Indian plasma fractionation industry: challenges and opportunities
Introduction
India is one of the fastest developing nations on the globe. It has made a very impressive progress in various areas of healthcare with world-class hospitals, large number of US-FDA approved pharmaceuticals plants, clinical research organizations, young and talented human resource and change in the regulatory environment. It attracts a large number of patients across the globe for medical tourism because of cost effectiveness, for the same quality of care as anywhere else in the world. Despite this progress and growth pattern it also faces many challenges. It is a unique country, located in South East Asia (SEA) with about 1.3 billion inhabitants, accounting for more than 20% of the global population. About 70% of population still lives in rural area and 30% in urban and semi-urban setting in very large cities and towns (1). This makes India, a country of contrast, where people with highly diverse socio-economic backgrounds live together. This highly multicultural society faces challenges of complex dynamics of competing priorities in the critical areas like health, education, infrastructure, etc. Indian healthcare system is largely managed by private sector, which is about 75%, while public healthcare accounts for 25%. It spends about 4.5% of GDP on health (2). Though the central government has many schemes to support its healthcare initiatives, still health is a state chapter, where each state decides its own priority based on the pattern of disease and budget allocated. The access to healthcare facilities is one of the very big challenges because of many factors. One of them being less than 5% Indian population has medical coverage and therefore most of the healthcare expenditure is out of pocket and this puts a huge financial burden to the individual or the families of the patients. These 5% population is largely urban based or in regular employment either in private or government sector. Though access to health is a fundamental right of every citizen and government must ensure it, but at present, there is wide disparity in the access and coverage of essential healthcare services to the citizens of India. To address these issues in the scope of health services delivery, the government has initiated several schemes under Universal Health Coverage viz. National Rural Health Mission (NRHM), Rashtriya Swasthya Bima Yojna (RSBY), etc. (3).
India is highly import dependent for its plasma product needs, which are considered to be life-saving medicines. At this point in time, India produces majorly only two plasma products, albumin and intravenous immunoglobulin (IVIG) from local (Indian) plasma but there are ten more plasma products, which are needed for various clinical conditions, are largely imported. Because of changing disease pattern, improved clinical diagnostic facilities, affordability, medical tourism and many other factors the demand of these products are so high that India is always in short supply, for some of these products (4). Various hyper-immune plasma proteins (Anti-D, tetanus immunoglobulin, hepatitis-B immunoglobulin, and rabies immunoglobulin) are 100% imported or prepared from imported bulk, as India does not produce any hyper-immune plasma domestically, which is the raw material to produce these specialized hyper immune proteins (5). This creates a huge demand and supply gap and many patients lose battle of life because of non-availability of these products.
Transfusion services
Blood, blood components and plasma are categorized as a “drug” under Section 3(b) of Drugs and Cosmetics Act, 1940 of India. This Act and the Rules thereof provide the legal framework for regulating the functions of blood banks, which provide recovered plasma for plasma fractionation to various fractionating companies in India (6). Under the ambit of the Drugs and Cosmetics Act 1940, has been expanded and the rules are frequently amended in the form of gazette notification to incorporate preparation/manufacturing/storage and usages and blood, blood components and plasma. Till early/mid 1990s, blood donation was very poorly regulated and main source of donation was professional donors to meet the demands of the plasma supply. These donors were compensated financially for blood and there were practically no proper guidelines to donate the blood and on quality of plasma obtained from these donations. During this time frame, very few small/mid-size plasma fractionation plants were operating in India. Most of these plants were collecting/sourcing plasma from the blood banks and making albumin, immunoglobulins and lyophilized cryoprecipitate, where the quality of plasma was not well defined. Both blood banks and plasma fractionation units, were largely operating under the same umbrella of regulation and the quality of plasma used for manufacturing was poorly monitored. With reporting of first case of transmission of HIV through blood transfusion in Mumbai in 1986, came a new era of transfusion transmitted infection (TTI). It created a major scare in public health arena and as a response in 1992 central government established National Aids Control Organization (NACO) with a mandate to oversee overall operation of blood banking services in India and work towards supply of safe blood and its products to the patients (7). During the same period the consumer movement was also picking up in India and on November 13, 1995, the Supreme Court of India, upheld the National Consumer Commission’s judgment of April 1992, whereby patients who received deficient/sub-standard services from medical professions and hospitals are entitled to claim damages under this Act. It still remains as a huge challenge as there is a reported case of transmission of HIV in 2018 (8). This clearly implies that there is a vast scope to improve blood transfusion services in the country (9).
