Human leukocyte antigen antibody detection technologies in platelet transfusion refractoriness, with special emphasis on functional test
Introduction
Thrombocytopenia is a condition known to be associated with various adverse outcomes, such as prolonged hospitalization, increased medical expenses, and poor survival rate (1-3). Platelet transfusion is indicated with the aim to either stop bleeding (therapeutic use) in patients with severe hemorrhage or prevent bleeding (preventive use) in patients with risk of hemorrhage, under conditions such as reduced platelet counts or altered platelet function. Generally, there is need to confirm the efficacy of platelet transfusion in the post-transfusion period by evaluating the improvement of the clinical symptoms/signs and by confirming the increase of platelet counts in the post-transfusion period. Corrected count increment (CCI) is the indicator generally used for the evaluation of the platelet count increment. CCI is obtained by comparing the platelet increment 1 hour (10 min–1 h) or 24 hours (16–24 h) post-transfusion with the platelet counts pre-transfusion (4). However, platelet transfusion refractoriness (PTR), a condition in which the expected increment is not achieved, is often observed. PTR may be of immunological or non-immunological etiologies, and the latter, which accounts for about 80% of the cases (2), is associated with conditions such as inflammation or infection, splenectomy, bone marrow suppression, hemolytic uremic syndrome (HUS), disseminated intravascular coagulation (DIC), hypersplenism, or use of drugs, among others (2,5). On the other hand, PTR of immunological etiology is dependent on the production of alloantibodies produced by the transfusion recipients which react with alloantigens expressed on the donor’s platelet surface including antigens of the ABO system, human leukocyte antigens (HLA class I) and human platelet antigens (HPA) (6,7). Among those immunological PTR, more than 80–90% are due to anti-HLA antibodies (8), which are considered of great importance in PTR. HLA antibodies are frequently identified in multiparous women or patients who received multiple blood transfusions (9), and it is reported that 42.9% of hematologic patients with multiple transfusions are positive for HLA antibodies (10). For these patients with HLA antibodies, platelet concentrates (PC) without the specific HLA antigen (HLA compatible PC) are effective. For this transfusion management, the identification of HLA antibodies, the determination of their specificities and the dissection of the specific antibody(ies) responsible for the PTR is mandatory.
With the advent of fluorescence beads technology, the detection of HLA antibodies with a higher sensitivity and the determination of the antibody specificity became feasible. However, it is also associated with the detection of antibodies with low clinical relevance including very low titer antibodies or naturally occurring ones, requiring careful analysis of the results. Moreover, the antibodies of clinical relevance in PTR, among those identified by the fluorescence beads technology, remain to be elucidated. For the characterization of functionally relevant antibodies involved in PTR, there is need to accumulate and analyze the clinical data, which has been a difficult task, mostly dependent on the heavy workload of the medical institutions. To solve these issues, the development of a simple and highly sensitive functional assay for the analysis of platelet clearance was required.
Here, we review on the diagnosis of PTR, especially focusing on the methods available for the detection of HLA antibodies, discuss on the advantages and disadvantages of each method, and describe a new functional assay for the analysis of platelet clearance, namely platelet phagocytosis assay, which we have published recently (11).
HLA antibody detection methods
To now, methods such as the complement dependent cytotoxicity (CDC) assays have been developed and applied in the HLA antibody testing (12). Either methodology has its advantages and disadvantages, so it is important to know their features for the selection of the most appropriate one. In addition to detection of the antibody, there is need to confirm the antibody specificity, and to interpret on its clinical relevance, and for this, there is need to deeply understand the patient’s background, that means fully understand the aim of the antibody testing, which requires high level of expertise and experience. HLA antibody detection methods can be divided into cell-based, which are based on the use of live intact cells, and solid-phase assays, such as ELISA or fluorescent-beads technologies, and they should be selected according to the needs, which may be antibody screening or antibody specificity identification.
Cell-based assay
Cell-based assays are methods in which intact cells are used, and include CDC, flow-cytometry assay (FCXM), and ICFA. They require a repertoire of antigens to be covered according to the target antibodies, and for the identification of antibody specificity, there is need to prepare panel cells with known HLA specificity. Moreover, these methods are useful for the crossmatch test, because they allow the detection of a reaction with intact cells.
