
Diagnosis and management of von Willebrand disease in Australia
Von Willebrand disease (VWD) and von Willebrand factor (VWF)
VWD is typically identified as being the most common inherited bleeding disorder. The exact prevalence of VWD is debated, but has been reported in epidemiological studies to affect up to 1% of the general population, but alternatively the prevalence may be as low as 0.01% (1 in 10,000) based on symptomatic patient presentations to clinics (1).
VWD is caused by deficiencies and/or defects in VWF, a large and complex multimeric protein, which otherwise would facilitate both primary and secondary hemostasis, by binding to platelets, factor VIII (FVIII), and subendothelial matrix components such as collagen (1-4).
Congenital VWD primarily arises due to mutations in VWF. Diagnosis requires evidence of personal or family history of (mainly) mucocutaneous bleeding, together with laboratory findings that demonstrate quantitative or qualitative defects in VWF (1-6).
Current classification of VWD, based on whether VWF quantitative deficiencies (VWD types 1 and 3), or qualitative defects (type 2 VWD) are present, defines 6 types (Table 1) (2).
Table 1
| VWD type | Description | Phenotypic presentation |
|---|---|---|
| 1 | Partial quantitative deficiency of VWF | Low levels of VWF, with VWF functional concordance (i.e., ratio of functional VWF/VWF:Ag approximates unity) |
| 2A | Decreased VWF-dependent platelet adhesion and a selective deficiency of high-molecular-weight (HMW) VWF multimers | Loss of HMW VWF. Usually low levels of VWF, with VWF functional discordance (i.e., ratios of RCo/Ag and CB/Ag typically <0.7) |
| 2B | Increased affinity of VWF for platelet glycoprotein 1b | Low to normal levels of VWF, typically with VWF functional discordance (i.e., ratios of RCo/Ag and CB/Ag generally <0.7), loss of HMW VWF and (mild) thrombocytopenia. Atypical cases may not show this pattern |
| 2M | Decreased VWF-dependent platelet adhesion without a selective deficiency of high-molecular-weight (HMW) VWF multimers | Low to normal levels of VWF, usually with VWF functional discordance detected by RCo/Ag (generally <0.7), but relatively normal CB/Ag ratio. HMW VWF present, but multimers may show other abnormalities |
| 2N | Markedly decreased binding affinity for factor VIII | Identified by VWF:FVIIIB assay, with low FVIIIB/VWF ratios |
| 3 | Virtually complete deficiency of VWF | Typically defined by VWF levels <2 U/dL and FVIII <10 U/dL |
Classification scheme derived and adapted from Sadler
Type 1 VWD is characterized by quantitative deficiency of an otherwise functionally normal VWF. Type 1 VWD is therefore confirmed by detection of reduced levels of VWF protein (‘antigen’; VWF:Ag), and similar proportionally decreased levels of functional VWF, which can be identified by various assays, including VWF ristocetin cofactor (VWF:RCo) and collagen binding (VWF:CB) (7-10) (Table 2). There is a corresponding fall in plasma levels of FVIII, since VWF acts to protect FVIII from proteolysis.
Table 2
| VWD type | VWF: Ag | VWF: RCo* | VWF: CB | FVIII:C | Multimers | RCo/Ag* | CB/Ag* | FVIII/VWF* | Comments/additional testing** |
|---|---|---|---|---|---|---|---|---|---|
| 1 | ↓to ↓↓ | ↓to ↓↓ | ↓to ↓↓ | N to ↓↓ | Normal pattern but reduced intensity | Normal | Normal | Normal | VWF levels between ~30–50 U/dL will generally not be associated with VWF mutations and may be considered as representing ‘low’ VWF as a risk factor for bleeding. VWF levels below ~30 U/dL will often be associated with VWF mutations and can be considered as representing ‘true’ type 1 VWD. |
| 2A | ↓to ↓↓ | ↓↓ to ↓↓↓ | ↓↓ to ↓↓↓ | ↓to ↓↓ | Loss of HMW VWF | Low | Low | Normal | 2A and 2B VWD can only be distinguished by means of RIPA. Platelet type (PT-) VWD phenotypically resembles 2B VWD; these can be distinguished by means of RIPA mixing studies, or by genetic analysis of VWF and/or platelet GPIb. |
| 2B | N to ↓↓ | ↓ to ↓↓↓ | ↓ to ↓↓↓ | N to ↓↓ | Loss of HMW VWF | Low | Low | Normal | |
| 2N | N to ↓↓ | N to ↓↓ | N to ↓↓ | ↓↓to ↓↓↓ | Normal pattern | Normal | Normal | Low | Phenotypically similar to haemophilia A; distinguish using VWF:FVIII binding assay or genetic analysis of FVIII and/or VWF |
| 2M | ↓to ↓↓ | (↓ to ↓↓↓) | (↓ to ↓↓↓) | ↓to ↓↓ | No loss of HMW VWF; some multimer defects may be present | Low (platelet binding defect) or normal (collagen binding defect) | Low (collagen binding defect) or normal (platelet binding defect) | Normal | 2A and 2M VWD can only be distinguished by comprehensive or composite panel testing, including VWF:Ag, VWF:RCo (or GPIb binding assay), plus VWF:CB or multimer analysis. |
| 3 | ↓↓↓ (absent) | ↓↓↓ (absent) | ↓↓↓ (absent) | ↓↓↓ | No VWF present | NA | NA | NA | Type 3 VWD can only be identified when VWF tests are performed and these are sensitive to very low levels of VWF |
*, VWF GPIb binding assays [including the Siemens ‘Innovance’ VWF Ac assay, recently named VWF:GPIbM (
Representing the most severe phenotype, Type 3 VWD essentially describes an absence of VWF, but in practice can also be identified by very low levels of VWF:Ag (i.e., <2-5 U/dL) should assays suffer from low level sensitivity issues (Table 2). Results of functional VWF assays are similarly low, but low limit of VWF detection issues may further affect correct diagnosis, and falsely higher values may be reported.