This Act automatically included functioning of all the blood banks for their product and services, and operating plasma fractionation units. Both donor and recipient may take the cover of this Act and challenge the establishment for any damage. In 1996 under the article 32 of the Constitution of India, a public interest litigation (PIL) was filed in the Supreme Court of India by a Delhi based organization, challenging government/regulatory agencies for this situation and were asked to respond. After a series of hearings and deliberations, came the historical and a milestone judgment by Hon’ble Justice S C Agarwal and Hon’ble Justice G B Pattanaik making selling of blood illegal effective from January 1, 1998. The honorable court also came with a series of recommendations to improve the overall quality of blood banking in India and making state more responsible and answerable. It was also suggested to formulate National Blood Transfusion Council (NBTC) and State Blood Transfusion Councils (SBTC), make central licensing mandatory, increase voluntary blood donation base, change/modification/amendment in the Drug and Cosmetic Act, improvised funding, better testing methods, set the new and modern standards of quality, build the human capital by introducing postgraduate courses in transfusion medicines at various premier institutes of India and involvement of civic bodies to bring overall change in the system (10).
This resulted in the formation of various committees and active groups, including people from various walks of life with a goal to provide safe blood and blood products to the people of India. Though all these activities did bring a substantial change and improvement in the quality of blood banking services in India and led to the establishment of large modern blood banks with better equipment, more voluntary blood collection, popularization of component therapy and implementation of quality system but it did not help much in the growth of plasma protein industry. As the issues related to blood banking and plasma protein therapies, are quite similar in one sense but are also very different in many ways. Blood banking is a relatively very small level of operation as compared to plasma fractionation, which is a very big industrial operation with a very heavy emphasis on GMP norms and viral safety concerns. The issues related to plasma fractionation industry did not come up for serious discussion at higher level of deliberation, as there were hardly any companies working in this field and that is why at that point in time it was very invisible and insignificant from the point of view of public healthcare system. This could be one of the reasons that many of the hemophiliacs who received contaminated lyophilized cryoprecipitate during late 1980s and early 1990s died silently without any large public outcry and any compensation. This was the time, when Hemophilia Federation of India (HFI) was in very initial stage of formation and most of the patients were leading isolated life without any support. With release of contaminated products in the market many blood banks and fractionating units were shut down and virtually without any alternative plan of supply. As there was no domestic production of anti-hemophiliac factor (AHF) and no import was not permitted, which led to a major crisis for Indian hemophiliacs. It affected Hemophilia community to a very great extent and as availability of factor VIII was very seriously compromised and, hemophiliacs started losing battle of life in absence of basic support from government. With this in view, in mid 2000s again, a PIL was filed by a representative of hemophilia community of India under the provision of right to health, which is guaranteed in the constitution of India (11).
Plasma fractionation operations
With closing down of various operating units in late 1980s and early 1990s, India did not have a single plasma fractionation plant to cater to its large population, which was about 890 million. The availability and affordability of safe product was a major challenge. During this time, with the help of Swedish International Development Agency (SIDA), a small plasma fractionation plant “National Plasma Fractionation Center (NPFC)” was established in the premise of King Edward Memorial Hospital, Mumbai with a fractionation capacity of 10,000 L/year. It is being run by a trust comprising of municipal corporation officials as ex-officio members and staff. It was envisaged that this center would receive safe plasma from various blood banks in India, which were getting into blood component therapy in 1990s (12). The plasma was to come at either no cost or notional price. It was thought that if this model becomes successful, it may form the basis of growth of industry in India as it would streamline plasma supply issues, initiate regulatory reforms required for growth of plasma fractionation industry in the country and bring awareness amongst clinicians and patients. This experiment looked very relevant and logical, as it would have helped India to explore a model, which is developed locally and sustainable from the point of view of technical, business and financial considerations.