Complement-dependent cytotoxicity (CDC)
CDC is a method which uses the donor lymphocytes, which are used for the identification of complement-dependent antibodies in the patient’s plasma, and is also known as lymphocyte cytotoxicity test (LCT). It was first reported in 1964 by Terasaki et al. (12) and since then, it has been the gold standard method for the pre-transplant prediction of hyperacute rejection (HAR) in patients receiving transplantation. However, it is associated with disadvantages such as the need to prepare raw cells with a good viability, the low sensitivity of the test, and the affection of the test results by non-HLA antibodies. In patients receiving blood transfusion, there are reported cases of PTR due to low titer antibodies not detectable by LCT (13), reason why recently the mainstream of HLA antibody detection has been shifted to methodologies such as FCXM and solid-phase assay, which have higher sensitivity for antibody detection.
Flow-cytometry assay
Flow-cytometry is based on the use of panel cells consisting of lymphocytes, which are reacted with the antisera, followed by a secondary fluorescence-labeled antibody, and the fluorescence intensity is measured in a flow-cytometer.
This method allows the identification of both class I and class II antibodies, by using T or B lymphocytes, respectively, and has been shown to be useful for patients with low-titer donor specific antibody (DSA) with high risk of antibody mediated rejection (AMR) or transplant rejection (14).
Moreover, its usefulness in detecting low titer antibodies undetectable by LCT in patients with PTR has been confirmed (13). On the other hand, the standardization of the flow-cytometry assay has been considered an issue, due to the inter-institutional variations of the flow-cytometer, the fluorescence dye, the secondary antibody, and the number of cells and the volume of antisera applied, in addition to the disadvantages of the method, which include the affection of the results by non-HLA antibodies, similar to CDC, the need of cells with a good viability and the affection by drugs such as the anti-thymocyte globulin (ATG).
ICFA
This method, originally reported by Fujiwara et al. (15), is based on the capture of the HLA antigen-antibody complex, formed by reaction of hemolyzed donor whole blood (leukocyte pellet) with the patient’s serum, by the anti-HLA antibody immobilized to the surface of fluorescence beads. Since antigen-specific antibodies are detected, the development of non-specific reactions due to patient’s factors, which was one important issue of the cell-based assays, could be importantly reduced. Moreover, the reactivity of naturally occurring antibodies, which is an issue of the fluorescence beads technology using genetically recombinant proteins, as described below, is not observed, which makes it a valuable method for use as a high sensitive crossmatch test.
Solid-phase assay
Solid-phase assays, including enzyme-linked immunosorbent assay (ELISA) and fluorescence-based technologies using Luminex, are known to have higher sensitivity compared to cell-based assays. The fluorescence-based technologies are nowadays the mainstream in HLA antibody detection. The fluorescence beads technology is composed by the original fluorescent polystyrene beads, and the specialized equipment to measure their fluorescence, and is recently widely diffused in various fields including blood transfusion, and organ and stem cell transplantation. The advantageous features of the fluorescent beads include the feasibility to conduct the test using small amounts of reagents, the multiple information obtained in a single assay, and the higher sensitivity and throughput compared with the other technologies.
Next, we describe the currently available fluorescence beads technologies in details.
Fluorescence beads technologies
The fluorescence beads technologies are based on the use of 100 different types of polystyrene beads, prepared by coating with two different fluorescent dyes in different contrasting densities, which are read in the Luminex system. In the Luminex system, the red laser is irradiated to the beads, and the reflected fluorescence intensity is identified, in addition to the irradiation of a green laser to identify the fluorescence intensity of phycoerythrin (PE). Thus, the fluorescence of the beads and that of the labeled antibody are simultaneously identified, making it feasible to differentiate even in case of a mixture of beads, allowing the simultaneous measurement of multiple specificities. The Luminex system allows the use of a maximum of 100 beads per assay, which means 100 types of information can be obtained simultaneously.