Type 2 VWD patients exhibit qualitative VWF defects; accordingly, levels of VWF protein (VWF:Ag) might be normal (although it is usually reduced), FVIII levels might also be normal or low, but most importantly, VWF function is somehow impaired. ‘Subtypes’ of type 2 VWD are identified according to the type of impaired function.
Type 2A VWD is defined by absence or deficiency of high molecular weight (HMW) VWF (2), representing the most biologically active forms, and specifically identified phenotypically by absence of HMW VWF on multimer analysis and/or by low VWF activity/Ag ratios, using various functional assays (e.g., low VWF:RCo/Ag and low VWF:CB/Ag; Table 2).
Patients with type 2B VWD have a hyper-adhesive form of VWF, which binds platelets with increased avidity and is then removed from circulation more rapidly, often resulting in a selective depletion of HMW VWF, and ‘classically’ also (mild) thrombocytopenia. A diagnosis of 2B VWD is confirmed by enhanced ristocetin induced platelet aggregation (RIPA) (10,12); patients also typically have reduced HMW VWF, with low ratios for VWF activity/Ag (e.g., low VWF:RCo/Ag and low VWF:CB/Ag; Table 2).
Patients with type 2N VWD have VWF defects that prevent it binding to FVIII, leading to more rapid proteolytic degradation and depletion of plasma FVIII. Consequent hemorrhagic manifestations and laboratory test patterns may therefore be mistaken for those of hemophilia A. Type 2N VWD is phenotypically discriminated from hemophilia A using VWF:FVIII binding assays (Table 2) (13).
Type 2M VWD characterizes different qualitative VWF defects that are not linked to depletion of HMW VWF (2). Often type 2M VWD is phenotypically identified by low VWF:RCo/Ag ratio without loss of HMW VWF by multimer analysis, although it can alternatively be identified by discordance between VWF:RCo/Ag and VWF:CB/Ag ratios [whereby one is normal (usually VWF:CB/Ag) and the other is low (usually VWF:RCo/Ag); Table 2].
Recent advances in VWD diagnostics
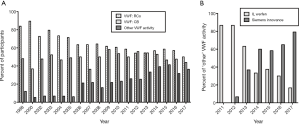
Worldwide, the mainly used assays in VWD diagnostics are FVIII, VWF:Ag, VWF:RCo, while VWF:CB is used in some geographies. The main recent advances relate to automation of VWF:CB, and commercialization of new assays that reflect platelet glycoprotein Ib (GPIb) binding but which do not use platelets and in some cases do not use ristocetin (5-16). Extensive descriptions of these assays are outside the remit of the present review, and have otherwise been reviewed or published (5-16). Suffice to say that these assays are likely to increase in usage, and otherwise replace or supplement existing test systems (Figure 1). Importantly, the newer GPIb binding assays will likely replace classical VWF:RCo assays in the immediate future, and essentially will be considered interchangeable with VWF:RCo when identifying VWD and defining VWD subtypes. Nevertheless, additional research may be required to further explore similarities and differences between assays before they can be defined to be identical. Such comparative evaluations may be facilitated with some new nomenclature devised by the International Society on Thrombosis and Haemostasis (ISTH) VWF Scientific Standardization Committee (SSC) (5,6,11).
Diagnosis of VWD in Australia
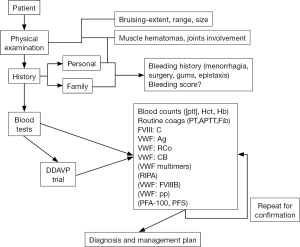
The current review essentially updates a previous analogous review published in 2011 (17). Our own approach to diagnosis/exclusion of VWD is represented in algorithmic form in Figures 2,3. In addition to personal and familial bleeding history and physical examination, a battery of laboratory studies may be required, including, but not necessarily limited to VWF and FVIII testing (Figures 2,3). For example, in urgent cases where access to VWF tests may be limited, the Platelet Function Analyzer (PFA)-100/200 may be useful in order to exclude VWD (18-21). The PFA-100/200 shows high sensitivity to deficiency of VWF (e.g., types 1 and 3 VWD), and also to selective loss of HMW VWF (i.e., type 2A VWD), and some defects in VWF (e.g., type 2M VWD). PFA-100/200 closure times (CTs) are therefore prolonged in virtually all cases of type 2A, 2M, 2B and 3, and in most cases of type 1 VWD, especially those with levels of VWF below 30U/dL. Thus, normal PFA CTs are generally inconsistent with VWD.
