
Laboratory testing for the diagnosis of immune-mediated thrombocytopenia
Introduction
Platelets play an important role in hemostasis and inflammation, and recently confirmed to be involved in innate and adaptive immune response (1,2). In addition to ABO blood group type antigens and human leukocyte antigen (HLA) class I, which are non-platelet specific antigens, human platelets also express human platelet antigens (HPA) on their surface. To date, 29 HPA systems carried on six platelet membrane glycoproteins (GPIa, GPIbα, GPIbβ, GPIIb, GPIIIa and CD109) have been identified, and among them, 12 are grouped into six biallelic systems (HPA-1, -2, -3, -4, -5, and -15) (refer to Immuno Polymorphism Database, http://www.ebi.ac.uk/ipd/hpa/). HPAs are numbered in order of finding and are designated alphabetically in order of the frequency from high to low (3). All but one of the characterized HPAs represents single nucleotide polymorphisms resulting in single amino acid substitution. The majority of HPAs is located on the GPIIb/IIIa (4). The frequency of HPA varies among the populations, thus different HPA are clinically relevant in different populations. Whereas anti-HPA-1a alloantibodies are the major cause of immune mediated thrombocytopenia in Caucasian, in Asian population, especially Japanese, HPA-4 and Naka (anti-CD36) antibodies are the predominant (5). Anti-HPA alloantibodies may be elicited by incompatible pregnancy, blood transfusion or, more rarely, by organ transplantation. HPA alloantibodies have been implicated in the pathogenesis of immune mediated thrombocytopenia, including fetal/neonatal alloimmune thrombocytopenia (FNAIT), platelet transfusion refractoriness (PTR), and post-transfusion purpura (PTP). The mechanism of FNAIT resembles that of hemolytic disease of the newborn (HDN), in which the mother produces alloantibodies against an incompatible antigen, inherited from the father, expressed on the surface of fetal cells (6). Maternal alloantibodies of the IgG type can cross the placenta, binding to fetal platelets. The antibody-bound platelets are removed from the circulation by phagocytic cells, resulting in thrombocytopenia. The presentation of FNAIT varies from an incidentally found mild thrombocytopenia to life-threatening intracranial hemorrhage and consequent neurological sequelae (7).
PTR is defined as a poor response to at least two consecutive platelet transfusions. PTR patients fail to achieve the appropriate platelet count increment because of anti-platelet antibodies produced by repeated blood transfusions (8,9). The most frequent alloantibodies involved in immunological PTR are anti-HLA class I antibodies (around 80–90%), followed by anti-HPA antibodies (around 10–20%) (10,11). Although platelet transfusions are necessary to prevent hemorrhagic complications, the onset of PTR during chemotherapy or during the regimen for hematopoietic stem cell transplant, increase the risk of hemorrhage and threaten the life of patients. Therefore, the detection and identification of the causative antibodies is crucial for the diagnosis, prevention and management of immune-mediated thrombocytopenia.
In this review, we will describe the presently available methods for the detection of anti-platelet antibodies and discuss on the advantages and disadvantages of each methodology.
Classical methods
Various techniques have been developed for the serological investigation of platelet immune disorders (Table 1). The earliest assays, such as platelet aggregation assay, platelet factor 3 (PF-3) release test and serotonin release test, were based on the platelet function-dependent endpoints (12,13). However, their sensitivity was low, because some antibodies elicit platelet activation, but most of them do not. Thereafter, binding assays using intact platelets have been developed, including platelet immuno-fluorescence test (PIFT) and mixed-passive hemagglutination assay (MPHA), which are described below. However, it is difficult to discriminate between platelet specific antigens and non-platelet specific antigens in these assays. In the 1980’s, GP specific antibodies were produced, which contributed for the development of antigen capture assays such as antigen capture ELISA (ACE), modified antigen-capture ELISA (MACE), and monoclonal antibody-specific immobilization of platelet antigen (MAIPA). These antigen-capture assays allow the identification of the antigenic epitopes on the specific GPs.