The HLA antibody detection by the Luminex system is based on the following principle: extracted and purified HLA or recombinant HLA antigens are coupled to the polystyrene beads, and the beads are reacted with the antisera. Then, the PE-labeled secondary antibody (anti-human IgG) is added to identify the HLA antibody specificity. The results are analyzed and interpreted using the software provided by the manufacturers, allowing the identification of the antibody specificities. A vast product lineup is available according to the purpose of the test, but all the commercialized products have high sensitivity and simple (easy) manipulability, in addition to the short-time required for the test. The fluorescence bead technology can be divided into screening products and antibody specificity identification products. In the screening products, multiple beads coated with multiple antigenic specificities are applied for the antibody detection. Since beads are coated with multiple specific HLA antigens, the antibody specificity cannot be identified. Also, products in which beads are coated with a “single specific” HLA antigen are available, which allows the antibody screening with a rough identification of antibody specificity, but the clear identification is not feasible. In these products, the HLA antigen extracted and purified from cultured B-cell lineage cell lines are coated to the beads. On the other hand, antibody screening products in which individual beads are coated with a single HLA antigen synthesized by recombinant technology are also available as the antibody specificity identification products (single antigen beads). These specificity identification products allow the identification of antibodies specific for a single HLA antigen, in addition to making feasible to estimate the antibody titer roughly. Thus, the HLA antibody tests based on the fluorescence beads technology vary in sensitivity or in the resolution of specificity identification, so they should be carefully selected according to the aim of the test. Compared to the previously described cell-based assays, this technology has many advantages, including the higher sensitivity, easier manipulability, and the availability of commercial kits, which are steadily subjected to quality control tests. However, dependent on the use of recombinant HLA antigens, it is known to detect antibodies with low clinical relevance or naturally occurring HLA antibodies (16,17).
Cautions when applying the fluorescence beads technologies
Many types of naturally occurring antibodies have been reported, which have the characteristic of being monospecific (16). Morales-Buenrostro et al. (17) reported that any type of HLA antibody (HLA class I Ab in 43%, class II antibody in 11%, class I + class II antibodies in 12%) is detected in the plasma of 63% of healthy male, and it was hypothesized to be dependent on a cross-reactive immunization against vaccination, any kind of bacteria, or the ingestion of any kind of food. These naturally occurring antibodies have been suggested to specifically react with a new epitope emerged on HLA denatured during extraction of the recombinant protein (18). Thus, it is estimated to be not reactive with the native HLA on live cells, and many reports have suggested the low clinical relevance of such antibodies (19,20). However, discriminating these non-HLA antibodies is not an easy task.
There were reports on the PTR caused by HLA antibodies undetectable by the conventional LCT (13), but the detection of such antibodies became feasible with the advent of fluorescence beads technologies, and the risk of PTR due to HLA antibodies significantly decreased. However, recent reports have shown that low titer HLA antibodies are not involved in the pathogenesis of PTR (21,22). Also, there are reports on the correlation between the intensity of the results in fluorescence beads technology and the severity of PTR, and Beligaswatte et al. reported that fluorescence intensity over 5,440 is associated with more than 90% risk of PTR (21). On the other hand, Linjama et al. have reported that the effectiveness of platelet transfusion is affected in case the cumulative MFI exceeds 1,000 (23), however, the discrimination of the causative antibody is not feasible, and presently, defining the appropriate cut-off is not feasible. In addition, recently, many factors, such as epitope sharing, oversaturation, or inhibition of secondary antibody binding by complement (prozone effect), have been shown to affect HLA antibody detection by fluorescence beads technologies, and it is suggested that the correlation with the mean fluorescence intensity (MFI) is lost in case a certain antibody titer is exceeded, making difficult to know the precise antibody titer (24-26). In fact, Tambur et al. reported that the prozone effect is observed in 71% of alloimmunized patients (27). For this reason, it is recommended that the results of fluorescence intensity using recombinant HLA antigens should be interpreted semi-quantitatively (28,29). As described above, the use of fluorescence beads technology allows the detailed identification of HLA antibody with high sensitivity, but on the other hand, there is need to pay special attention to the possibility of detecting naturally occurring antibodies or low titer antibodies without clinical relevance. Moreover, many uncertainties, such as the correlation between antibody titer and the effectiveness of platelet transfusion and the determination of the cut-off value of the test, remain to be solved. To solve these issues, it is essential to accumulate clinical data, but for reasons such as the burden for the patients or the health insurance coverage, or the busy schedule of the medical staff, the clinical data of platelet transfusion is not necessarily available post-transfusion, which may be one reason why the understanding on the clinical significance of HLA antibodies has not improved. For the better understanding of the importance of HLA antibodies in the pathogenesis of PTR, we developed a functional assay based on the phagocytosis of platelets, and using this assay, we are trying to improve the understanding of such antibodies in PTR.