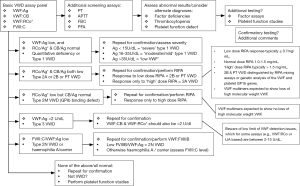
However, where more specific VWF testing is indicated, especially for diagnosis and typing of VWD, then our recommended approach is as per Figure 3. For all patients we recommend a minimum four test (‘basic’) panel of investigations, and namely FVIII, VWF:Ag, VWF:CB and a GPIb binding assay—be it classical VWF:RCo or one of the modern ‘alternatives’ (e.g., VWF:GPIbM). This recommendation is also in line with those of the United Kingdom Haemophilia Centre Doctors Organization (4). Based on the plasma levels identified, further testing may be required. Mandatory repeat testing using a new sample is undertaken before VWD is categorically diagnosed, or even excluded in some cases, due to the possibility of both pre-analytical issues (22) and analytical (5) test limitations. The test levels and test patterns provide clues to the VWD diagnosis and which approach to testing should subsequently be followed to make a final diagnosis and characterize VWD type.
Based on national statistics and a locally maintained database, the breakdown of VWD types is as shown in Tables 3,4. Patients identified to have low VWF (as a risk factor for bleeding) or type 1 VWD predominate in Australia, as per all other geographies. Type 3 VWD is relatively rare. Type 2 is less common than type 1, but these patients still comprise a significant proportion of VWD cases, and typically represent the category of patients most likely to be misdiagnosed (either as another type of VWD, or as not suffering from VWD) (23-25).
Table 3
| VWD type | 2011 data | 2015–2016 data | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | Total | % of total VWD | % of type 2 VWD | Female | Male | Total | % of total VWD | % of type 2 VWD | ||
| VWD type 1 | 420 | 601 | 1,021 | 75.4 | 851 | 477 | 1,328 | 63.5 | |||
| VWD type 2A | 28 | 34 | 62 | 4.6 | 21.2 | 62 | 56 | 118 | 5.6 | 23.5 | |
| VWD type 2B | 26 | 17 | 43 | 3.2 | 14.7 | 31 | 31 | 62 | 3.0 | 12.4 | |
| VWD type 2M | 36 | 33 | 69 | 5.1 | 23.5 | 103 | 80 | 183 | 8.7 | 36.5 | |
| VWD type 2N | 11 | 7 | 18 | 1.3 | 6.1 | 20 | 10 | 30 | 1.4 | 6.0 | |
| VWD type 3 | 16 | 25 | 41 | 3.0 | – | 22 | 21 | 43 | 2.1 | – | |
| VWD type 2—uncharacterized | 49 | 52 | 101 | 7.5 | 34.5 | 61 | 48 | 109 | 5.2 | 21.7 | |
| VWD type 2 total | 150 | 143 | 293 | 16.0 | 100 | 100 | |||||
| VWD—uncharacterized | 272 | 189 | 461 | 25.2 | 125 | 94 | 219 | 10.5 | |||
| Totals | 1,047 | 780 | 1,827 | 100.0 | 100.0 | 1275 | 817 | 2,092 | 100.0 | – | |
*, From Australian Bleeding Disorders Registry (ABDR). 2011 data as per previously published (
Table 4
| VWD Type | Description/Comments | Number in database | % of total | % of subtotals |
|---|---|---|---|---|
| Main categories | ||||
| 1 | Quantitative deficiency of VWF | 1,254 | 82.4 | |
| 2 | Qualitative defects in VWF | 241 | 15.8 | |
| 3 | Absence of VWF | 23 | 1.5 | |
| PT-VWD | Platelet GPIBA defect | 3 | 0.2 | |
| Totals | (excludes undefined VWD; n=311) | 1,521 | 100 | |
| Subcategories | % of type 1 | |||
| ‘1p’ | ‘plausible’ Type 1 VWD (VWF ‘low’ or borderline normal group) (VWF =36–64 U/dL) | 1069 | 70.3 | 85.2 |
| ‘1m’ | Moderate/mild type 1 VWD (VWF =16–35 U/dL) | 136 | 8.9 | 10.8 |
| ‘1s’ | Severe type 1 VWD (VWF =2–15 U/dL) | 49 | 3.2 | 3.9 |
| Totals | (for type 1 VWD) | 1,254 | 82.4 | 100 |
| Type 2 VWD | % of type 2 | |||
| 2A | Loss of HMW VWF (e.g., CB/Ag and RCo/Ag <0.7) | 55 | 3.6 | 22.8 |
| 2M | Loss of VWF function not due to loss of HMW VWF (e.g., CB/Ag ≥0.7 but RCo/Ag <0.7) | 51 | 3.4 | 21.2 |
| 2B | Enhanced RIPA (low dose ristocetin response) | 20 | 1.3 | 8.3 |
| 2N | Defective VWF-FVIII binding (low FVIIIB/VWF ratio) | 31 | 2.0 | 12.9 |
| 2U | Undefined—VWD/qualitative defects as yet not completely characterized | 84 | 5.5 | 34.9 |
| Totals | (for type 2 VWD) | 241 | 15.8 | 100 |
*, As per 2011 data as previously published (
Therapeutic rationale in VWD
Primary treatment of VWD involves restoring the depleted or dysfunctional VWF, and also in some circumstances the lost FVIII (26,27). Additional therapeutic measures might also be necessary in a proportion of patients. Type 1 VWD is usually effectively managed using desmopressin (1-deamino-8-d-arginine vasopressin, DDAVP); this facilitates release of endogenous VWF, stored in endothelial cells (Table 5) (27). Prior to use, it is recommended to first trial DDAVP and assess both responsiveness and tolerance, either in the patient or a close family member suffering the same disorder (27-29). DDAVP may also be effective in a subset of patients with type 2 VWD, but is not useful in type 3 VWD, and may only be partially useful in any VWD patient with limited DDAVP responsiveness or requiring longer duration of therapy, for instance after major surgery.