Unfortunately, for varieties of the reasons it did not work the way it was planned. Eventually, in early 2000s, it was found that it is not meeting with mandatory GMP norms, it stopped its operation and since then it has not been functioning (13). Although there was no problem with technology of the plant but because of poor management/regulatory inputs, this project failed. As this plant was first of its kind in India, it needed support from operational point of view as well as management system. Though by any standard, this plant would have not been in position to cater such a large Indian population, but it definitely would have provided impetus to the growth of the industry and much desired confidence to regulators, public in general and patients, those who have gone through very difficult time because of unsafe products in the past. Its success would have changed the landscape of plasma fractionation in India, sadly its failure was a huge setback to the country and delayed the progress of plasma fractionation in India. During this time, a private plasma fractionation plant came up in India and another company started working on contract plasma fractionation model. It did ease the situation to some extent as the products coming from western world were very expensive, considering average Indian salary, very low or no medical insurance and fluctuating exchange rate of international currency, these products were/are unaffordable by a large number of patients (14). However, in order to make a plan for successful plasma fractionation program, following three major issues have to be discussed and resolved;
- Availability of safe plasma on consistent basis;
- Linkage between blood bank and plasma fractionation operations through a well-defined regulatory system;
- Public health policies.
Plasma for fractionation
As per government records India has more than 2,700 blood banks collecting about 10–11 million units of blood on annual basis (15).
Quantity of plasma
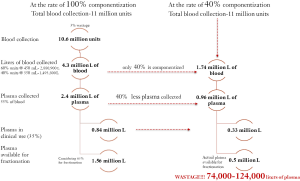
The amount of blood collected from an individual depends upon his body weight. So out of 11 million units collected annually about 60% is collected as 450 mL and 40% as 350 mL. It is estimated that about 3% of blood gets wasted in the process of collection, storage, componentization process and distribution. Considering if each unit of remained blood undergoes componentization, it can give about 1.5 million L of plasma. Even if the entire blood collected undergoes componentization, the plasma available will be far more inadequate looking at the out growing need of plasma products in India (16). Unfortunately, due to rampant whole blood therapy only 40% of blood is componentized to blood cells and plasma and excessive clinical usages of plasma and regulatory hurdles leaves only 0.50–0.55 million L of plasma is available for fractionation, as explained in the Figure 1.
Low rate of componentization could be due to complexity of licensing process, lack of infrastructure or management inputs. Also, some amount of plasma gets wasted because of poor guidelines or understanding of directives for transfer of plasma from blood banks to a fractionating company (17).
Quality of plasma
Each unit of blood collected in India must be tested for presence of five blood borne pathogens: HIV I and II, hepatitis C, hepatitis B, syphilis and malaria. Under current regulation serological methods are mandatory, as Nuclear Acid Test (NAT) is not mandatory.
- Though there is thrust to increase the voluntary blood donation base but India still has sizable of proportion of blood donor who either come under replacement and directed donation. These donations are done without any expectation of financial or material gain;
- As India has more than 2,700 blood banks and very complicated regulatory approval process where the licensing being under the control of the Drug Controller General of India (DCGI), policy under NBTC and SBTC and its implementation being monitored by SBTC. Many times, it leads to poor implementation of policy, which eventually affects all aspects of blood banking, including the quality of plasma;
- Though each blood bank is expected to follow guidelines set by various regulatory agencies, but each blood bank has different format for recruitment and introducing questionnaire to donors. This is very critical from the point of safety of blood, because while recruiting donor if each question, its interpretation and impact is not explained in detail it may have impact on quality of blood and therefore plasma;
- One of the most challenging part is that, where most of the donations in India are one-time donation and repeat donor base is less than 20%. This very clearly implies that donor recruitment and proper testing of plasma is far more important and because of this reason probably there is no provision for inventory hold in current India laws;
- Under current regulation each unit must be tested for serological method but does not explicitly mentions the generation of kit and relevance of newer methods and quality control. Therefore, different blood banks use different quality and generation of kits and this variation may affect the quality of plasma;
- Most of the blood banks having license of preparing blood components, have good infrastructural support and equipment for preparation of cellular components and its storage but when it comes to plasma preservation, it is not available. Most of the blood banks do not freeze the plasma by blast or snap freezing. This is one of the very important reasons for poor yield of factor VIII out of Indian plasma. This also has a serious financial impact whether the cryoprecipitate is used by the patient or utilized for manufacturing of lyophilized factor VIII.