Table 1
| Generation | Principle | Assays |
|---|---|---|
| First | Platelet function | Platelet aggregation |
| Complement fixation | ||
| Platelet factor 3 (PF-3) release test | ||
| Serotonin release test | ||
| Second | Measurement of PAIgG and PBIgG | Radioimmunoassay |
| Immunofluorescence test | ||
| Mixed passive hemagglutination (MPHA) | ||
| Solid-phase RBC adherence (SPRCA) | ||
| Enzyme-linked immunosorbent assay (ELISA) | ||
| Third | Use of platelet glycoproteins as targets | Immunobead assay |
| Immunoblotting | ||
| Monoclonal antibody immobilization of platelet antigens (MAIPA) | ||
| Antigen capture ELISA (ACE) | ||
| Modified antigen capture ELISA (MACE) | ||
| Fourth | – | Beads based method |
| ❖ Simultaneous analysis of specific platelet antibodies (SASPA) | ||
| ❖ Immune-complex capture fluorescence analysis (ICFA) | ||
| ❖ Platelet antibody beads array (PABA) | ||
| ❖ PAKLX (commercial kit) | ||
| Application of new tools | ||
| ❖ Transfected cell lines | ||
| ❖ iPSC-derived HPCs expressing allele-specific forms of HPA | ||
| ❖ Endothelial cells |
iPSC, induced pluripotent stem cell; HPC, hematopoietic progenitor cell; PAIgG, platelet-associated IgG; PbIgG, platelet-binding IgG; RBC, red blood cell.
PIFT
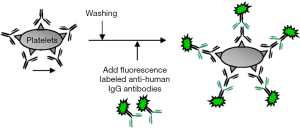
In the PIFT, intact platelets are incubated with the patient’s or the control serum and allowed to bind to the antigenic epitopes. Then, fluorescence-labeled anti-human IgG/IgM are added as the secondary antibody, and allowed to bind to the antibody bound to the antigenic epitope. The fluorescence-labeled platelets are then analyzed by fluorescence microscopy or by flow-cytometry (Figure 1). Although initially the fluorescence microscope was applied for the evaluation of the fluorescence intensity of the stained platelets, recently, it has been replaced by flow-cytometry because of the lack of the objectivity of the former (14). The measurement of the fluorescence intensity by flow-cytometer allows the more objective interpretation of the data. The result can be quantitatively expressed and judged by calculating the ratio of the fluorescence intensity of samples to that of the negative control. The flow-cytometry method is one of the widely applied techniques based on the use of intact platelets, and it is highly sensitive for the detection of most of the anti-HPA antibodies, except for HPA-5 and HPA-15 antibodies, which are expressed at lower levels on the platelet surface. Around 3,000–5,000 antigenic sites of HPA-5, and only 1,000 sites of HPA-15 are expressed on platelets (4). The detection of such low expression antigens by this method is challenging. The binding assays are associated with the issue of difficult identification of multiple antibodies, especially the coexistence of anti-HLA with anti-HPA antibodies. For the elimination of the reactivity of anti-HLA antibodies, treatment of platelets with chloroquine or acid can be used, which cause destruction of the β2 microglobulin on the surface of platelets. However, the complete elimination of the reactivity with anti-HLA antibodies may be difficult in case of a coexisting high titer anti-HLA antibody in the serum (15,16). Also, anti-A and anti-B antibodies are detected, so blood group O platelets are chosen for the assay.