Table 5
| VWD type | Summary of main therapies | Therapy—general considerations | Additional therapies |
|---|---|---|---|
| Type 1 | DDAVP; VWF(/FVIII) concentrate | Usually respond well to DDAVP, unless VWF <10 U/dL. VWF concentrate required for DDAVP non-responders or for long-term therapy. Need to replace VWF and sometimes also FVIII | Anti-fibrinolytic therapy (e.g., tranexamic acid & aminocaproic acid) may be used for less severe forms of mucosal bleeding, menorrhagia, epistaxis, dental procedures; hormonal treatments effectively helps manage menorrhagia in some cases |
| Type 2A | VWF(/FVIII) concentrate; DDAVP | Variable clinical response to DDAVP. VWF concentrate represents most common therapy. Need to replace (HMW) VWF and sometimes also FVIII | |
| Type 2B | VWF(/FVIII) concentrate; (DDAVP) | DDAVP use is contentious (believed contraindicated by some; whereas others feel this may represent an effective treatment in a proportion of patients). VWF concentrate represents most common therapy. Need to replace (HMW) VWF and only rarely also FVIII | |
| Type 2M | VWF(/FVIII) concentrate; DDAVP | Variable clinical response to DDAVP and VWF concentrate represents most common therapy. Need to replace functional VWF and sometimes also FVIII | |
| Type 2N | VWF(/FVIII) concentrate; DDAVP | Variable clinical response to DDAVP and VWF concentrate represents most common therapy. Need to replace functional VWF and also sometimes FVIII (perhaps at least initially. Once stable infused VWF levels (‘steady state’) reached, FVIII levels will rise due to stabilization of endogenous FVIII, and FVIII transfusion will no longer be required) | |
| Type 3 | VWF(/FVIII) concentrate | DDAVP ineffective, and VWF concentrate represents only effective therapy. Need to replace VWF and also FVIII, at least initially. Once stable infused VWF levels (‘steady state’) reached, FVIII levels will rise due to stabilization of endogenous FVIII, and FVIII transfusion will no longer be required |
Additional/alternate therapies for VWD may be applied in distinct geographies, based on relative availability of main treatments, including DDAVP and/or VWF(/FVIII) concentrates. Summarized from references (
DDAVP has a number of potential adverse effects including facial flushing, tachycardia, headache, hypotension or hypertension, gastrointestinal upset and hyponatremia, which if severe can rarely lead seizures (3). The risk of hyponatremia is significant after repeat doses; therefore, fluid restriction and monitoring of electrolytes is recommended in this setting. DDAVP should also be avoided in patients with symptomatic cardiovascular and cerebrovascular disease (3).
Where DDAVP is contraindicated or in patients with a DDAVP response that is sub-therapeutic or short-lived, exogenous replacement of VWF (/FVIII) is used (2,3,27). Our treatment protocol is identified in Table 6, and is based on a phase II/III open-label multicenter study conducted in Australia and New Zealand, as using the only Australian available concentrate (named Biostate; CSL Limited; a double virus inactivated plasma-derived product which has retained HMW VWF multimers and where the FVIII:C to VWF:RCo ratio is at least 1:2) (30). Pharmacokinetic studies may be used to personalize individual therapy if required (e.g., if patients show increased clearance of VWF).