In vitro platelet phagocytosis assay
First reported by Lim et al. in 2002 (30), the platelet phagocytosis assay is an effective tool for the evaluation of PTR, also recently confirmed by Sayyadi et al. (31). However, in their protocol, platelets are pre-stained with a CellTracker Orange CMTMR (5-(and-6)-(((4-chloromethyl)benzoyl)amino) tetramethylrhodamine) or a CellTracker Green CMFDA (5-chloromethylfluorescein diacetate) followed by the phagocytosis, which may result in increased background, dependent on the platelets attached to the external membrane of phagocytic cells. For this reason, we have modified the protocol to use pHrodo (Thermo Scientific), a rhodamine pH indicator, as the fluorescence dye, and as a result we obtained an in vitro assay of platelet phagocytosis with higher sensitivity and specificity (11).
pHrodo is a fluorescence dye that emits fluorescence only under acidic conditions, and is widely applied in the phagocytosis assays of bacteria or apoptotic cells (32,33).
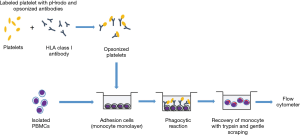
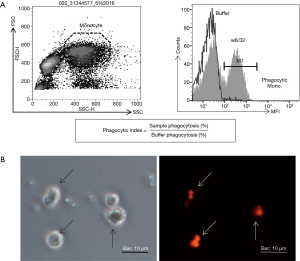
The principle of this test is based on the fluorescence emission by pre-stained platelets only under acidic condition, i.e., after phagocytosed by monocytes, which allows the detection of only the internalized stained cells. With this method, we can accurately detect platelet phagocytosis. The details of the method are shown in Figure 1. First, washed platelets are stained with the pHrodo dye, then reacted with the antiserum, and washed with buffer solution. In case the antiserum contains an antibody, it binds to the surface of platelets, becoming opsonized platelets. Separately, peripheral blood mononuclear cells (PBMC) isolated by gradient centrifugation are seeded onto a plastic plate, and incubated for a period of time to allow them to adhere to the bottom of the wells. The non-adherent cells are removed by washing, and the remaining monocytes are used for the assay. Opsonized platelets are seeded onto the monocyte layer, and incubated. In case antibodies are bound to the surface of the platelets, the Fc fragment of the immunoglobulin is recognized by the FcγR of the monocytes, and platelets are phagocytozed by monocytes. Opsonized platelets are translocated to the lysosome, and under the acidic environment of the lysosome, the pHrodo emits fluorescence. Then, monocytes are removed from the bottom of the wells and analyzed by flow-cytometry. Monocytes emitting fluorescence can be recognized, allowing the analysis of antibody-dependent platelet clearance (Figure 2). The phagocytosis assay using pHrodo allows the accurate identification of phagocytosis of the platelets specifically reactive with the antibodies, and thus, it is not associated with increased background reactivity as in previous phagocytosis assay. Also, compared to the previous assays, there are many advantages, such as the very sensitive detection ability, a wide dynamic range, allowing the more precise detection of platelet phagocytosis. This method will allow us to better understand the relationship between antibody titer and transfusion effectiveness, as well as the association with the antibody features, as discussed in the next section.
