
Low prevalence of human T lymphocyte virus in blood donors in Guangdong, China
Introduction
Human T lymphocyte virus (HTLV) was the first human retrovirus discovered in the late 1970s. HTLV belongs to RNA oncornavirus of retrovirus family and has two subtypes (Type I and II HTLV) (1). HTLV-I has been associated with adult T cell leukemia (ATL) and tropical spastic paraparesis (TSP) or HTLV-associated myelopathy (HAM) (2-4). Currently, the clinical significance of HTLV-II in disease has not been identified clearly. However, there are a few literatures which indicate that HLTV-II may involve in T cell hairy cell leukemia (THCL). It is reported that HTLV-infected population in the world is up to 15 to 20 million (5). Blood transfusion, needle injection, sexual contact, placental blood exchange, natural childbirth, and lactation are the main modes of HTLV infection. Since the incubation period of asymptomatic carriers can be longer than 20 years, the risk of transfusion transmission should be considered. Therefore, since 1988, serological screening of HTLV has been included in routine blood screening in some developed countries and regions such as United States, Western European countries, Japan, South Korea, Hong Kong, Macao, and Taiwan (6). However, HTLV serological screening is not mandated in mainland China. Herein, we aimed to understand the prevalence of HTLV among blood donors in Guangdong and conducted a series of serological testing.
Methods
Blood samples
Samples of blood donors were collected from 25 blood stations of 21 cities in Guangdong province. Two mL of plasma and 1 mL of buffy coat were taken for each sample. The experimental data from 5 blood stations were not complete and excluded. A total of 2,058,790 blood samples from 20 blood stations were included in this study.
Reagents and instruments
HTLV-I/II Antibodies Diagnostic ELISA Kits (Wantai Bio-Pharm, Beijing, China; Abbott Laboratories, USA; ACON Biotech, Hangzhou, China) were used for blood screening. HTLV-I/II Elecsys and cobas e analyzers (Roche, Germany) were used for electrochemiluminescence immunoassay (ECLIA). HTLV BLOT 2.4 Western Blot Assay kits (MP Biomedicals, USA) were used in confirmation experiments.
QuickGene DNA whole blood kit L (Kurabo, Japan) and Diagnostic kits for Type I HTLV nucleic acid (Wantai Bio-Pharm, Beijing, China) were used in our study. Seventy-five hundred fast Real-Time PCR System (Applied Biosystems, USA) and Nucleic Acid Isolation System QuickGene-610L (Fujifilm, Japan) were applied in the study.
Screening of HTLV antibody
The collected samples were tested using HTLV antibodies diagnostic ELISA kits independently by each blood station in Guangdong province according to instructions. Samples with second reactive results were further tested with Roche ECLIA.
Confirmation of HTLV infection
Confirmatory tests for the samples with both reactive results of ELISA and ECLIA were performed by nucleic acid testing (NAT) and Western-blot (WB). If either of the two results is positive, the donor is identified as an HTLV infected individual.
Results
Primary screening of blood donation by ELISA
Of 2,058,790 blood samples collected from 20 blood stations in Guangdong province, 758 samples were antibody reactive to HTLV by the ELISA, resulting in a rate of 0.037%. The results provided from the blood stations are presented in Table 1.
Table 1
| Region | Number of blood donors | ELISA positive (‱) | ECLIA positive (‱) | NAT/WB positive (‱) |
|---|---|---|---|---|
| Guangzhou | 635,456 | 109 (1.72) | 33 (0.52) | 9 (0.14) |
| Zhanjiang | 113,304 | 77 (6.80) | 17 (1.50) | 7 (0.62) |
| Dongguan | 116,951 | 56 (4.79) | 15 (1.28) | 3 (0.26) |
| Jiangmen | 82,399 | 34 (4.13) | 8 (0.97) | 1 (0.12) |
| Baoan | 61,501 | 11 (1.79) | 5 (0.81) | 1 (0.16) |
| Maoming | 100,718 | 33 (3.28) | 10 (0.99) | 2 (0.20) |
| Shantou | 58,543 | 13 (2.22) | 3 (0.51) | 2 (0.34) |
| Huizhou | 131,945 | 64 (4.85) | 16 (1.21) | 4 (0.30) |
| Yangjiang | 48,624 | 8 (1.65) | 4 (0.82) | 0 (0.00) |
| Panyu | 64,879 | 19 (2.93) | 3 (0.46) | 0 (0.00) |
| Meizhou | 67,387 | 13 (1.93) | 2 (0.30) | 1 (0.15) |
| Yunfu | 49,286 | 18 (3.65) | 3 (0.61) | 2 (0.41) |
| Jieyang | 61,112 | 35 (5.73) | 8 (1.31) | 2 (0.33) |
| Chaozhou | 17,260 | 7 (4.06) | 2 (1.16) | 0 (0.00) |
| Shanwei | 30,604 | 25 (8.17) | 6 (1.96) | 4 (1.31) |
| Heyuan | 20,530 | 37 (18.02) | 6 (2.92) | 0 (0.00) |
| Zhongshan | 96,142 | 68 (7.07) | 13 (1.35) | 2 (0.21) |
| Foshan | 160,980 | 66 (4.10) | 19 (1.18) | 3 (0.19) |
| Shunde | 65,969 | 10 (1.52) | 2 (0.30) | 0 (0.00) |
| Zhaoqing | 75,200 | 55 (7.31) | 7 (0.93) | 0 (0.00) |
| Total | 2,058,790 | 758 (3.68) | 182 (0.88) | 43 (0.20) |
HTLV, human T lymphocyte virus; ELISA, enzyme-linked immunosorbent assay; ECLIA, electrochemiluminescence immunoassay; NAT, nucleic acid testing; WB, Western-blot.