Linkage between blood bank and plasma fractionation operations
After many PILs, transmission of infection and non-availability of products, led to major debate at higher level and the importance of availability of plasma products in public healthcare system. It was found to be very urgent and important to create bridge between plasma fractionation industry and blood bank community, who is responsible for supply of safe plasma and plasma fractionation industry to make sure that is proper understanding of their roles and responsibilities. Government started very seriously considering all possible ways to ease the situation and support industry and patients groups (18). The last one decade saw a dramatic change in the perception of all stake holders in this field and it resulted into development plasma fractionation industry in India. It paved the road for future development and growth.
- Plasma fractionation project in public sector: within short period of time following PIL and its discussion, cabinet gave its approval for setting up a plasma fractionation center in Chennai. A detailed project report was prepared a group of experts appointed by Blood Transfusion Services (BTS), NACO and submitted for further approval. The mandate was to provide free or subsidized factor VIII and IX to hemophiliacs of India. This plant was planned to fractionate about 150,000 L plasma and manufacture albumin, IVIG, factor VIII and IX and budget allocated was 250 Cr (around US$ 35 M). This project was to come up by end of 2014 but unfortunately it has not taken off for various reasons (19);
- Approval of plasma collection and contract plasma fractionation: with ongoing reform and modern blood banks coming up, component therapy started getting more and more acceptance from clinicians resulted into generation of recovered plasma. A part was utilized for treating the patients but still a fair amount of plasma started accumulating in blood banks, which could be used for fractionation. In mid 2000s, government started attending the request for allowing collection of plasma for fractionation by fractionating companies which was pending for number of years. After a very careful consideration the permission came in the form of No Objection Certificate (NoC) and it gave a very big boost to Industry. By now one private plasma fractionation plant started its operation and one other private company applied to DCGI to get permission for starting contract fractionation of Indian plasma. Unfortunately, Indian Drug and Cosmetic Act does not have very clear provision for contract plasma fractionation, a special very high-level committee was formed under the Ministry of Science and Technology to look into possible implications and impact. This committee gave its report to Ministry of Health and Family Welfare and for the first time in the history of India, permission for contract plasma fractionation was granted in 2005. Initially this permission was granted for 10,000 L on experimental basis and based on the success of the project, further permission was granted and regulatory provisions were made;
- More plasma fractionation units: with more conducive environment 3 more companies started on setting up plasma fractionation plant. These small/mid-size plants are based on Cohn and Chromatography or pure Chromatography technology. Recently, one more company has announced setting up of a plasma fractionation plant in the southern part of India and with this addition, the capacity of fractionation could go up to 700,000 L/year. Today India has four plasma fractionation units and fractionates albumin, immunoglobulins, factor VIII, factor IX and fibrin glue. Since source plasma collection is not permitted in India, India remains completely import dependent as far specialized immunoglobulins are concerned. This dependency could be either in the form of getting full finished products or import in the form of bulk or plasma;
- Reform on testing protocols of plasma products: National Institutes of Biologicals (NIB) is the nodal governmental organization under Ministry of Health and Family Welfare responsible for release of biologicals in India, including blood plasma products. Along with very change in regulatory process at DCGI level, NIB also started working on improvement in the testing protocols and process of release of blood plasma products in India. Various committees were constituted comprising of representatives from NIB, DCGI office, Indian Pharmacopeia Commission (IPC) and Industry to make recommendation and sent it to appropriate authorities for its implementation;
- Policy for access to plasma-derived medicinal products from human plasma for clinical/therapeutic use: This policy was formulated to facilitate movement of plasma from blood banks to fractionation companies (20);
- Change in Indian pharmacopeia: pharmacopeia is a very key document for any country/region in order to make sure that products which are made available to people are of set standard and meet safety norms. As plasma fractionation industry is developing, IPC has formed a committee of expert to review and revise IP, add new products, based on international protocols of testing either add new methods of testing or remove obsolete protocols from IP (21);