The correlation between antibody features and PTR
It is suggested that not only HLA antibody titers, but also antibody features and antibody class, affect antibody-mediated platelet clearance. As an example, the IgG subclass is considered to be an important factor regulating the antibody-mediated platelet clearance. Presently, IgG1, IgG2, IgG3 and IgG4 are the known subclasses of IgG. IgG1 and IgG3, but not IgG2 and IgG4, bind to complement and play important roles in effector functions, such as antibody-dependent cellular cytotoxicity (ADCC) activity (Table 1). In the field of organ transplantation, humoral immunity is considered to play an important role, and many reports have shown the role of antibody features, including IgG subclass, in transplant outcomes (34-36) but, presently, little evidence is found in the literature related to PTR. Also, it is known that the affinity of the FcγR expressed on effector cells, such as macrophages, and the Fc fragment of the antibody play an important role. The many types of Fc fragment which interact with the FcγR are known (Table 2), and the affinity of each IgG subclass with the FcγR and their effector function are reported (37,38). The effector function of IgG subclasses is reported to be stronger in the following sequence: IgG3>IgG1>>IgG2>IgG4 (39), but presently there are no reports on the correlation between HLA antibody subclass and PTR. Recently, the ratio of the expression level of FcγRIIa carrying the ITAM motif to that of FcγRIIb carrying immunoreceptor tyrosine-based inhibitory motif (ITIM) motif importantly has been reported to importantly regulate cells activation (40). Since FcγRIIb is reported to negatively regulate phagocytosis (41), by evaluating the correlation between the IgG subclass or the diversity of Fcγ receptor on monocytes and the phagocytosis may help improve our knowledge on the correlation between alloantibodies, such as HLA antibodies, and PTR. Also, recently, deletion of core fucose, a component of the sugar chain of IgG1, has been reported to be associated with severity of neonatal alloimmune thrombocytopenia (NAIT) and with platelet clearance, and shown to associate with a more than 50 times stronger ADCC activity (42). In addition, increase of sialic acid is reported to associate with stronger ADCC or phagocytic activities (43), suggesting the importance of the modification of IgG Fc glycans in modulating effector activities (44). For this reason, we believe there is need to investigate not only on the IgG subclasses, but also on the correlation of the IgG sugar chain modification and PTR. Moreover, although IgM type antibodies are also reported to be involved in the pathogenesis of PTR (45), presently its mechanism remains to be elucidated. Recently, C3b deposited on the membrane of cells through complement activation has been shown to have high affinity to the complement receptor present on hepatic Kupffer cells (46), and that this binding increases the cell clearance (47), thus complement activation is also an important mechanism to be considered, and future studies to clarify on the association of antibody subclass and complement activation, using reagents for the detection of complement activation, such as C1q Screen (One Lambda) and LIFECODE C3d Detection (Immucor), are required. According to the phenotype, HLA antigen expression is reported to vary (48-51), but recently, it was shown that there is individual difference not only in the phenotype but also in the HLA antigen expression on platelets, and it has been demonstrated that those platelets from donors with low HLA antigen expression are not the target of antibody-mediated clearance (52). This knowledge confers great advantage for the selection of HLA compatible platelet donors, so there is need to further investigate on the importance of expression level of HLA on platelets in the pathogenesis of PTR.
Table 1
| Biological function | IgG1 | IgG2 | IgG3 | IgG4 | IgM | IgA | IgE | IgD |
|---|---|---|---|---|---|---|---|---|
| Pathogen neutralization | ++ | ++ | ++ | ++ | + | ++ | − | − |
| Opsonization | +++ | +/− | ++ | + | + | + | − | − |
| NK cell ADCC | ++ | − | ++ | − | − | − | − | − |
| Surface of mast cells and basophils | + | − | + | − | − | − | +++ | − |
| Classical complement activation | ++ | + | +++ | − | +++ | + | − | − |
| Transplacental transfer | +++ | + | ++ | +/− | − | − | − | − |
| Serum level (mg/mL) | 9 | 3 | 1 | 0.5 | 1.5 | 2.1 | 3×10−5 | 0.04 |
−, none; +, weak activity; ++, moderate activity; +++, strong activity.