Secondary screening of ELISA-reactive blood donors by ECLIA
A number of 758 ELISA-reactive samples were further tested by the ECLIA. Among them, 182 (24%) samples were antibody positive for HTLV, resulting in a positive rate of 0.00884% (1:11,312).
Confirmatory test of HTLV antibody reactive samples by real-time quantitative polymerase chain reaction (qPCR) and WB
The 182 samples reactive for both ECLIA and ELISA were further detected by qPCR and WB, respectively. A total of 43 (23.6%) samples were identified reactive by either qPCR or WB, which were highly consistent except for one blood sample positive for WB but negative for qPCR. An overall positive viremia rate was detected in 0.002% (1:47,879) of Guangdong blood donor population. All HTLV samples were classified as subtype I by WB.
Regional analysis
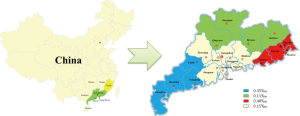
Guangdong province has four regions: East district where the borders neighbor Fujian province; North district where borders next to Hunan and Jiangxi province; West district where borders connect to Guangxi Zhuang autonomous region and face to Hainan province by across the gap of Qiongzhou Strait. The Pearl River Delta area is well developed in Guangdong, where the borders close to Hong Kong and Macao. We found that Eastern Guangdong had the highest prevalence rate of HTLV-I, followed by western Guangdong. The HTLV positive rate in eastern and western Guangdong was significantly higher than that in the Pearl River Delta area (Table 2). Specific locations of HTLV prevalence are shown in Figure 1.
Table 2
| Region | Number of blood donors | ELISA positive (‱) | ECLIA positive (‱) | NAT/WB positive (‱) |
|---|---|---|---|---|
| East Guangdong | 167,519 | 80 (4.78) | 19 (1.13) | 8 (0.48)* |
| West Guangdong | 311,932 | 136 (4.36) | 34 (1.09) | 11 (0.35)* |
| North Guangdong | 87,917 | 50 (5.69) | 8 (0.91) | 1 (0.11) |
| Pearl River Delta | 1,491,422 | 492 (3.30) | 121 (0.81) | 23 (0.15) |
*, the NAT positive rate in East Guangdong and West Guangdong is higher than that in the Pearl River Delta (P<0.05). HTLV, human T lymphocyte virus; ELISA, enzyme-linked immunosorbent assay; ECLIA, electrochemiluminescence immunoassay; NAT, nucleic acid testing; WB, Western-blot.
Gender analysis
The gender information is obtained from 1,665,185 samples collected from 14 blood stations, which is showed in Table 3. We found that the reactive rate of HTLV was higher in female donors (14/506,927 or 1:36,209) than in male donors (22/1,158,258 or 1:52,648), but this difference was not statistically significant.
Table 3
| Gender | NAT/WB positive (‱) | NAT/WB negative | Total number of blood donors | χ2 | P value |
|---|---|---|---|---|---|
| Male | 22 (0.190) | 1,158,236 | 1,158,258 | 1.213 | 0.271 |
| Female | 14 (0.276) | 506,913 | 506,927 |
HTLV, human T lymphocyte virus; NAT, nucleic acid testing; WB, Western-blot.
Discussion
Similar with several other blood-borne infectious viruses such as hepatitis B, hepatitis C, and HIV, transmission via transfusion is an important route of HTLV infection. It had been estimated that up to 40–60% of HTLV infection were through blood transfusion. Among the infected people, 5% will exhibit symptoms, while most are asymptomatic carriers (7). Therefore, in an effort to prevent the transmission of HTLV to the public as a result of blood transfusion, HTLV antibody has been listed in routine screening items for decades in Japan [1986], USA [1988], Canada [1990], Australia [1993], Sweden [1994], and South Korea [2009] (1,8). However, HTLV antibody testing has not been included in the routine screening for blood donors in mainland China.
