units pass the testing criteria, then the units are labeled and released to the supply department for storage. Upon request, final products are transported to hospitals for patient transfusion. Several critical control points are in place to ensure delicate handling. In addition, blood system needs quality control to ensure that reagents, equipment and methods perform as expected. And all “Component QC” will ensure that blood products are passed the criteria for each kind of product. This is the scope of QC, which span from “Testing” to “Storage”. At the GMP level, the scope will widen to include everything from “Donor Selection” to “Distribution”. Each step along the process will now be subjected to facilities, equipment, computer software validation and maintenance, process validation, document control, process control, trained staff, and record management. As the requirement of GMP, internal auditing and corrective action and preventive action (CAPA) must be applied to oversee the entire process. Once the blood products are issued, they should be subjected to standards for traceability. Finally, QA encompasses all of the GMP elements as well as QC on this figure. A “Quality Plan and Quality Performance Review” should be drafted for the whole quality system. All these items should be included within a quality system and well organized by QAP. Our quality system was set up based on ISO 9001 quality system and following FDA’s “five layer protections for donated blood” (donor screening, donation testing, donor deferral lists, quarantine, problems and deficiencies) (4) and AABB’s quality system component (5).
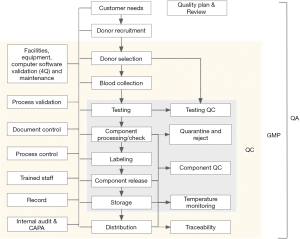
QAP can use a process approach to develop, implement and improve the effectiveness of BE’s quality system. This approach enables all organization to control the interrelationships and interdependencies among the processes of the system, and overall performance of BE can be enhanced. QAP can monitor and measure the check points, which are necessary for control in each process based on its related risks. The overall strategy, which is used to ensure that all PDMPs are produced from safe starting materials, is based on the following principles:
- Systematic approach to donor selection;
- Ensures appropriate testing methods and test kits as well as suitable reagents to standardize testing protocols for all blood donations;
- Requires the use of suitable facilities, equipment and materials, especially enhance the cold chain and freezer storage capacity;
- Reduces errors and technical problems;
- Ensures the existence of validated and robust processes;
- Guarantees the release of products comply with safety and quality requirements;
- Ensures adequate documentation and full traceability for each donation;
- Strengthens the competency of personnel;
- Promotes continuous improvement through QA measures.
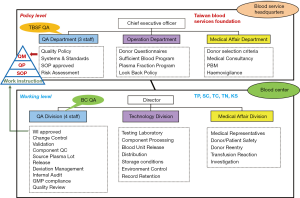
To ensure that the quality system in place can assure product quality, there is a requirement that QAP interact strongly with all departments. The QA department should be independent from other departments within BE. The role of the QAP can be described as a supervisor, mediator, initiator, and coordinator for all QA aspects. Figure 2 shows the organization chart and QA function in Taiwan’s BEs.
Duties of QAP
The duties of QAP are influenced by the activities performed in BE; however, some duties are common to all. A minimum of duties should include:
- Documentation management (e.g., administration, review, and approval of SOPs, master documents, and batch files);
- Qualification of equipment, validation of processes and analytical methods;
- Audit and inspection management;
- Change control procedures;
- Batch disposition and batch release;
- Batch record review;
- Deviations (investigation, evaluation, and CAPA);
- Product quality reviews/trending of critical process indicators;
- Training program (new personal and existing staff);
- Process improvement (if not done by CAPA);
- Customer complaint handling, recall procedure;
- Risk analysis and risk management.
Table 1 lists the example of routine duties of QAP in our center.
Table 1
| Activity item |
| Daily |
| Monitor deviation reporting system |
| Weekly |
| Participate in the investigation of deviation |
| Monthly |
| Review all quality indicators |
| Monitor testing performance |
| Approval change control |
| Approval all equipment validation reports |
| Approval competence evaluation reports |
| Approval and review the plan and report of validation |
| Communication the change of SOP and management |
| Make sure all invalidate plasma units are excluded during storage |
| Quarterly |
| Batch release of recovered plasma for fractionation |
| Review all quality indicators |
| Conduct staff GMP training |
| Review deviation or customer complaint report |
| Semiannually |
| Management review |
| Conduct internal audits |
| Conduct internal auditor training |
| Computer system validation |
| Annually |
| Hosting of fractionator audits |
| Hosting ISO 9001 and 15189 accreditation audits |
| Approval QA plan for all equipment |
| Approval all calibration plan |
| Approval quality goal for each process |
| SOP annually review |
| Regulatory update communication |
| Review the plasma specification and agreement with fractionator |
| Biennially |
| Hosting of GMP, GLP external inspection |
| Critical material supplier audits |
| Evaluate internal auditors |
QAP, quality assurance person; GMP, Good Manufacturing Practice; QA, quality assurance; SOP, standard operating procedure; GLP, good laboratory practice.