Table 2
| Receptor | FcγRI (CD64) | FcγRIIa (CD32A) | FcγRIIb (CD32B) | FcγRIIIa (CD16A) | FcγRIIIb (CD16B) |
|---|---|---|---|---|---|
| Structure | |||||
| Affinity | IgG1≥IgG3>IgG4>>IgG2 | IgG3≥IgG1, IgG2>>IgG4 | IgG3≥IgG1>IgG4>IgG2 | IgG1, IgG3>>IgG2, IgG4 | IgG1, IgG3>>IgG2, IgG4 |
| Cellular distribution | Macrophage; neutrophil; eosinophil; dendritic cell | Macrophage; neutrophil; eosinophil; platelet; dendritic cell | Macrophage; neutrophil; eosinophil; dendritic cell; B-cell; mast cell; basophil | NK cell; eosinophil; macrophage; mast cell | Neutrophil; eosinophil |
| Function | Phagocytosis; ROS release; cytotoxicity; cytokine release | Phagocytosis; ROS release; cytotoxicity; cytokine release | Inhibition of signal | Phagocytosis; ROS release; cytotoxicity; cytokine release; degranulation | ROS release; Cytotoxicity |
Concluding remarks
The antibody detection test and cross-matching are important test for the provision of HLA compatible platelets, but also analysis of the effectiveness of platelet transfusion in the post-transfusion period is essential. However, due to reasons such as the burden for the patient, the health insurance payment coverage, and the heavy duty of the medical staff, the effectiveness of platelet transfusion is not appropriately analyzed in the post-transfusion period. With the advances of the laboratory technologies, although we have the benefits of highly sensitive tests on one side, the above issue on the other side lead to a dissociation between the antibody testing and the clinical results, which is the present issue of the HLA antibody testing. To solve this, there is need to confirm the validity of the test results in the clinical setting, that means, there is need to accumulate data on the antibody titers that are clinically relevant in PTR, and simultaneously, apply in vitro tools for the prediction or evaluation of the clinical effectiveness, especially focusing on the antibody features.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Sentot Santoso) for the series “Platelet Immunology” published in Annals of Blood. The article has undergone external peer review.
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob.2018.10.01). The series “Platelet Immunology” was commissioned by the editorial office without any funding or sponsorship. NHT serves as an unpaid editorial board member of Annals of Blood from March 2018 to March 2020. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the manuscript and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Meehan KR, Matias CO, Rathore SS, et al. Platelet transfusions: utilization and associated costs in a tertiary care hospital. Am J Hematol 2000;64:251-6. [Crossref] [PubMed]
- Kerkhoffs JL, Eikenboom JC, van de Watering LM, et al. The clinical impact of platelet refractoriness: correlation with bleeding and survival. Transfusion 2008;48:1959-65. [Crossref] [PubMed]
- Toor AA, Choo SY, Little JA. Bleeding risk and platelet transfusion refractoriness in patients with acute myelogenous leukemia who undergo autologous stem cell transplantation. Bone Marrow Transplant 2000;26:315-20. [Crossref] [PubMed]
- Rebulla P. A mini-review on platelet refractoriness. Haematologica 2005;90:247-53. [PubMed]
- Slichter SJ, Davis K, Enright H, et al. Factors affecting post transfusion platelet increments, platelet refractoriness, and platelet transfusion intervals in thrombocytopenic patients. Blood 2005;105:4106-14. [Crossref] [PubMed]
- Novotny VMJ. Prevention and management of platelet transfusion refractoriness. Vox Sang 1999;76:1-13. [Crossref] [PubMed]
- Slichter SJ. Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusion. The Trial to Reduce Alloimmunization to Platelets Study Group. N Engl J Med 1997;37:1861-9.
- Pavenski K, Freedman J, Semple JW. HLA alloimmunization against platelet transfusions: pathophysiology, significance, prevention and management. Tissue Antigens 2012;79:237-45. [Crossref] [PubMed]
- Laundy GJ, Bradley BA, Rees BM, et al. Incidence and specificity of HLA antibodies in multitransfused patients with acquired aplastic anemia. Transfusion 2004;44:814-25. [Crossref] [PubMed]