- Price control of plasma products: under the Ministry of Chemicals and Fertilizer, Department of Pharmaceuticals, the prices of medicines are controlled by National Pharmaceutical Pricing Authority (NPPA). This organization has many roles and works with various stakes holders in order to achieve logical pricing mechanism. Currently, plasma products, which are under price control, are albumin, AHF, anti-D immunoglobulin, anti-tetanus immunoglobulin, anti-rabies immunoglobulin, however, plasma is not under price control;
- Price control of plasma: in October of 2014 NACO had organized a meeting of all stake holders to discuss the various issues related to self-sufficiency of plasma products in India. Along with varieties of issues, pricing mechanism and payment issues for the movement of plasma from blood bank to fractionation, were also discussed. After almost 1 year in October 2015, a guiding document was published by NACO, which is progressive and a step forward but still lacks the clarity on many of the very critical issues as it does not clearly define the quality of plasma, payment mode, logical role of SBTCs in this matter, etc. This could have been made more contemporary keeping global developments in this field;
- Source plasma: Indian law prohibits payment for source plasma collection for any commercial activity like plasma fractionation. This has historical view of before 1996, when compensation to blood donors was legal. This resulted into transmission of HIV and other blood borne diseases to many patients. How to develop source plasma collection model has come up at various forums but no concrete plan has been proposed. However, in the 26th General Body meeting of NBTC, held on June 1, 2017 discussed many relevant issues including recommendation for use of generation IV kit for testing of viral markers and permission to start plasmapheresis centers for collection of source plasma. A national consultation meeting was held under chairmanship of Director General of Health Services (DGHS) which deliberated on this subject in meeting held on July 18, 2016 and the suggestion from this meeting came for further discussion to NBTC core coordination committee meeting held on September 29, 2016. It clearly states that recovered plasma collection should be considered a major source for plasma fractionation, however collection of source plasma could be encouraged only as voluntary activity and donors cannot be compensated (22,23);
- Hemovigilance: As NIB is a nodal agency for testing and release of biologicals in India. It started its pilot project on Hemovigilance in December 2012. This is of first of its kind, as not many Asian countries have this surveillance program. At this point in time, it largely focuses on blood donors and recipients of blood components but has provision of inclusion of plasma products in the next phase of implementation. It is also now a part of International Hemovigilance Network (IHN). It collects the data from various medical centers, hospitals and regularly updates its finding, which will help in understanding the pattern of adverse drug reaction and how to minimize it (24).
Self-sufficiency of plasma products
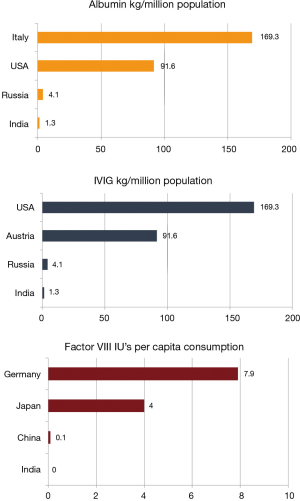
A report assessed the self-sufficiency levels for human albumin, IVIG & plasma-derived factor VIII in 15 countries (Austria, Brazil, Canada, China, Czech Republic, France, Germany, Greece, India, Italy, Japan, Mexico, Russia, Spain, United States) which had varying degrees of self-sufficiency achievement (25). Self-sufficiency ratio is the key parameter to assess the ability of a country to meet its need for plasma derived medicines, through utilization of locally collected plasma, either fractionated domestically or abroad. This ratio is product specific and can vary from country to country based on the requirement for the product in question. Figure 2 shows the consumption of plasma products in India.
One of the key observations of the report was the importance of a mature & consistent plasma supply to achieve self-sufficiency levels in plasma derived medicines. For instance, although the average self-sufficiency in IVIG & human albumin for these 15 countries was over 100% as a group, but when five key countries (USA, Germany, Czech Republic, China & Austria) which contribute to over 85% of the global plasma requirement, were excluded, the self-sufficiency of the remaining countries drastically dropped.
- IVIG: decreases from 137% to 57%;
- Human albumin: decreases from 171% to 63%;
- Factor VIII: decreases from 287% to 48%.
In the context of India, the self-sufficiency ratio of IVIG was 46% and 24% for human albumin. On calculating optimum global consumption pattern, a theoretical average demand of 60 kg per million inhabitants was derived for IVIG & 270 kg per million inhabitants was derived for human albumin, it dropped down to just 1% for both albumin and IVIG. For calculation of self-sufficiency ratio for factor VIII, the report considers that all the plasma which is collected, gets used for manufacturing of AHF and that is why the self-sufficiency ratio comes to 75%, while in the reality, India largely (90%) depends on imports of AHF because of very poor yield of AHF/L of Indian plasma. The following table show the number of patients based on global prevalence rate and actual number of patients identified or registered with patient groups (Table 1).