Table 6
| Indication | Dose* of VWF:RCo (IU/kg) | Number of infusions | Target plasma VWF:RCo level |
|---|---|---|---|
| Type 1 VWD Major surgery or haemorrhage | Loading dose 40, then 40–50 | Every 8–12 hours for 3 days then daily for up to 7 days | > 50 U/dL; maintain levels for 7–10 days |
| Type 1 VWD Minor surgery or haemorrhage | 40–50 | 1 or 2 doses | >30 U/dL; maintain levels for 2–4 days |
| Type 2 or 3 VWD Major surgery or haemorrhage | Loading dose 50–60 then 40–60 | Every 8–12 hours for 3 days then daily for up to 7 days | > 50 U/dL; maintain levels for 7–10 days |
| Type 2 or 3 VWD Minor surgery or haemorrhage | 40–50 | 1 or 2 doses | >30 U/dL; maintain levels for 2–4 days |
*, Our local practice based on a phase II/III Australian and New Zealand study (
Since FVIII levels may rise excessively when administering concentrates containing both VWF and FVIII for longer duration treatment, monitoring of therapy is critical, and ideally, concentrates devoid of FVIII may be preferred for some treatment applications (2,3,27,31), although these are not currently available in Australia. Similarly, although recombinant VWF has recently been cleared for use in the USA, this is also not yet available in Australia.
Monitoring of therapy in VWD
The tests used to diagnose VWD can also be used to monitor therapy or treatment of VWD (Table 7) (28,32). We have recently published an extensive review on this topic (28). In brief, as mentioned, DDAVP administration triggers release of endogenous VWF. As cellular stored VWF is usually normal in type 1 VWD, most of these patients achieve sufficient increases in VWF to allow use of DDAVP as first-line therapy for bleeding or minor surgery and procedures. Further, as the response pattern is consistent within families, a parent’s response can predict that of an affected child. Responses to DDAVP are highly variable in type 2 VWD, although usually better in type 2M than 2A. DDAVP response may be short-lived in type 2N VWD, and DDAVP is usually considered contraindicated in type 2B VWD, because increasing the level of ‘abnormal’ VWF with enhanced affinity for platelet GPIb may result in thrombocytopenia, potentially increasing the risk of bleeding.
Table 7
| Therapy | Clinical monitoring | Laboratory monitoring | Notes |
|---|---|---|---|
| DDAVP Trial | Facial flushing, hypertension or hypotension, tachycardia, headache, gastrointestinal upset and hyponatremia (rarely complicated by seizures) | Minimum: pre and post DDAVP testing of FVIII:C and VWF:RCo | PFA-100/200 closure times tend to correct if functional VWF levels normalize, and/or if functional VWF/Ag ratios are normal (select type 1 VWD and type 2 VWD patients) |
| Recommended: pre and post DDAVP testing of FVIII:C, VWF:Ag, VWF:RCo and VWF:CB (and if indicated PFA-100/200 closure time). Assessment of functional VWF/Ag (e.g., RCo/Ag and CB/Ag) ratios | |||
| Timepoints: pre-dose plus repeat testing performed at 1 hour, 2 and/or 4 hours, and finally 24 hours post infusion | |||
| Other: pre- and post-DDAVP assessment of standard blood counts, especially platelet count, may be useful (e.g., 2B VWD). Pre- and post-DDAVP monitoring of electrolytes (especially sodium) may be useful in select patients (up to 24h post) | |||
| DDAVP therapy | Efficacy of bleeding treatment (has bleeding stopped or slowed?) | Minimum: pre and post DDAVP testing of FVIII:C and VWF:RCo | Be aware of tachyphylaxis |
| Recommended: pre and post DDAVP testing of FVIII:C, VWF:Ag, VWF:RCo and VWF:CB (and if indicated PFA-100/200 closure time). Assessment of functional VWF/Ag (e.g., RCo/Ag and CB/Ag) ratios | |||
| Timepoints: pre-dose plus repeat testing performed at 1 hour | |||
| VWF/FVIII concentrate—pharmacokinetic assessment | – | Minimum: pre and post concentrate testing of FVIII:C and VWF:RCo | In our experience, PFA-100/200 closure times do not tend to correct when VWF concentrates are used, and so this testing can be omitted |
| Recommended: pre and post concentrate dose testing of FVIII:C, VWF:Ag, VWF:RCo and VWF:CB. Assessment of functional VWF/Ag (e.g., RCo/Ag and CB/Ag) ratios | |||
| Timepoints: pre-dose plus repeat testing performed at 1 hour, 2 and/or 4 hours, and finally 24 hours post infusion | |||
| VWF/FVIII concentrate | Efficacy of bleeding treatment (has bleeding stopped or slowed?) | Minimum: pre and post concentrate testing of FVIII:C and VWF:RCo | In our experience, PFA-100/200 closure times do not tend to correct when VWF concentrates are used, and so this testing can be omitted |
| Recommended: pre and post concentrate testing of FVIII:C, VWF:Ag, VWF:RCo and VWF:CB. Assessment of functional VWF/Ag (e.g., RCo/Ag and CB/Ag) ratios. | |||
| Timepoints: pre-dose plus repeat testing performed at 1 hour. | |||
| Antifibrinolytic agents | Efficacy of bleeding treatment (has bleeding stopped or slowed?) | No specific laboratory monitoring when applied to VWD | Monitor for any potential adverse events: nausea, vomiting, diarrhea and rarely thrombotic events |
| Oral contraceptive agents | Efficacy of bleeding treatment (has bleeding stopped or slowed?) | No specific laboratory monitoring when applied to VWD. | Consider the use of the PBAC |
| Iron therapy | Assessment of pre-, and post-, iron related parameters may be useful? | – | |
| Normal platelets | Efficacy of bleeding treatment (has bleeding stopped or slowed?) | Assessment of VWD patient platelet VWF level (VWF:Ag and VWF:RCo; also VWF:CB if available) may be useful | FVIII:C can be omitted, as not present in platelets |
| Topical thrombin | Efficacy of bleeding treatment (has bleeding stopped or slowed?) | No specific laboratory monitoring when applied to VWD | Beware of the rare risk of development of anti-factor V antibodies if using bovine thrombin |
Summarized from references (
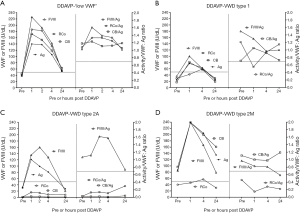
In addition to determining potential therapeutic utility, a DDAVP response profile, which is often characteristic for a given VWD type (examples shown in Figure 4) (28,29) can assist diagnosis for some patients with otherwise unclear diagnosis.
