
Novel approaches to quality control and external quality assessment for platelet function testing with a focus on the platelet function analyser (PFA-100 and PFA-200)
Introduction
Platelets represent a key component of primary hemostasis, and deficiency and/or defects in platelets, be they congenital or acquired, lead to bleeding diatheses in affected individuals (1-3). In turn, platelet function tests (PFTs), aiming to identify if platelets are functionally active or instead defective or impaired in some way, represent key diagnostic test processes within hematology laboratories associated with advanced hemostasis diagnostic facilities (4-7).
There are various levels of platelet function tests, from simple screening tests, to complex functional assays and molecular analysis (3-7). Platelet function testing has evolved to now incorporate a wide variety of test processes. These processes include (I) whole blood aggregometry (WBA), which in turn may be driven by a variety of instrumentation (3-8), (II) light transmission aggregometry (LTA) (3-7,9), representing a kind of ‘gold-standard’ in testing by nature of long historical use and wide user experience, (III) testing by automated platelet function analyser (PFA) -100 (or -200) (3-7,10), (IV) assessments by flow cytometry (3-7,11-13), and ‘(V)’ to ‘(XXVI)’ representing a large number of additional methodologies (3,6,7). The reader is referred to Table 1 for a summary of the main types of platelet function tests currently available in diagnostics. However, many other procedures may also be used to monitor anti-platelet therapies and/or in research settings. Therefore, should readers be interested in more detailed listings, they are referred to other excellent reviews (3,6,7,14).
Table 1
| Procedure | What it measures/detects | Strengths/benefits/advantages | Limitations/weaknesses/disadvantages |
|---|---|---|---|
| Light transmission aggregometry (LTA) | Low shear platelet-to-platelet aggregation in response to a range of agonists and concentrations | Gold standard. Widely used in specialized laboratories. High publication and evidence base around usage | Time-consuming, complex, sample preparation, poorly standardized, and requires specialized equipment. Limited IQC and EQA |
| Whole blood aggregometry (WBA) | Monitors changes in impedance in response to a range of agonists, sometimes including a range of agonist concentrations | Simplified whole blood test, multichannel version available. Widely used in specialized laboratories although less than LTA | Dependent on platelet count, older instruments require electrodes to be cleaned and recycled. Simplified system has limited utility in diagnostics, and perhaps has some utility in monitoring anti-platelet therapy. Requires specialized equipment. Not standardised. Limited IQC and no EQA available |
| Lumi-aggregometry | Combines LTA or WBA with measurement of nucleotide release | Monitors release reaction with secondary aggregation. Widely used in specialized labs, although less than LTA or WBA | Semiquantitative. Requires specialized equipment. Not standardised. Limited IQC and no EQA available |
| PFA-100/-200 | High-shear platelet adhesion and aggregation during formation of a platelet plug | Whole blood test, high shear, small blood volumes, simple, rapid, POC feasibility. Very sensitive to VWD. Widely used. EQA available | Inflexible; VWF, hematocrit and platelet count dependent, meaning not specific for platelet function. Requires specialized equipment. May miss some forms of mild VWD (e.g., mild type 1, alternatively called ‘low VWF as a cause of bleeding’) |
| Flow cytometry | Measurement of platelet glycoproteins and activation markers by fluorescence | Whole blood test, small blood volumes, wide variety of tests. Increasingly used in specialized labs | Specialized operator, expensive, samples prone to artifact unless carefully prepared. Mainly in realm of research at the moment. Not standardised. Requires specialized equipment. Limited IQC and no EQA available |
*Table is not meant to be an exhaustive list of options, but rather expresses the main procedures used in diagnostics for platelet function assessment. Many other procedures may also be used to monitor anti-platelet therapies and/or in research settings. For a detailed listing, please refer to other excellent reviews (
Despite many of these tests being in diagnostic use now for decades, and/or continuously evolving, internal quality control (IQC) and external quality assessment (EQA) for them is limited and made specifically difficult for diagnostic PFTs by the nature of the tests themselves, as well the test material used, with this typically representing functional cellular material (i.e., platelets). The current review therefore aims to overview platelet function testing from the perspective of diagnostic screening and highlights current limitations in IQC and EQA for PFTs, as well as highlighting potential solutions that will enable more effective and accurate testing in the future. However, the primary focus of the review is on IQC and EQA for the platelet function analyser (PFA-100 and PFA-200).
What are PFTs and what are the problems associated with these tests?
Simplistically, PFTs aim to investigate the function of platelets. Although this seems a straightforward statement of fact, how do laboratories actually achieve this, and how can they ensure the quality of PFTs?