Skills and awareness of QAP
QAP is essential in the quality system of BEs and is responsible for ensuring that GMP is in place. If there are any changes in manufacturing or quality control the QAP will assess these impacts on product quality. QA manager or the authorized QAP is also legally responsible for certifying that each batch of recovered plasma is suitable for release for fractionation. To be named as a QAP of BEs, they need to be deemed eligible. In Taiwan, pharmacists or medical technologists can become QAP. Specific knowledge and practical experience are needed to work as a QAP. A pharmacy or medical technologist degree can provide the initial knowledge needed to become a QAP and certain skills. A publication by Dr. Vuk (6) lists some desired skills for QAP including leadership, the ability to motivate others, organizational abilities, planning, decision-making, teamwork, communication, and excellent interpersonal skills. Besides, QAP needs to have a good understanding of all aspects or professional knowledge of blood processing, supply cold chain and transfusion medicine. Strong leadership and oversight are necessary, especially before certifying a batch release of plasma for fractionation, the QAP must be satisfied that all relevant checks and tests have been performed and documented. QAP also need to be confident in the judgements they make, especially when “grey areas” arise, and be prepared to act decisively when things go wrong. If one donor has been identified retrospectively as presenting deferral criteria making his/her donation unsuitable for fractionation, the QAP should calmly apply the approved recall procedure of the plasma. The QAP needs to cope well under pressure, be flexible and be able to take appropriate steps based on the information available. QAP must have professional competency and a strong sense of confidence to establish their credibility. They also need to have a good knowledge of the regulations, guidelines and resources to maintain an up-to-date knowledge of blood regulations. Table 2 lists the competence and knowledge of QAP required in our center.
Table 2
| Competences |
| Design criteria of QM Systems (QMS) |
| |
| Process and equipment validation |
| |
| |
| Materials management |
| |
| Documentation control |
| |
| Distribution |
| |
| Complaints and recall |
| |
| Deviation and change control |
| |
| Provision of training |
| |
| Assessment and inspection |
| |
| Associated skills and knowledge |
| Content and practice of audit |
| Impact and risk assessment methods and evaluation |
| Underlying principles of validation design and evaluation |
| Defective products evaluation and categorization |
| Demonstrate knowledge of the blood-related regulations |
| Demonstrate knowledge of statistical tools such as: statistical process control, common statistical tools, principles of six sigma and lean project |
| Must read references |
| WHO—Recommendations for the production, control and regulation of human plasma for fractionation ( |
| US FDA—Guideline for Quality Assurance in Blood Establishments ( |
| AABB—Technical Manual and Accreditation standard ( |
| EC—Guide to the preparation, use and quality assurance of blood components ( |
| PICS—PIC/S GMP Guide for Blood Establishments ( |
| National regulation—cGMP guide: Human plasma for fractionation (In Chinese) |
QAP, quality assurance person; DQ, design qualification; IQ, installation qualification; OQ, operational qualification; PQ, performance qualification.
Ethics and data integrity
In addition to traits and skills, QAP should have strong ethics. QAP are responsible for their conduct, personal behavior and professional practice and must be able to justify their actions and decisions. As they are guided by the ethical principles of no maleficence, beneficence, autonomy, and justice, QAP needs to evaluate their actions in the context of overall blood safety rather than single area. An excerpt from the Code of ethics for BE personnel, modified from WHO National Standards for Blood Transfusion Service, indicates that:
- Blood should be collected under the overall responsibility of either a registered medical practitioner who can manage a donor adverse reaction;
- Anonymity between the donor and the recipient must be ensured and the confidentiality of donor information assured;
- Blood is a public resource and access should not be restricted. Wastage of blood and blood components should be avoided at all times;
- A profit motive shall not be the basis for the establishment.
Data integrity has been and currently is a major global concern of NRA in pharmaceutical industry. For BEs concern focus on donor/patient confidentiality, traceability, and potential for blood contamination. These have resulted in increasing pressure on BEs to assure the consistency and reliability of blood operations, security and data integrity. Data integrity can result from lack of awareness of regulatory requirements, employee errors, failure to check accuracy of data, software or system malfunction, configuration problems with electronic data handling, or malfeasance by employees. All data recorded in electronic or paper formats should be attributable, legible, contemporaneously, true copy, and accurate. QAP needs to be able to assess data integrity to assure that the data supporting all blood product quality decisions can be trusted. The BEs shall maintain and establish data integrity procedures including elements of a code of conduct for data integrity, data integrity training for all employees, and periodic monitoring of data integrity.