Efficacy of VWF concentrates is judged clinically by assessing whether treatments have achieved normal haemostasis during surgery and halted or reduced active bleeding, and is also monitored using the same suite of laboratory tests as for diagnosis (Table 2) (1-5,27,28).
As reported, the PFA-100/200 is also used, both to exclude severe VWD in urgent cases, as well as to help assess the efficacy of therapy. Prolonged CTs in type 1 VWD tend to normalize following DDAVP, effects that parallel rising VWF, particularly HMW VWF, or VWF forms otherwise identified by testing with VWF:CB and VWF:RCo (26,27,29). However, while DDAVP might restore VWF:Ag in type 2A VWD, it may not correct VWF:CB or VWF:RCo, nor PFA CTs. Thus, performance of PFA CTs, especially during DDAVP trials, can be a useful and quick measure of treatment ‘efficacy’, with CT normalization reflecting a reasonable surrogate of adequate treatment response, often also associated with correction of VWF:CB and VWF:RCo, as well as typically normalization of RC/Ag and CB/Ag. PFA CT data does not appear to be useful to monitor VWF concentrate therapy, at least reflective of our clinical experience with Biostate in either type 1 or 2A VWD. The normalization of CTs with DDAVP, but not with VWF concentrate, at least in type 1 VWD, may be related to the higher levels of HMW VWF released by DDAVP compared to HMW VWF composition of Biostate. Consequently, the change in CTs may vary with different VWF/FVIII concentrates. Conversely, DDAVP typically fails to normalize CTs in type 2A VWD, since it does not yield release of HMW VWF. In summary, although CT normalization might reflect evidence of efficacious treatment, failure to normalize CTs may not indicate lack of clinical treatment efficacy.
Clinical monitoring in VWD
Clinical monitoring should be individualized according to bleeding phenotype (28). Clinically, effectiveness of bleeding management may be monitored by visual inspection (e.g., cessation in visible hemorrhage such as epistaxis or gingival bleeding), maintenance of adequate haemoglobin levels without iron supplementation or transfusion support in cases presenting with gastrointestinal or uterine bleeding or reduced pain/swelling for muskuloskeletal bleeding for severe VWD. For most VWD patients, therapy will only be required at the time of surgery or procedures, when clinical monitoring entails assessment of adequacy of haemostasis achieved by perioperative therapy. Special considerations may apply to women, and situations involving menorrhagia, pregnancy and delivery (28). Other notable special situations include gastrointestinal bleeding/angiodysplasia (especially relevant in type 2 VWD) and prophylaxis (especially relevant in type 3 VWD) (28,33).
Other adjunctive therapies
Antifibrinolytic agents, such as tranexamic acid, are useful for management of mucocutaneous bleeding and in the periprocedural setting, particularly for dental procedures. Occasionally, transfusion of normal platelets containing normal VWF content may help bleeding patients despite ‘adequate’ VWF replacement therapy (34). Topical thrombin may help minor wound bleeding and fibrin sealants may be useful in dental procedures (3).
Conclusions and future perspectives
In line with the United Kingdom Haemophilia Centre Doctors Organization guideline (4), we believe that diagnosis of VWD requires a minimum four test panel covering FVIII, VWF:Ag, GPIb binding (e.g., VWF:RCo or VWF:GPIbM), and collagen binding (VWF:CB). Additional testing may be required to provide VWD typing, or differential diagnoses, including exclusion of VWD. Such testing can be described by means of an algorithm, such as per Figure 3. ‘Standard’ therapy to manage VWD, in most developed countries, including Australia, utilizes DDAVP wherever possible, otherwise VWF/FVIII concentrates, and adjunct therapy (e.g., antifibrinolytic) as needed (27). Monitoring of therapy typically involves laboratory measurement of baseline and post therapy levels of various VWF parameters and FVIII:C at select intervals, and using the same four test panel used for initial VWD diagnosis (28). We integrate PFA-100/200 testing in select patients, both in diagnosis/exclusion of VWD, as well as in DDAVP therapy (27-29,32).