MPHA and magnetic-MPHA
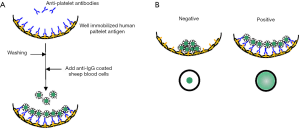
In MPHA, intact platelets or platelet membrane extracts are coated to the wells of the round-bottom microtiter plates, and human antisera is added to the microtiter wells to allow binding to the platelet antigens (17-19). For the detection of the reaction, indicator cells, consisting of sheep red cells (MPHA) or magnetic beads (M-MPHA), coated with anti-human IgG, are added to the wells and allowed to sediment spontaneously (MPHA) or pulled-down by a magnetic plate (M-MPHA). As shown in Figure 2, in case anti-platelet antibodies are present, they bind to the correspondent antigens immobilized to the bottom of the microtiter wells, and are recognized by the anti-human IgG present on the surface of indicator cells. In case of a positive reaction, the indicator cells bind to the human IgG bound to the specific antigens and consequently remain disperse, thus, do not sediment. In the negative reaction, the indicator cells sediment to the bottom of the wells, creating a clear “ring”. For the judgement of the reaction, sample wells are compared with the pattern of the negative control wells. The MPHA has high sensitivity for most HPA antibodies, including HPA-5, and is largely used in Japan for the platelet serological testing. It has many advantages, including the handling of various samples in a single assay, easy manipulability, and the very short time of the assay, especially with magnetic beads, which allows the reaction to develop in about 3 min after the antibody binding to platelet antigens. The main limitation of this method is the interference of the antibody identification by a coexistent strong HLA antibody. The treatment with chloroquine or acid, as described in PIFT, can remove HLA class I antigenicity, however, some antigenicities, such as HPA-15 antigen, are also destroyed by this treatment.
Antigen capture assays
Presently, there are three types of antigen capture assay; ACE, modified ACE (MACE) and MAIPA (20-22). These methods differ in the way the glycoprotein antigens are captured, as shown in Figure 3. MAIPA is widely used in Europe and other countries, whereas MACE is preferred in the U.S. (23,24).
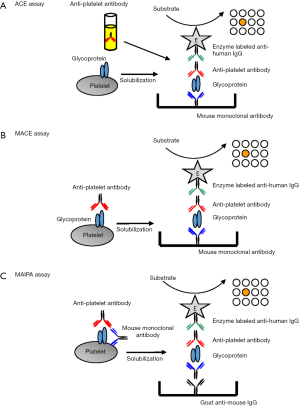
In the MAIPA, platelets are sensitized with patient’s serum, washed, and then incubated with a mouse monoclonal antibody recognizing the desired target glycoprotein on the platelet surface. After sensitization with both antibodies, platelets are washed and solubilized using a nonionic detergent such as Triton. After centrifugation to remove cytoskeletal fragments, an aliquot of the supernatant lysate containing the trimolecular complex, which consists of GPs-specific mAb/GPs/anti-platelet antibody, is added to the wells of the microtiter plate previously immobilized with goat anti-mouse IgG. This immobilized IgG captures the complex. For the detection of the captured complex, a peroxidase-labeled anti-human IgG is added, followed by an appropriate substrate. This method is highly sensitive and specific, and considered the gold standard method in platelet immunology. The antigen capture methods, including MAIPA, allow the discrimination of HPA and HLA antibodies. However, the selection of the mouse monoclonal antibodies is important, because some monoclonal antibodies may compete with anti-HPA alloantibodies, especially Naka antibodies, in binding to the antigenic epitope (25). It is not appropriate as a screening test, because of the time-consuming protocol, and the need of multiple microplates and large panels of HPA-typed platelets in order to identify antibodies reactive with HPA located on all the major GPs. The MAIPA protocol and reagents have been modified in different laboratories; therefore, there are inter-laboratory variations of the sensitivity for the detection of HPA antibodies (26,27).
New approaches for the detection of HPA antibodies
Beads-based technologies
Recently, several different beads-based techniques, which are highly sensitivity and have high throughput, have been developed. As anti-HPA-1a antibodies are the most clinically relevant among Caucasian, beads-based assays focused on the detection of HPA-1a antibodies have been reported (28-30). However, presently, it is also available for the testing of the major HPA antibodies (31-34). The great advantage of the beads-based technologies is the feasibility of simultaneous analysis of various antibody specificities in a single tube or well. Additionally, these assays eliminate the laborious and time-consuming work, which are the major disadvantages of the classical methods. These technologies are known to be associated with the detection of antibodies with low clinical relevance or naturally occurring antibodies when applied for HLA antibody testing (35,36), dependent on the use of recombinant HLA antigens. However, presently, there is no evidence of such issues when these technologies are applied for the detection of anti-HPA antibodies.