- Kiefel V, König C, Kroll H, et al. Platelet alloantibodies in transfused patients. Transfusion 2001;41:766-70. [Crossref] [PubMed]
- Takahashi D, Fujihara M, Miyazaki T, et al. Flow cytometric quantitation of platelet phagocytosis by monocytes using a pH-sensitive dye, pHrodo-SE. J Immunol Methods 2017;447:57-64. [Crossref] [PubMed]
- Terasaki PI, McClelland JD. Microdroplet assay of human serum cytotoxins. Nature 1964;204:998-1000. [Crossref] [PubMed]
- Sato S, Sakurai T, Yamamoto Y, et al. Earlier detection of HLA alloimmunization in platelet transfusion refractoriness by flow cytometric analysis. Transfusion 2005;45:1399-401. [Crossref] [PubMed]
- Couzi L, Araujo C, Guidicelli G, et al. Interpretation of positive flow cytometric crossmatch in the era of the single-antigen bead assay. Transplantation 2011;91:527-35. [Crossref] [PubMed]
- Fujiwara K, Shimano K, Tanaka H, et al. Application of bead array technology to simultaneous detection of human leucocyte antigen and human platelet antigen antibodies. Vox Sang 2009;96:244-51. [Crossref] [PubMed]
- Lachmann N, Toorova K, Schulze H, et al. Luminex and its applications for solid organ transplantation, hematopoietic stem cell transplantation, and transfusion. Transfus Med Hemother 2013;40:182-89. [Crossref] [PubMed]
- Morales-Buenrostro LE, Terasaki PI, Marino-Vázquez LA, et al. "Natural" human leukocyte antigen antibodies found in nonalloimmunized healthy males. Transplantation 2008;86:1111-5. [Crossref] [PubMed]
- El-Awar N, Terasaki PI, Nguyen A, et al. Epitopes of HLA antibodies found in sera of normal healthy males and cord blood. Hum Immunol 2009;70:844-53. [Crossref] [PubMed]
- Poli F, Benazzi E, Innocente A, et al. Heart transplantation with donor-specific antibodies directed toward denatured HLA-A*02:01:a case report. Hum Immunol 2011;72:1045-8. [Crossref] [PubMed]
- Pereira S, Perkins S, Lee JH, et al. Donor-specific antibody against denatured HLA-A1:clinically nonsignificant? Hum Immunol 2011;72:492-8. [Crossref] [PubMed]
- Beligaswatte A, Tsiopelas E, Humphreys I, et al. The mean fluorescence intensities of anti-HLA antibodies detected using micro-bead flow cytometry predict the risk of platelet transfusion refractoriness. Br J Haematol 2013;162:409-12. [Crossref] [PubMed]
- Jackman RP, Deng X, Bolgiano D. Low-level HLA antibodies do not predict platelet transfusion failure in TRAP study participants. Blood 2013;121:3261-6. [Crossref] [PubMed]
- Linjama T, Niittyvuopio R, Tuimala J, et al. Platelet donor selection for HLA-immunised patients; the impact of donor-specific HLA antibody levels. Transfus Med 2017;27:375-83. [Crossref] [PubMed]
- Schnaidt M, Weinstock C, Jurisic M, et al. HLA antibody specification using single-antigen beads- a technical solution for the prozone effect. Transplantation 2011;92:510-5. [Crossref] [PubMed]
- Visentin J, Vigata M, Daburon S, et al. Deciphering complement interference in anti-human leukocyte antigen antibody detection with flow beads assays. Transplantation 2014;98:625-31. [Crossref] [PubMed]
- Schwaiger E, Wahrmann M, Bond G, et al. Complement component C3 activation: the leading cause of the prozone phenomenon affecting HLA antibody detection on single-antigen beads. Transplantation 2014;97:1279-85. [Crossref] [PubMed]
- Tambur AR, Herrera ND, Haarberg KM, et al. Assessing antibody strength: comparison of MFI, c1q, and titer information. Am J Transplant 2015;15:2421-30. [Crossref] [PubMed]
- Tambur AR, Wiebe C. HLA Diagnostics: Evaluating DSA Strength by Titration. Transplantation 2018;102:S23-S30. [Crossref] [PubMed]
- Sullivan HC, Liwski RS, Bray RA, et al. The Road to HLA Antibody Evaluation:Do Not Rely on MFI. Am J Transplant 2017;17:1455-61. [Crossref] [PubMed]
- Lim J, Kim Y, Han K, et al. Flow cytometric monocyte phagocytic assay for predicting platelet transfusion outcome. Transfusion 2002;42:309-16. [Crossref] [PubMed]
- Sayyadi M, Shaiegan M, Nikougoftar Zarif M, et al. Platelet Transfusion Outcome and Flow Cytometric Monocyte Phagocytic Assay (FMPA). Arch Iran Med 2016;19:426-9. [PubMed]