Genetic testing also has a role in diagnosis of VWD, although (outside of a research setting) we use this selectively in our own practice (35,36). However, given the great strides being made by next generation sequencing (37), we will be rethinking this practice over the next few years.
Our recommendations here as related to our own therapeutic use and monitoring of VWF concentrate might need modification to suit local needs, particularly where different laboratory test panels are in use or concentrates differ substantially from Biostate with respect to VWF:FVIII content (or Humate P, which is structurally similar to Biostate) (38). These differences are sometimes overlooked, but represent a major obstacle to international ‘standardization’ of diagnosis or biological therapy for VWD. Although recombinant VWF has been successfully developed, and recently approved for use in the USA (31), this is not yet available in Australia and many other jurisdictions. When eventually available more globally than the USA, recombinant VWF use may also impose on us refinements to ‘standard’ monitoring of therapy, as well as further refinement of patient management alongside concepts of personalized medicine (31).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Editorial Office, Annals of Blood for the series “Diagnosis and Management of von Willebrand Disease: Diverse Approaches to a Global and Common Bleeding Disorder”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob.2018.03.05). The series “Diagnosis and Management of von Willebrand Disease: Diverse Approaches to a Global and Common Bleeding Disorder” was commissioned by the editorial office without any funding or sponsorship. Emmanuel J. Favaloro served as an unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the manuscript and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclaimer: The views expressed herein are those of the authors and not necessarily those of NSW Health Pathology.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Favaloro EJ. Von Willebrand disease: local diagnosis and management of a globally distributed bleeding disorder. Semin Thromb Hemost 2011;37:440-55. [Crossref] [PubMed]
- Sadler JE, Budde U, Eikenboom JCWorking Party on von Willebrand Disease Classification, et al. Update on the pathophysiology and classification of von Willebrand disease: a report of the Subcommittee on von Willebrand Factor. J Thromb Haemost 2006;4:2103-14. [Crossref] [PubMed]
- Nichols WL, Hultin MB, James AH, et al. von Willebrand disease (VWD): evidence-based diagnosis and management guidelines, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA). Haemophilia 2008;14:171-232. [Crossref] [PubMed]
- Laffan MA, Lester W, O'Donnell JS, et al. The diagnosis and management of von Willebrand disease: a United Kingdom Haemophilia Centre Doctors Organization guideline approved by the British Committee for Standards in Haematology. Br J Haematol 2014;167:453-65. [Crossref] [PubMed]
- Favaloro EJ, Pasalic L, Curnow J. Laboratory tests used to help diagnose von Willebrand disease: an update. Pathology 2016;48:303-18. [Crossref] [PubMed]
- Just S. Laboratory Testing for von Willebrand Disease: The past, present, and future state of play for von willebrand factor assays that measure platelet binding activity, with or without ristocetin. Semin Thromb Hemost 2017;43:75-91. [PubMed]
- Favaloro EJ, Mohammed S, Patzke J. Laboratory Testing for von Willebrand Factor Antigen (VWF:Ag). Methods Mol Biol 2017;1646:403-16. [Crossref] [PubMed]
- Mohammed S, Favaloro EJ. Laboratory Testing for von Willebrand Factor Ristocetin Cofactor (VWF:RCo). Methods Mol Biol 2017;1646:435-51. [Crossref] [PubMed]
- Favaloro EJ, Mohammed S. Laboratory Testing for von Willebrand Factor Collagen Binding (VWF:CB). Methods Mol Biol 2017;1646:417-33. [Crossref] [PubMed]
- Favaloro EJ. Diagnosis or Exclusion of von Willebrand Disease Using Laboratory Testing. Methods Mol Biol 2017;1646:391-402. [Crossref] [PubMed]
- Bodó I, Eikenboom J, Montgomery R, et al. Platelet-dependent von Willebrand factor activity. Nomenclature and methodology: communication from the SSC of the ISTH. J Thromb Haemost 2015;13:1345-50. [Crossref] [PubMed]
- Frontroth JP, Favaloro EJ. Ristocetin-Induced Platelet Aggregation (RIPA) and RIPA Mixing Studies. Methods Mol Biol 2017;1646:473-94. [Crossref] [PubMed]
- Mohammed S, Favaloro EJ. Laboratory Testing for von Willebrand Factor: Factor VIII Binding (for 2N VWD). Methods Mol Biol 2017;1646:461-72. [Crossref] [PubMed]