Immune-complex capture fluorescence analysis (ICFA)
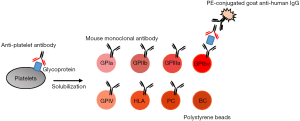
ICFA is a methodology based on the Luminex technology combined with the antigen capture method (32). Platelets are sensitized with patient’s serum, then washed and solubilized with a nonionic detergent, similar to the MACE assay. Then, the aliquot of the supernatant lysate, obtained after centrifugation, is incubated with the polystyrene beads coupled with GP specific mouse monoclonal antibodies, instead of microtiter plate in MACE. The beads are then washed and incubated with PE-conjugated goat anti-human IgG antibodies. After washing, the beads are read on the Luminex system (Luminex Co, Austin, TX, USA) (Figure 4). This method requires a repertoire of donor platelets for the detection of HPA and HLA antibodies, however at a lower volume than in classical methods. In the previous report, the detection of HPA-1a, -2b, -3a, -3b, -4a, -4b, -5a, -5b, -6b and Naka antibodies by this technology has been confirmed, but HPA-15 antibodies were not tested because of the lack of anti-sera (32). Similar methodologies, such as the platelet antibody beads array (PABA), have been reported (37), and applied for the detection of HPA-15b alloantibodies. Cell lines expressing recombinant CD109 proteins, and fresh and frozen HPA-15 typed platelets were tested, but only the cell lines and the fresh platelets were able to identify HPA-15b antibodies. As the ICFA assay is based on antigen capture assay, the selection of the capture monoclonal antibodies is essential for the prevention of false negative reactions.
PAKLx
PAKLx (Lifecodes, HOROGIC/Genprobe) is a beads-based method for the detection and identification of anti-platelet antibodies. In this kit, purified GPs from HPA-typed platelet donors and HLA class I antigen from pooled platelets, consisting of 100 Caucasian, 100 African American, and 100 Hispanic blood donors, are immobilized to the polystyrene beads. The beads are sensitized with patient serum, followed by washing. Then, beads are incubated with PE-labeled anti-human IgG antibodies, and the mean fluorescence intensity (MFI) of the beads is measured using the Luminex equipment. The MFI of each sample bead is compared with that of negative control beads, and the results judged as negative or positive. Presently, HPA-1a, -1b, -2a, -2b, -3a, -3b, -4a, -4b, -5a, -5b and Naka antibodies can be detected, but not anti-HPA-15a and -15b antibodies, because HPA-15 antibody identification beads are not available in the kit (33). It is well known that anti-HPA-15 antibodies are clinically significant in NAIT and PTR, thus, the beads assay including anti-HPA-15 antibody detection is greatly desired. Because of the easy manipulability, not requiring especial skills, it is a good screening method. Additionally, HPA-typed platelets are not required and only very low amount of sera is enough for the testing. It is also reported to have a high sensitivity. However, it has the disadvantage of not been able to detect some antibody specificities, such as anti-HPA-3a, which could be detected only by the PIFT and MAIPA using appropriate monoclonal antibodies, and -5b antibodies (33). In addition, it could not detect low titer and low avidity antibodies. Therefore, they should be used in combination with other methods.
Transfected cell lines, induced pluripotent stem cell (iPSC)-derived hematopoietic progenitor cells (HPCs) expressing allele-specific forms of HPA and endothelial cells (ECs)
An important issue in the laboratory diagnosis of platelet alloantibodies is the difficulty in obtaining the appropriate platelets, especially those expressing the low frequency HPA antigens.