- Miksa M, Komura H, Wu R, et al. A novel method to determine the engulfment of apoptotic cells by macrophages using pHrodo succinimidyl ester. J Immunol Methods 2009;342:71-7. [Crossref] [PubMed]
- Kapellos TS, Taylor L, Lee H, et al. A novel real time imaging platform to quantify macrophage phagocytosis. Biochem Pharmacol 2016;116:107-19. [Crossref] [PubMed]
- Gao ZH, McAlister VC, Wright JR Jr, et al. Immunoglobulin-G subclass antidonor reactivity in transplant recipients. Liver Transpl 2004;10:1055-9. [Crossref] [PubMed]
- Kaneku H, O'Leary JG, Taniguchi M, et al. Donor specific human leukocyte antigen antibodies of the immunoglobulin G3 subclass are associated with Chronic rejection and graft loss after liver transplantation. Liver Transpl 2012;18:984-92. [Crossref] [PubMed]
- Lowe D, Higgins R, Zehnder D, et al. Significant IgG subclass heterogeneity in HLA-specific antibodies: Implications for pathogenicity, prognosis, and the rejection response. Hum Immunol 2013;74:666-72. [Crossref] [PubMed]
- Anderson CL, Shen L, Eicher DM, et al. Phagocytosis mediated by three distinct Fc gamma receptor classes on human leukocytes. J Exp Med 1990;171:1333-45. [Crossref] [PubMed]
- Bruhns P, Iannascoli B, England P, et al. Specificity and affinity of human Fcγ receptors and their polymorphic variants for human IgG subclasses. Blood 2009;113:3716-25. [Crossref] [PubMed]
- Bruhns P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood 2012;119:5640-9. [Crossref] [PubMed]
- Hunter S, Indik ZK, Kim MK, et al. Inhibition of Fcgamma receptor mediated phagocytosis by a non-phagocytic Fcgamma receptor. Blood 1998;91:1762-8. [PubMed]
- van Mirre E, Breunis WB, Geissler J, et al. Neutrophil responsiveness to IgG, as determined by fixed ratios of mRNA levels for activating and inhibitory FcγRII (CD32), is stable over time and unaffected by cytokines. Blood 2006;108:584-90. [Crossref] [PubMed]
- Kapur R, Kustiawan I, Vestrheim A, et al. A prominent lack of IgG1-Fc fucosylation of platelet alloantibodies in pregnancy. Blood 2014;123:471-80. [Crossref] [PubMed]
- Nagelkerke SQ, Dekkers G, Kustiawan I, et al. Inhibition of FcgR-mediated phagocytosis by IVIg is independent of IgG-Fc sialylation and FcgRIIb in human macrophages. Blood 2014;124:3709-18. [Crossref] [PubMed]
- Shinkawa T, Nakamura K, Yamane N, et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex type oligosaccharides shows the critical role of enhancing antibody dependent cellular cytotoxicity. J Biol Chem 2003;278:3466-73. [Crossref] [PubMed]
- Saito S, Tamai T, Ohta M, et al. Platelet transfusion refractoriness associated with IgM-HLA-class I antibodies-Immunoglobulin class heterogeneity of HLA class I antibodies in PTR patients. Japanese Journal of Transfusion Medicine 2006;52:405-13.
- Helmy KY, Katschke KJ Jr, Gorgani NN, et al. CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell 2006;124:915-27. [Crossref] [PubMed]
- He JQ, Wiesmann C, van Lookeren Campagne M. A role of macrophage complement receptor CRIg in immune clearance and inflammation. Mol Immunol 2008;45:4041-47. [Crossref] [PubMed]
- Apps R, Qi Y, Carlson JM, et al. Influence of HLA-C expression level on HIV control. Science 2013;340:87-91. [Crossref] [PubMed]
- Ramsuran V, Kulkarni S, O'Huigin C, et al. Epigenetic regulation of differential HLA-A allelic expression levels. Hum Mol Genet 2015;24:4268-75. [Crossref] [PubMed]
- Isa A, Nehlin JO, Sabir HJ, et al. Impaired cell surface expression of HLA-B antigens on mesenchymal stem cells and muscle cell progenitors. PloS One 2010;5:e10900 [Crossref] [PubMed]
- Kaur G, Gras S, Mobbs J, et al. Structural and regulatory diversity shape HLA-C protein expression levels. Nat Commun 2017;8:15924. [Crossref] [PubMed]
- Saris A, Tomson B, Brand A, et al. Platelets from donors with consistently low HLA-B8, -B12, or -B35 expression do not undergo antibody-mediated internalization. Blood 2018;131:144-52. [PubMed]
Cite this article as: Takahashi D, Nakajima F, Tsuno NH. Human leukocyte antigen antibody detection technologies in platelet transfusion refractoriness, with special emphasis on functional test. Ann Blood 2018;3:42.