- Patzke J, Favaloro EJ. Laboratory Testing for von Willebrand Factor Activity by Glycoprotein Ib Binding Assays (VWF:GPIb). Methods Mol Biol 2017;1646:453-60. [Crossref] [PubMed]
- Favaloro EJ, Mohammed S. Evaluation of a von Willebrand factor three test panel and chemiluminescent-based assay system for identification of, and therapy monitoring in, von Willebrand disease. Thromb Res 2016;141:202-11. [Crossref] [PubMed]
- Favaloro EJ, Mohammed S. Towards improved diagnosis of von Willebrand disease: comparative evaluations of several automated von Willebrand factor antigen and activity assays. Thromb Res 2014;134:1292-1300. [Crossref] [PubMed]
- Favaloro EJ, Bonar R, Favaloro J, et al. Diagnosis and management of von Willebrand disease in Australia. Semin Thromb Hemost 2011;37:542-54. [Crossref] [PubMed]
- Favaloro EJ. Clinical utility of closure times using the Platelet Function Analyzer (PFA)-100/200. Am J Hematol 2017;92:398-404. [Crossref] [PubMed]
- Favaloro EJ. Commentary: The Platelet Function Analyser (PFA)-100 and von Willebrand disease: A story well over 16 years in the making. Haemophilia 2015;21:642-5. [Crossref] [PubMed]
- Ardillon L, Ternisien C, Fouassier M, et al. Platelet function analyser (PFA-100) results and von Willebrand factor deficiency: a 16-year ‘realworld’ experience. Haemophilia 2015;21:646-52. [Crossref] [PubMed]
- Favaloro EJ. Clinical Utility of the PFA-100. Semin Thromb Hemost 2008;34:709-733. [Crossref] [PubMed]
- Favaloro EJ, Lippi G. Pre-analytical issues that may cause misdiagnosis in haemophilia and von Willebrand disease. Haemophilia 2017; [Epub ahead of print]. [Crossref]
- Favaloro EJ, Bonar RA, Meiring M, et al. Evaluating errors in the laboratory identification of von Willebrand disease in the real world. Thromb Res 2014;134:393-403. [Crossref] [PubMed]
- Favaloro EJ, Bonar RA, Mohammed S, et al. Type 2M von Willebrand disease – more often misidentified than correctly identified. Haemophilia 2016;22:e145-55. [Crossref] [PubMed]
- Favaloro EJ. Detailed von Willebrand factor multimer analysis in patients with von Willebrand disease in the European study, molecular and clinical markers for the diagnosis and management of type 1 von Willebrand disease (MCMDM-1VWD) – a rebuttal. J Thromb Haemost 2008;6:1999-2001. [Crossref] [PubMed]
- Favaloro EJ, Franchini M, Lippi G. Biological therapies for von Willebrand Disease. Expert Opin Biol Ther 2012;12:551-64. [Crossref] [PubMed]
- Curnow J, Pasalic L, Favaloro EJ. Treatment of von Willebrand Disease. Semin Thromb Hemost 2016;42:133-46. [Crossref] [PubMed]
- Favaloro EJ, Pasalic L, Curnow J. Monitoring Therapy during Treatment of von Willebrand Disease. Semin Thromb Hemost 2017;43:338-354. [PubMed]
- Favaloro EJ. Rethinking the diagnosis of von Willebrand Disease. Thromb Res 2011;127:S17-21. [Crossref] [PubMed]
- Dunkley S, Baker RI, Pidcock M, et al. Clinical efficacy and safety of the factor VIII/von Willebrand factor concentrate BIOSTATE in patients with von Willebrand's disease: a prospective multi-centre study. Haemophilia 2010;16:615-24. [PubMed]
- Favaloro EJ. Towards personalised therapy for von Willebrand disease: a future role for recombinant products. Blood Transfus 2016;14:262-76. [PubMed]
- Favaloro EJ, Kershaw G, Bukuya M, et al. Laboratory diagnosis of von Willebrand Disorder (VWD) and monitoring of DDAVP therapy: Efficacy of the PFA-100® and VWF:CBA as combined diagnostic strategies. Haemophilia 2001;7:180-189. [Crossref] [PubMed]
- Saccullo G, Makris M. Prophylaxis in von Willebrand Disease: Coming of Age? Semin Thromb Hemost 2016;42:498-506. [Crossref] [PubMed]
- Castillo R, Monteagudo J, Escolar G, et al. Hemostatic effect of normal platelet transfusion in severe von Willebrand disease patients. Blood 1991;77:1901-5. [PubMed]
- Favaloro EJ. Genetic testing for von Willebrand disease: the case against. J Thromb Haemost 2010;8:6-12. [Crossref] [PubMed]
- Favaloro EJ, Krigstein M, Koutts J, et al. Genetic testing for the diagnosis of von Willebrand Disease: benefits and limitations. J Coagul Disord 2010;2:37-47.
- Batlle J, Pérez-Rodríguez A, Corrales I, et al. Diagnosis and management of von Willebrand disease in Spain. Ann Blood 2018;3:5. [Crossref]
- Favaloro EJ, Bukuya M, Martinelli T, et al. A comparative multi-laboratory assessment of factor concentrate and implications for clinical efficacy as potential replacement therapy in von Willebrand’s Disease (VWD). Thromb Haemost 2002;87:466-76. [Crossref] [PubMed]
Cite this article as: Favaloro EJ, Pasalic L, Curnow J. Diagnosis and management of von Willebrand disease in Australia. Ann Blood 2018;3:31.