In the last decade, the stably transfected Chinese hamster ovary (CHO) cell lines expressing HPAs were generated. The validity of these cell lines was confirmed by MAIPA; however, it was observed that the expression level of HPAs on the cells was reduced over a period of time (38,39). Recently, the HPA panels of transfected K562 cells, which do not express HLA or HPA, have been developed. The reactivity of these cells with HPA antibodies is very specific and sensitive, and no reactivity with normal human sera was confirmed. Until now, the transfected cell lines of HPA-1a, -1b, -2a, -2b, -3b, -4b, -5b, -6b, -7b, -7variant, -12a, -13b, -15a, -15b, -18b, -21b, and CD36 have been developed (40). Transfectant cells are valuable tools for the detection and identification of HPA antibodies, since they allow the identification of single specificities even in samples containing concomitant HLA antibodies, which hamper the antibody identification in the binding assays such as PIFT and MPHA.
More recently, the group from Milwaukee reported on the successful production of pluripotent stem cells (iPSCs) from the megakaryocyte-like DAMI cells, using the CRISPR/Cas9 (clustered regularly interspaced short palindromic repeats/CRISPR associated protein 9) gene-editing technology (41). They were able to produce cells expressing the HPA-1b alloantigenic epitope, which is the low frequency antigen of HPA-1. These designer platelets are promising tools for the diagnostic, investigative and ultimately therapeutic use in clinical conditions associated with platelet alloantibodies.
In addition, an important target of anti-HPA alloantibodies seems to be the GPIIIa (integrin β3), which is also present on ECs in a complex with the integrin alpha v (αvβ3). Alloantibodies reactive with the αvβ3 may play an important role in the pathogenesis of intracranial hemorrhage (ICH), the most severe complication of FNAIT (42,43). Antibody against β3 integrin, but not GPIbα, reduced brain and retina vessel density, impaired angiogenic signaling, and increased EC apoptosis, in a model of FNAIT (42). Using serum samples from mothers of FNAIT with and without ICH, it was confirmed that anti-HPA-1a antibodies of anti-αvβ3 specificity were present among those FNAIT with ICH, and these antibodies induced EC apoptosis of HPA-1a positive ECs by caspase-3/7 activation, mediated by reactive oxygen species and interfered with EC adhesion to vitronectin and with EC tube formation (43). From these results, it can be suggested that the identification of those alloantibodies of anti-αvβ3 specificity may contribute for the determination of severe cases of FNAIT, and may help implementing the early and more aggressive preventive measures.
All these cell lines can be applied not only in the classical methods, including MAIPA, MACE and immunofluorescence test, but also in the novel technologies such as ICFA, and their application will open new premises for the appropriate diagnosis of immune thrombocytopenia. The use of ECs as the target will be important for the diagnosis of severe cases of FNAIT, especially those associated with ICH.
Conclusions
New methodologies for the detection of anti-platelet antibodies have been developed, and the validation studies have been conducted. However, since the number of the serum samples used in the validation test is limited, as well as the antibody specificity, and some discrepancies between the classical and the novel methods have been confirmed, further investigation is required to validate these novel technologies. Most alloantibodies directed against the major HPAs shall be detected using the existing technology. However, the detection of some antibodies, such as anti-HPA-3, is still problematic. Previous reports indicated there may be different structural requirements of the HPA-3 epitopes, some antibodies being detected only when whole platelets are used, and not with the beads-based technology (33,44,45). Additionally, the groups from Germany and Milwaukee reported that surface plasmon resonance (SPR) allowed to detect the low-avidity anti-HPA-1a antibodies involved in severe FNAIT cases, which were not identified by the MAIPA assay (46,47). Recent reports indicated that low-avidity anti-HPA-1a antibodies are present in a large number of maternal sera, in which antibodies were not previously detected by the MAIPA assay (47,48). Presently, no single technique alone is sufficient to detect all clinically relevant alloantibodies, because of the complexity of platelet membrane glycoproteins carrying platelet alloantigenic determinants, and the nature of the alloantibodies themselves. Many low frequency HPAs have been found in the last years (49), and it is believed that new HPA antigens will continue to be identified in the future. By applying these novel beads-based assays, alloantibodies against new or low frequency HPA antigens, against conformation-dependent antigenic determinants formed only on intact platelets, or against endothelial specific epitopes may be overlooked. Although significant progress has been made in platelet serology, the advantages and disadvantages of each method should be taken into account for the further improvement of the technologies for platelet antibody detection.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Sentot Santoso) for the series “Platelet Immunology” published in Annals of Blood. The article has undergone external peer review.