Examples of QA activities: Taiwan experiences
Some examples of QA activities and the role of QAP for improving the effectiveness of quality system are provided below.
Example of a critical material release process
For instance, for the release process of blood bags, the QAP first check the supplier documents to ensure the compliance with local regulation and review the certificate of analysis for each lot. Then, QAP will conduct a random sampling test, which includes sending three lots each year to an outsourced laboratory. The sampled bag will be subjected to three tests including pack leakage test, endotoxin test and sterilization test for ensuring the quality and safety of blood bags. In addition to documents review and random sampling test, QAP also conduct supplier onsite audit every 3 years. QAP prepare supplier auditing checklist based on ISO 13485 and ISO 3826 standards.
In 2011, after our first supplier audit, we recommended blood bag manufacturer should provide us the transport temperature records to ensure quality of blood bag products, because ocean freight shipment often results in higher shipping temperature due to changing weather conditions. In response to our request, they provided the transport temperature chart and chemical analysis for the shipment to Taiwan. In their validation report, the temperature profile of the transportation (less than 2 weeks) clearly demonstrated that the shipping temperature did not exceed the transport temperature range stated in the package insert. And there was no any effect of the chemical analysis result for blood bags with anticoagulant. These data ensure that our blood bags, which contain anticoagulant, were not exposed to extreme temperature during transportation. Currently, the manufacturer provides us with the transportation temperature validation report twice per year in winter (Jan. to Feb.) and summer (Jul. to Sep.).
Example of deviation reporting process
BE needed to demonstrate a capability to execute CAPA to address the response to deviations errors, accidents, complaints and adverse events. QAP must monitor a corrective action dealing with the affected plasma batch, and take some preventive actions to avoid repeated deviations of the same nature. The main requirements for CAPA system include reliable detection and notification system, highly motivated employees, a company culture which promotes error detection and process improvement, experts in different areas with a high degree of process understanding, and a clear deviation processing work flow. In 2011, during Taiwan FDA (TFDA) inspection, we were cited for deficiencies that include the lack of records to trace the disposal of event-related blood components and QAP did not verify the completeness and effectiveness of the CAPA plan. After we reviewed the citation, we took some actions. We revised SOP to include QA final review and effectiveness check, added blood product disposal records for each case, and set some check points for deviation reporting system for record review by QAP. Our deviation reporting system is now completely online based. Once the deviation has been reported, we devise an immediate response action as well as a root cause analysis (RCA). Then, we draft a CAPA plan and QAP review all the information. Due to the citation from the TFDA, we now check the disposal of blood components and at least use one investigation tool at RCA stage. After QAP review the CAPA plan and they will follow-up these actions. At the end, the QAP conduct an effectiveness check. Besides, QAP can illustrate some instances of formal application of the CAPA process for review at regular management review meetings and assesses for the appearance of trends that may justify continual improvement measures. By enforcing QAP review the CAPA plan, a significant reduction in repeat non-conformances or events was seen.
Example of internal and external audit or inspection
For internal audit, we trained 20 auditors by providing auditor training every 3 years in our blood center and qualified them to establish an effective auditing system. All auditors have in-depth experience in technical topics of the BEs. After conducting an internal audit program, all auditors would provide a written report and submitted to QA department within 14 days. The audit report would list all observations or findings categorized into major or minor deficiencies and recommendations. The auditees would initiate corrective and preventive actions and approval by QAP. All the responsibilities and timelines would be defined and enforced by QAP. Auditees would be expected to assist the auditors with required information or documentation during all phases including the notification about completion of corrective and preventive measures. Besides, audit plans, reports, and the evaluation of every critical audit results would be regularly reported to the senior management of BEs by QAP.
For external inspection, all BEs including blood collection sites and random selected mobile blood collection units are inspected every 3 years by TFDA since 2006. The inspection team includes 3–4 GMP inspectors and 4 external experts. Prior to each inspection, BE has to provide the master file. The inspection itself takes about 3–4 days. After the inspection, the BE’s QA department will receive the inspection report. Then, QAP review any citation and respond with a CAPA plan to the inspection team. Assuming all the issues has been resolved; the Taiwan regulatory authority will extend the manufacturing license. In our experience, inspections always provide a great opportunity to interact with inspectors to achieve better GMP-compliance. During routine inspections, QAP cherish this opportunity to communicate and reach an optimize standard that will meet the GMP criteria as well as the needs of the BEs.