HTLV-I infection is prevalent in various countries and regions including South of Japan, Caribbean, Central Africa, Central and South America, Papua New Guinea, and Australia (9), with significantly varied infection rates among blood donor populations in different regions. HTLV-I infection rate in Taiwan, China was reported as 0.06% between 1996 and 1998 (10), 0.007% in South Korea in 2006 (11), and 0.317% among first-time blood donors in Japan from 2006 to 2007 (12). Furthermore, 0.0025% HTLV-I infection rate was reported among USA blood donors from 2002 to 2010 (13), 0.24% in Argentina in 2008 (14), 0.1% in Brazil between 1993 and 2004 (15), 0.041/10,000 in Netherlands from 2001 to 2010 (16), 0.09/10,000 in the UK from 2002 to 2006 (17), and reported up to 6% among donors in Jamaica and Trinidad. Compared with HTLV-I, prevalence of HTLV-II may be relevant with specific exposure factors or special populations. For example, HTLV-II mainly infects intravenous drug users. It is more common among African Americans and Indians in the USA, and there is also a relatively high infection rate among those populations in Panama, Italy, Brazil, France, and Sweden. Interestingly, HTLV-II infection has been rarely reported among other populations.
During the recent years, some studies have been conducted in China about epidemiology of HTLV and have found that the infection rate was relatively higher in the southeast coastal areas, especially in Putian (0.14%), Ningde (0.4%), and other areas in Fujian province (18), compared with populations in other regions (19). Currently, there is no large-size survey results reported for HTLV-I/II prevalence in Guangdong province. Results of our study indicated that an overall prevalence of HTLV among blood donors in Guangdong is very low. The HTLV NAT positive rate was 0.002%, which was lower than that in Taiwan, Fujian, Japan and South Korea. It was similar with the infection rate of developed European countries and the USA. However, higher prevalence of HTLV has been noted in eastern Guangdong, especially in Shanwei area with a prevalence of up to 0.013%. High prevalence in eastern Guangdong may be attributed by the geographical position adjacent to Fujian and frequent folk communication which lead to mass population movements. In our study, 43 blood donors were confirmed to have been infected with HTLV-I. The number of infected donors (five) from Fujian province was second only to the number of local infected Cantonese (thirty-five). Nevertheless, the reasons for the high prevalence rate in western Guangdong (especially in Zhanjiang) and whether there is a link between lifestyle and high prevalence remains to be further studied.
HTLV is also transmitted through sexual intercourse or from mother to child besides blood transfusion. Sexually transmitted diseases that cause ulcers and result in mucosal ruptures, such as syphilis, herpes simplex type 2, and chancroid might enhance the transmission of HTLV. Other sexually transmitted diseases might result in the recruitment of inflammatory cells and could increase the risk of HTLV-1 acquisition and transmission. It is reported that sexual transmission occurs more efficiently from men to women than women to men while the mechanisms are not very clear. Our results of higher prevalence in women than in men also showed a consistent assumption. The non-significant difference may be due to the low positive rate.
HTLV is a retrovirus with a capacity to integrate its reverse transcription-generated DNA into the host DNA, which greatly hinders the identification and treatment of HTLV infection. The integration of HTLV DNA into host cells can evade identification by the immune system and reduce the effect of antiretroviral drugs, which is considered the main obstacle in complete elimination of the virus (20). Thus far, no effective treatment or prophylactic vaccine has been developed targeting HTLV-I/II infection. Therefore, the only proactive and effective method in preventing infection to a healthy populace is to identify HTLV-I/II-infected individuals in order to cut off the route of transmission. In addition to screening of HTLV antibody in blood products, infection rate and risks can be effectively reduced by filtering out white blood cells from blood and blood components products. Currently, in addition to the countries which have been performing HTLV screening, blood donations are treated by filtering out white blood cells in Belgium, Germany, Finland, Austria, Malta, Norway, Spain, and Switzerland due to the low infection rate in these regions. Both screening of HTLV-I/II antibody and filtering treatment are performed in France, Ireland, Holland, Portugal, Romania, and UK. In China, no systematic prevention and control strategy has been established, indicating that the risk of HTLV infection via blood transfusion cannot be effectively inhibited. Nonetheless, our results showed that Guangdong province should be regarded as a low HTLV epidemic area. The cost of mass screening may be a bit higher. However, the reduction of white blood cells by filtration and/or screening for first-time blood donors should be considered as a preventive strategy against HTLV infection transmitted via blood transfusion in Guangdong and other areas in China.
Acknowledgments
We thank surveyors from 25 blood stations in Guangdong province for the support in this study. We would like to thank Shenshier for English language editing and data collection.