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob.2018.09.02). The series “Platelet Immunology” was commissioned by the editorial office without any funding or sponsorship. Nelson Hirokazu Tsuno serves as an unpaid editorial board member of Annals of Blood from March 2018 to March 2020. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the manuscript and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Semple JW, Italiano JE Jr, Freedman J. Platelets and the immune continuum. Nat Rev Immunol 2011;11:264-74. [Crossref] [PubMed]
- Morrell CN, Aggrey AA, Chapman LM, et al. Emerging roles for platelets as immune and inflammatory cells. Blood 2014;123:2759-67. [Crossref] [PubMed]
- Metcalfe P, Watkins NA, Ouwehand WH, et al. Nomenclature of human platelet antigens. Vox Sang 2003;85:240-5. [Crossref] [PubMed]
- Curtis BR, McFarland JG. Human platelet antigens - 2013. Vox Sang 2014;106:93-102. [Crossref] [PubMed]
- Tsuno NH, Matsuhashi M, Iino J, et al. The importance of platelet antigens and antibodies in immune‐mediated thrombocytopenia. ISBT Science Sereis 2014;9:104-11. [Crossref]
- Serrarens-Janssen VM, Semmekrot BA, Novotny VM, et al. Fetal/neonatal allo-immune thrombocytopenia (FNAIT): past, present, and future. Obstet Gynecol Surv 2008;63:239-52. [Crossref] [PubMed]
- Risson DC, Davies MW, Williams BA. Review of neonatal alloimmune thrombocytopenia. J Paediatr Child Health 2012;48:816-22. [Crossref] [PubMed]
- Friedberg RC, Mintz PD. Causes of refractoriness to platelet transfusion. Curr Opin Hematol 1995;2:493-8. [Crossref] [PubMed]
- Hod E, Schwartz J. Platelet transfusion refractoriness. Br J Haematol 2008;142:348-60. [Crossref] [PubMed]
- Pavenski K, Freedman J, Semple JW. HLA alloimmunization against platelet transfusions: pathophysiology, significance, prevention and management. Tissue Antigens 2012;79:237-45. [Crossref] [PubMed]
- Trial to Reduce Alloimmunization to Platelets Study Group. Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. N Engl J Med 1997;337:1861-9. [Crossref] [PubMed]
- Warner M, Kelton JG. Laboratory investigation of immune thrombocytopenia. J Clin Pathol 1997;50:5-12. [Crossref] [PubMed]
- McFarland JG. Detection and identification of platelet antibodies in clinical disorders. Transfus Apher Sci 2003;28:297-305. [Crossref] [PubMed]
- Allen DL, Chapman J, Phillips PK, et al. Sensitivity of the platelet immunofluorescence test (PIFT) and the MAIPA assay for the detection of platelet-reactive alloantibodies: a report on two U.K. National Platelet Workshop exercises. Transfus Med 1994;4:157-64. [Crossref] [PubMed]
- Kurata Y, Oshida M, Take H, et al. New approach to eliminate HLA class I antigens from platelet surface without cell damage: acid treatment at pH 3.0. Vox Sang 1989;57:199-204. [Crossref] [PubMed]
- Kurata Y, Oshida M, Take H, et al. Acid treatment of platelets as a simple procedure for distinguishing platelet-specific antibodies from anti-HLA antibodies: comparison with chloroquine treatment. Vox Sang 1990;59:106-11. [Crossref] [PubMed]
- Shibata Y, Juji T, Nishizawa Y, et al. Detection of platelet antibodies by a newly developed mixed agglutination with platelets. Vox Sang 1981;41:25-31. [Crossref] [PubMed]
- Shibata Y, Matsuda I, Miyaji T, et al. Yuka, a new platelet antigen involved in two cases of neonatal alloimmune thrombocytopenia. Vox Sang 1986;50:177-80. [PubMed]