Table 1
| Diseases | Estimated prevalence | Diagnosed patients |
|---|---|---|
| Hemophilia A | 100,000 | ~15,000 |
| Hemophilia B | 10,000 | ~2,500 |
| Primary immunodeficiency diseases | 1,000,000 | ~1,000 |
The low usages of products could be attributed to either shortage of products or low rate diagnosis of diseases. As a country India, fractionates about 500,000 L of plasma every year and mainly makes albumin and immunoglobulins, while almost all other products are imported in one form or the other.
Future roadmap for plasma fractionation industry
If one looks at the global plasma fractionation program, it clearly indicates a quintessential necessity for building plasma collection capacity across the world to meet the growing demand of plasma products in the future (26). Globally, increase in diagnosis of patients dependent upon plasma protein therapies & newer clinical indications for use of these products, has led to a rapidly increasing demand. But, limited amount of plasma available for fractionation and fractionation capacities, could lead to various challenges in availability of these products (6). Hence, every country should place importance upon evaluating its demand for plasma products, which would help in defining self-sufficiency & work towards collecting enough plasma and its processing capacity to meet its needs (27).
Unfortunately, plasma fractionation program did not get enough attention and focus in India because of other pressing challenges and priorities and, most importantly political will to change. This has led to an outdated regulatory system and low governance, which resulted into unavailability of safe and consistent plasma. Though, with the passage of time there has been a significant of improvement in the overall of blood banking scene and availability of plasma products in India but still much needs to be done considering clinical needs of the country. There is a need to create a vision documents for short-term, mid-term and long-term document for addressing the issue of self-sufficiency of plasma products in India (28). This can be divided into following two categories.
- Availability of safe plasma: how to overall increase the quantity and quality of plasma for fractionation;
- Increase voluntary blood collection, with increased repeat donor base;
- As a part of the licensing process make blood component separation mandatory and make a plan for current licensed blood banks to migrate to complete blood separation in the next 3 years;
- Making blood component therapy mandatory in clinical practice, so that blood therapy can be minimized, and patients can use plasma products, which are relatively safer and easily accessible;
- Create a regulatory path for permitting source plasma collection.
- Regulatory system reform: create a facilitative regulatory landscape for plasma industry to grow and evolve which would involve office of DCGI, NIB, Department of AIDS Control (DAC), IPC, NPPA, clinicians, patient groups and industry representatives.
- Forming a national level body for steering plasma protein initiative in India;
- Make a very robust regulatory system for collection and pricing mechanism of plasma and Indigenous manufacturing capabilities;
- Scientific mechanism of price control;
- More funding for products and patient care;
- Regulatory approval for importing different categories of plasma/fractions/intermediate and paste.
By addressing some of the above-mentioned issues, the huge gap between demand and supply can be bridged. India must work towards evolving and developing a sustainable model of transfusion medicine, where transfusion medicine specialist, regulators, industry and patient groups can work together and form a forum for public-private partnership. This model should be developed keeping local conditions in view and by optimally utilizing all its resources, as western model of plasma fractionation may not be viable and sustainable in Indian conditions. A sustained supply of safe blood and plasma products is just not economic necessity but it’s a scientific obligation also, as these proteins are used as life-saving medicines.