Funding: This research was supported by the grant from Science and Technology Project of Guangdong province (No. 2016A020216001).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob.2018.08.03). YF serves as the Editor-in-Chief of Annals of Blood. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the manuscript and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed written consent was obtained from all voluntary blood donors. This study strictly followed the ethical guidelines and was approved by the Medical Ethics Committee of Guangzhou Blood Center (No. GZBC-2018-08).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen Changrong XJ, Zhang Yongchang, et al. HTLV infection and HTLV genetic typing among voluntary blood donors in Xiamen, China. Chinese Journal of Blood Transfusion 2012;25:1257-61.
- Einsiedel L, Cassar O, Bardy P, et al. Variant human T-cell lymphotropic virus type 1c and adult T-cell leukemia, Australia. Emerg Infect Dis 2013;19:1639-41. [Crossref] [PubMed]
- Einsiedel L, Spelman T, Goeman E, et al. Clinical associations of Human T-Lymphotropic Virus type 1 infection in an indigenous Australian population. PLoS Negl Trop Dis 2014;8:e2643 [Crossref] [PubMed]
- Rajabalendaran N, Burns R, Mollison LC, et al. Tropical spastic paraparesis in an aborigine. Med J Aust 1993;159:28-9. [PubMed]
- Abrams A, Akahata Y, Jacobson S. The prevalence and significance of HTLV-I/II seroindeterminate Western blot patterns. Viruses 2011;3:1320-31. [Crossref] [PubMed]
- Jeang KT. HTLV-1 and adult T-cell leukemia: insights into viral transformation of cells 30 years after virus discovery. J Formos Med Assoc 2010;109:688-93. [Crossref] [PubMed]
- Zhang C, Yin H, Li D. Research status of human T cell leukemia virus type I. Prog Microbiol Immunol 2003;1:57-60.
- Li L, Liu Z. HTLV and Blood Safety. Zhongguo shu xue za zhi 2017;30:221-3.
- Proietti FA, Carneiro-Proietti AB, Catalan-Soares BC, et al. Global epidemiology of HTLV-I infection and associated diseases. Oncogene 2005;24:6058-68. [Crossref] [PubMed]
- Lu SC, Chen BH. Seroindeterminate HTLV-1 prevalence and characteristics in blood donors in Taiwan. Int J Hematol 2003;77:412-3. [Crossref] [PubMed]
- Kwon SY, Lim AH, Park JY, et al. Seroprevalence of human T-lymphotropic virus type 1 and 2 in Korean blood donors. J Med Virol 2008;80:1864-7. [Crossref] [PubMed]
- Satake M, Yamaguchi K, Tadokoro K. Current prevalence of HTLV-1 in Japan as determined by screening of blood donors. J Med Virol 2012;84:327-35. [Crossref] [PubMed]
- Dorsey KA, Moritz ED, Steele WR, et al. A comparison of human immunodeficiency virus, hepatitis C virus, hepatitis B virus, and human T-lymphotropic virus marker rates for directed versus volunteer blood donations to the American Red Cross during 2005 to 2010. Transfusion 2013;53:1250-6. [Crossref] [PubMed]
- Berini CA, Gendler SA, Pascuccio S, et al. Decreasing trends in HTLV-1/2 but stable HIV-1 infection among replacement donors in Argentina. J Med Virol 2010;82:873-7. [Crossref] [PubMed]
- Namen-Lopes MS, Martins ML, Drummond PC, et al. Lookback study of HTLV-1 and 2 seropositive donors and their recipients in Belo Horizonte, Brazil. Transfus Med 2009;19:180-8. [Crossref] [PubMed]
- Prinsze FJ, Zaaijer HL. The outcome of donor screening for human T-cell lymphotropic virus infection in The Netherlands. Vox Sang 2012;102:198-203. [Crossref] [PubMed]
- Davison KL, Dow B, Barbara JA, et al. The introduction of anti-HTLV testing of blood donations and the risk of transfusion-transmitted HTLV, UK: 2002-2006. Transfus Med 2009;19:24-34. [Crossref] [PubMed]
- Xie J, Ge S, Zhang Y, et al. The prevalence of human T-lymphotropic virus infection among blood donors in southeast China, 2004-2013. PLoS Negl Trop Dis 2015;9:e0003685 [Crossref] [PubMed]
- Liu W, Wang F, Wang X, et al. Investigation of HTLV-I/II infection in 9050 blood donors in Shenyang. Chinese Journal of Health Inspection 2014;24:588-90.
- Satou Y, Miyazato P, Ishihara K, et al. The retrovirus HTLV-1 inserts an ectopic CTCF-binding site into the human genome. Proc Natl Acad Sci U S A 2016;113:3054-9. [Crossref] [PubMed]
Cite this article as: Shan Z, Liao Q, Huang J, Xu R, Wang M, Huang K, Tang X, Li T, Nelson K, Li C, Rong X, Fu Y. Low prevalence of human T lymphocyte virus in blood donors in Guangdong, China. Ann Blood 2018;3:40.