Contribution for being a good role of QAP
In 2001, for the protection of domestic blood systems, Taiwan declared a policy of “self-sufficiency” in blood and blood components. A plasma quality work plan was established in our center and a fractionation project of “source plasma” has been initiated with a UK fractionator, the volume fractionated was about 20,000 liters/year at that time. However, there was a financial burden on Taiwan’s BEs due to the cost of storage and discard of “recovered plasma”. Improvement in the quality of recovered plasma to meet requirements for fractionation was imperative. Investments were necessary to reach GMP required for the manufacture of plasma for fractionation, in particular in the production methods and control of plasma (facilities, equipment, personnel, screening tests, quality system, traceability, etc.). Since 2005, TFDA started to inspect BEs producing plasma for fractionation. The role of QAP is essential during setting up and maintaining the quality system and GMP compliance. Today, Taiwan has been implemented a contract plasma fractionation program with CSL from Australia for many years, and will initiate another program with a Japanese fractionator in 2018. The yearly total volume of recovered plasma for fractionation was 200,000 liters in 2016 and is expected to reach 300,000 liters in 2020. The QAP can benefit the BEs in reaching various benefits including an increased plasma revenue thanks to an increasing portion of plasma units used for fractionation, increasing organization’s confidence to meet the requirements of regulations, and enhancing a common understanding for better collaboration between NRA, BEs and fractionators. The most important contribution is to optimize the quality, safety and utilization of all blood components, which are strategic resources, and contribute to meet the national needs for blood and blood components.
Conclusions
For the quality system to be effective, QAP need to monitor the system performance through real time data analysis and scheduled audits. Secondly, they need to ensure the validation of procedures and supplies before they are placed into use. Third, insist on realistic timescales for effective CAPA, in particular, defining and addressing the root cause of (human) errors. Finally, they need to act as the bridge between the organization and external auditors. It is also a good way to benchmark your performance against other organizations. A QAP should truly enjoy his role in QA, and ensure that the blood products manufactured under his/her supervision exhibit an optimal safety margin for all patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Thierry Burnouf) for the series “Plasma Fractionation” published in Annals of Blood. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob.2018.04.01). The series “Plasma Fractionation” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the manuscript and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Klein HG. Should blood be an essential medicine? N Engl J Med 2013;368:199-201. [Crossref] [PubMed]
- Strengers PF, Klein HG. Plasma is a strategic resource. Transfusion 2016;56:3133-7. [Crossref] [PubMed]
- Padilla A, Page P, Burnouf T. Improving access to safe blood products through local production and technology transfer in blood establishments. WHO Press, 2015:20-2. Available online: http://www.who.int/phi/publications/blood-prods_technology_transfer.pdf
- United States Food and Drug Administration. Keeping Blood Transfusions Safe: FDA’s Multi-layered Protections for Donated Blood. [published 06 November 2015, accessed 14 September 2017]. Available online: https://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/BloodSafety/ucm095522.htm
- Motschman TL, Jett BW, Wilkinson SL. Quality Management System: Theory and Practice. In: Roback JD, Combs MR, Grossman BJ, et al. editors. Technical manual, 18th edition. Maryland: American Association of Blood Banks, 2008:1-38.
- Vuk T. Skills and tasks of quality manager at a blood establishment. ISBT Science Series 2010;5:179-83. [Crossref]
- WHO. ANNEX 4: Recommendations for the production, control and regulation of human plasma for fractionation. WHO Technical Report Series [published 2005, accessed 14 September 2017]. Available online: http://www.who.int/biologicals/publications/
- United States Food and Drug Administration. Guideline for Quality Assurance in Blood Establishments. [published 1995, accessed 14 September 2017]. Available online: https://www.fda.gov/downloads/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/blood/ucm164981.pdf
- EDQM. Guide to the preparation, use and quality assurance of blood components. EDQM Council of Europe; [published 2017, accessed 14 September 2017]. Available online: https://www.edqm.eu/en/blood-transfusion-guides-1608.html
- PIC/S GMP Guide for Blood Establishments (PE 005-3). [published 2007, accessed 14 September 2017]. Available online: https://www.picscheme.org/en/publications
Cite this article as: Pai SC. Role of the quality assurance person in the production of recovered plasma for fractionation. Ann Blood 2018;3:25.