Acknowledgments
The author would like to thank Manpreet Ajmani for her assistance in editing of the manuscript. The author would also extend sincere gratitude to Rakin Khan and Kaustubh Karan Sharma for the help in making figures and charts.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Thierry Burnouf) for the series “Plasma Fractionation” published in Annals of Blood. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob.2018.04.03). The series “Plasma Fractionation” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the manuscript and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
-
India Population (LIVE) -
Healthcare in India - Millennium Development Goals-2015 only partially achieved: CAG. Available online: http://www.india.com/news/agencies/millennium-development-goals-2015-only-partially-achieved-cag-2342369/
- Albumin in short supply, patients, doctors on edge. Available online: https://timesofindia.indiatimes.com/city/mumbai/Albumin-in-short-supply-patients-doctors-on-edge/articleshow/48520664.cms
- Francis PA. Albumin shortage. Available online: http://www.pharmabiz.com/ArticleDetails.aspx?aid=86664&sid=3
- Central drugs standard control organization. Blood bank. Available online: http://www.cdsco.nic.in/forms/list.aspx?lid=1813&Id=1
-
National AIDS Control Programme - After blood transfusion at government hospital, 6-year-old tests HIV+. Available online: https://timesofindia.indiatimes.com/city/dehradun/after-blood-transfusion-at-government-hospital-6-year-old-tests-hiv/articleshow/62478692.cms
- Most govt blood banks don’t comply with best healthcare standards: report. Available online: http://www.livemint.com/Politics/B5j4i8W4VRNVsqOFFm7YbJ/Most-govt-blood-banks-dont-comply-with-best-healthcare-stan.html
-
National Blood Transfusion Council - Haemophilia Federation India vs. Union of India. Available online: http://www.hrln.org/hrln/disability-rights/pils-a-cases/129-haemophilia-federation-india-vs-union-of-india.html
- Plasma centre grant deferred. Available online: http://www.dnaindia.com/mumbai/report-plasma-centre-grant-deferred-1154624
- 7 years on, no place to house RS 5 crore life-saving machine. Available online: https://mumbaimirror.indiatimes.com/mumbai/other/articleshow/26347881.cms
- Health insurance in India. Available online: https://en.wikipedia.org/wiki/Health_insurance_in_India
- Despite shortage, India discards 1m blood units per year. Available online: https://timesofindia.indiatimes.com/india/despite-shortage-india-discards-1m-blood-units-per-year/articleshow/62274732.cms
- Ajmani R. Achieving self-sufficiency in plasma products: where do we stand? Presentation presented at Transmedcon 2014, Ahmedabad; 2014.
- Curling JM, Bryant CP, Hayes TK, et al. Ajmani: Development of Plasma-Derived Biopharmaceuticals in India. In: Langer ES. Advances in Biopharmaceutical Technology in India. 1st edition. BioPlan Associates Inc., 2008:465-518.
-
Developments on Plasma Fractionation in India - Ajmani R. Challenges of availability and affordability of plasma products: Asian Perspective. Presentation presented at Bioplasma World Asia 2014; 2014.
- Department of Aids Control, Ministry of Health & Family Welfare. National Policy for Access to Plasma Derived Medicinal Products from Human Plasma for Clinical/Therapeutic Use: Addendum to National Blood Policy 2003. 2014. Available online: http://naco.gov.in/sites/default/files/plasma%20policy_1.pdf
- work plan & road map for IP addendum-2019 to IP-2018. Available online: http://ipc.nic.in/showfile.asp?lid=889&EncHid
- National Aids Control Organisation. Blood Transfusion Services. Available online: http://www.naco.gov.in/blood-transfusion-services-publications
- Minutes of the 26th Governing Body Meeting of National Blood Transfusion Council. Available online: http://naco.gov.in/sites/default/files/Minutes%20of%2026th%20GB%20Meeting%20of%20NBTC-2017.pdf
- Haemovigilance Programme of India. National Institute of Biologicals & Indian Pharmacopoeia Commission Collaboration. Available online: http://nib.gov.in/haemovigilance1.html
- Robert P. Self Sufficiency–Facts and Pitfall, The Source, 2014:32-8. Available online: https://www.pptaglobal.org/images/source/2014/FALL/PPTA_14006TheSource_Fall_FINALweb.pdf
- Burnouf T. Plasma fractionation in Asia-Pacific: challenges and perspectives. ISBT Science Series 2011;6:366-72. [Crossref]
- Blood supply improves, but India still faces a shortfall of 10 per cent. Available online: http://www.thehindu.com/news/national/Blood-supply-improves-but-India-still-faces-a-shortfall-of-10-per-cent/article14517254.ece
- PlasmaGen raises $25M from Eight Roads Ventures and F-Prime Capital Partners. Available online: https://yourstory.com/2017/08/plasmagen-raises-25m-eight-roads-ventures-f-prime-capital-partners/, August 2017.
Cite this article as: Ajmani RS. Indian plasma fractionation industry: challenges and opportunities. Ann Blood 2018;3:30.