- Shibata Y, Miyaji T, Ichikawa Y, et al. A new platelet antigen system, Yuka/Yukb. Vox Sang 1986;51:334-6. [PubMed]
- Furihata K, Nugent DJ, Bissonette A, et al. On the association of the platelet-specific alloantigen, Pena, with glycoprotein IIIa. Evidence for heterogeneity of glycoprotein IIIa. J Clin Invest 1987;80:1624-30. [Crossref] [PubMed]
- Kiefel V, Santoso S, Weisheit M, et al. Monoclonal antibody-specific immobilization of platelet antigens (MAIPA): a new tool for the identification of platelet-reactive antibodies. Blood 1987;70:1722-6. [PubMed]
- Menitove JE, Pereira J, Hoffman R, et al. Cyclic thrombocytopenia of apparent autoimmune etiology. Blood 1989;73:1561-9. [PubMed]
- Davoren A, Curtis BR, Aster RH, et al. Human platelet antigen-specific alloantibodies implicated in 1162 cases of neonatal alloimmune thrombocytopenia. Transfusion 2004;44:1220-5. [Crossref] [PubMed]
- Ghevaert C, Campbell K, Walton J, et al. Management and outcome of 200 cases of fetomaternal alloimmune thrombocytopenia. Transfusion 2007;47:901-10. [Crossref] [PubMed]
- Morel-Kopp MC, Daviet L, McGregor J, et al. Drawbacks of the MAIPA technique in characterising human antiplatelet antibodies. Blood Coagul Fibrinolysis 1996;7:144-6. [Crossref] [PubMed]
- Metcalfe P, Allen D, Chapman J, et al. Interlaboratory variation in the detection of clinically significant alloantibodies against human platelet alloantigens. Br J Haematol 1997;97:204-7. [Crossref] [PubMed]
- Allen D, Ouwehand WH, de Haas M, et al. Interlaboratory variation in the detection of HPA-specific alloantibodies and in molecular HPA typing. Vox Sang 2007;93:316-24. [Crossref] [PubMed]
- Bakchoul T, Meyer O, Agaylan A, et al. Rapid detection of HPA-1 alloantibodies by platelet antigens immobilized onto microbeads. Transfusion 2007;47:1363-8. [Crossref] [PubMed]
- Skaik Y, Battermann A, Hiller O, et al. Development of a single-antigen magnetic bead assay (SAMBA) for the sensitive detection of HPA-1a alloantibodies using tag-engineered recombinant soluble beta3 integrin. J Immunol Methods 2013;391:72-80. [Crossref] [PubMed]
- Chong W, Metcalfe P, Mushens R, et al. Detection of human platelet antigen-1a alloantibodies in cases of fetomaternal alloimmune thrombocytopenia using recombinant beta3 integrin fragments coupled to fluorescently labeled beads. Transfusion 2011;51:1261-70. [Crossref] [PubMed]
- Nguyen XD, Dugrillon A, Beck C, et al. A novel method for simultaneous analysis of specific platelet antibodies: SASPA. Br J Haematol 2004;127:552-60. [Crossref] [PubMed]
- Fujiwara K, Shimano K, Tanaka H, et al. Application of bead array technology to simultaneous detection of human leucocyte antigen and human platelet antigen antibodies. Vox Sang 2009;96:244-51. [Crossref] [PubMed]
- Porcelijn L, Huiskes E, Comijs-van Osselen I, et al. A new bead-based human platelet antigen antibodies detection assay versus the monoclonal antibody immobilization of platelet antigens assay. Transfusion 2014;54:1486-92. [Crossref] [PubMed]
- Rockenbauer L, Eichelberger B, Panzer S. Comparison of the bead-based simultaneous analysis of specific platelet antibodies assay (SASPA) and Pak Lx Luminex technology with the monoclonal antibody immobilization of platelet antigens assay (MAIPA) to detect platelet alloantibodies. Clin Chem Lab Med 2015;53:1779-83. [Crossref] [PubMed]
- Lachmann N, Todorova K, Schulze H, et al. Luminex((R)) and its applications for solid organ transplantation, hematopoietic stem cell transplantation, and transfusion. Transfus Med Hemother 2013;40:182-9. [Crossref] [PubMed]
- Morales-Buenrostro LE, Terasaki PI, Marino-Vázquez LA, et al. "Natural" human leukocyte antigen antibodies found in nonalloimmunized healthy males. Transplantation 2008;86:1111-5. [Crossref] [PubMed]
- Metzner K, Bauer J, Ponzi H, et al. Detection and identification of platelet antibodies using a sensitive multiplex assay system-platelet antibody bead array. Transfusion 2017;57:1724-33. [Crossref] [PubMed]
- Kroll H, Yates J, Santoso S. Immunization against a low-frequency human platelet alloantigen in fetal alloimmune thrombocytopenia is not a single event: characterization by the combined use of reference DNA and novel allele-specific cell lines expressing recombinant antigens. Transfusion 2005;45:353-8. [Crossref] [PubMed]
- Santoso S, Tsuno NH. Progress and challenges in platelet serology. ISBT Science Sereis 2015;10:211-8. [Crossref]
- Hayashi T, Hirayama F. Advances in alloimmune thrombocytopenia: perspectives on current concepts of human platelet antigens, antibody detection strategies, and genotyping. Blood Transfus 2015;13:380-90. [PubMed]
- Zhang N, Zhi H, Curtis BR, et al. CRISPR/Cas9-mediated conversion of human platelet alloantigen allotypes. Blood 2016;127:675-80. [Crossref] [PubMed]
- Yougbare I, Lang S, Yang H, et al. Maternal anti-platelet beta3 integrins impair angiogenesis and cause intracranial hemorrhage. J Clin Invest 2015;125:1545-56. [Crossref] [PubMed]
- Santoso S, Wihadmadyatami H, Bakchoul T, et al. Antiendothelial αvβ3 Antibodies Are a Major Cause of Intracranial Bleeding in Fetal/Neonatal Alloimmune Thrombocytopenia. Arterioscler Thromb Vasc Biol 2016;36:1517-24. [Crossref] [PubMed]
- Lin M, Shieh SH, Liang DC, et al. Neonatal alloimmune thrombocytopenia in Taiwan due to an antibody against a labile component of HPA-3a (Baka). Vox Sang 1995;69:336-40. [PubMed]
- Kataoka S, Kobayashi H, Chiba K, et al. Neonatal alloimmune thrombocytopenia due to an antibody against a labile component of human platelet antigen-3b (Bakb). Transfus Med 2004;14:419-23. [Crossref] [PubMed]
- Socher I, Andrei-Selmer C, Bein G, et al. Low-avidity HPA-1a alloantibodies in severe neonatal alloimmune thrombocytopenia are detectable with surface plasmon resonance technology. Transfusion 2009;49:943-52. [Crossref] [PubMed]
- Peterson JA, Kanack A, Nayak D, et al. Prevalence and clinical significance of low-avidity HPA-1a antibodies in women exposed to HPA-1a during pregnancy. Transfusion 2013;53:1309-18. [Crossref] [PubMed]
- Bakchoul T, Kubiak S, Krautwurst A, et al. Low-avidity anti-HPA-1a alloantibodies are capable of antigen-positive platelet destruction in the NOD/SCID mouse model of alloimmune thrombocytopenia. Transfusion 2011;51:2455-61. [Crossref] [PubMed]
- Peterson JA, Gitter M, Bougie DW, et al. Low-frequency human platelet antigens as triggers for neonatal alloimmune thrombocytopenia. Transfusion 2014;54:1286-93. [Crossref] [PubMed]
Cite this article as: Matsuhashi M, Tsuno NH. Laboratory testing for the diagnosis of immune-mediated thrombocytopenia. Ann Blood 2018;3:41.