To some extent, the answer to such questions depends on the type of tests that are performed. Platelets are complex cellular components of hemostasis (1). There are over 100 million platelets in each mL of our blood, and each platelet expresses a large number of cell surface receptors, and also houses several internal granule types that contain a myriad of (primarily pro-) haemostatic components. Simplistically, then, PFTs aim to investigate whether the platelets are working by investigation of the activity of either the cell surface receptors, or its internal components. In brief, platelets are chiefly involved in so-called primary hemostasis—meaning platelet aided formation of platelet ‘plugs’ to seal sites of vascular injury, and thereby stop bleeding (1,3,15,16). They also assist in secondary hemostasis by providing a scaffold for assembly of coagulation, and by delivery of various hemostasis proteins (as contained in their storage granules) to the site of injury (1,15,16). These activities are achieved by a sequence of steps that involves platelet adhesion [via various surface receptors and von Willebrand factor (VWF; present within plasma and also stored inside the platelets)], platelet activation (which then causes release of the internal storage granule components) and finally platelet aggregation (in which the platelets aggregate (clump together) to help form the ‘brickwork’ that seals the site of injury. Various plasma proteins, some of which are also included within the platelets, but primarily fibrinogen, help to act as the ‘mortar’ for this brickwork.
Irrespective, the important piece of information to remember in terms of IQC and EQA is that platelets represent small cellular components derived from blood. Although collection of platelets (or of whole blood) is very strait forward, platelets are very easily activated, and once activated can no longer be assessed for their activity or their function (since the activity has already essentially taken place). Platelets can easily become activated by (poor) blood collection (procedures), including use of too small-bore needles, and usage of extended stasis or tourniquets (17). Platelets can also become activated by (poor) blood transport, including excessive agitation (‘shaking’) or pressure (e.g., tube transport systems), delays in transport, and extremes of temperature during transport (both high and low temperatures can adversely affect platelet activity).
In general, PFTs need to be completed within a few hours of (a well-managed) blood collection, so that tests usually need to be performed within the same general location as the blood collection, and after collection of blood by experienced phlebotomists. This is unlike most assays of hemostasis, which can either be performed soon after collection, or else, the plasma can be separated from the centrifuged blood and then frozen for later (even geographically distant) testing. Platelets cannot be frozen for later testing, as freezing platelets destroys them. Platelets cannot be transported over large distances.
IQC and EQA for most tests of hemostasis can typically be achieved using lyophilised or frozen plasma control material. Again, this is simply not possible for PFTs. For most tests of hemostasis, before patient samples can be tested, the process needs to be ‘controlled’ by use of IQC material that can identify whether or not the test processes are working appropriately. The IQC samples must yield test results that are within an acceptable pre-defined range of expected values. Typically, IQC is performed using commercially available (plasma) materials representing several ‘levels’ of the analyte to be controlled, usually meaning a normal sample to control test results around the normal range (or reference interval) and also a ‘pathological’ sample, to control test results above or below the normal range. Sometimes, laboratories utilise a sample that mimics an ‘anticoagulated sample’ to control testing performed around the ‘therapeutic range’ (as an alternative to a ‘pathological’ sample). Such processes can be exemplified, for example, for routine tests of hemostasis, such as prothrombin time (PT), international normalized ratio (INR) and activated partial thromboplastin time (APTT) (18,19), with 2–3 levels of IQC normally applied (20). Indeed, the same set of commercial materials can typically be used for all routine tests, as well as additional tests of hemostasis (e.g., same control set can be used for PT, APTT, thrombin time and fibrinogen).
For PFTs, there are no such commercial controls, simply because as stated, lyophilisation or freezing of platelets, or whole blood, effectively destroys the platelets (21), and there are no commercial sources of stabilised native platelets. Thus, the only way to control PFTs is to collect fresh whole blood or platelets from human donors. To control testing that provides ‘normal’ results, an ostensibly normal individual would need to be collected. This needs to be done each and every time that PFTs are performed—meaning that either a normal individual is punctured every day, or else that the laboratory needs an armamentarium of normal individuals from which to regularly source fresh whole blood/platelets. Neither of these options is ethical or sustainable. Even should such options be available, the arising IQC does not provide control cover for ‘pathological’, ‘therapeutic’ or ‘abnormal’ test results, which for PFTs may involve a multitude of distinct activities depending on the test performed. As a comparative example, for the INR, a ‘therapeutic’ IQC could be a warfarin-like plasma to cover vitamin K antagonist (VKA) therapy, and for the APTT, a ‘therapeutic’ IQC could be a (unfractionated) heparin-like sample (18,19). For PFTs involving LTA and WBA, for example, this might need to include ‘aspirin-treated’ platelets to assess arachidonic acid/cyclooxygenase pathway defects, ‘clopidogrel-treated’ platelets to assess P2Y12 defects and ADP responsiveness, ‘GPIb-denuded’ platelets to assess platelet glycoprotein 1b pathway defects, and so on and so forth. Thus, even if ‘normal’ platelet function testing could be controlled by collection of normal individuals, the results of ‘abnormal’ PFTs cannot be easily controlled, since this would require collection of a wide range of ‘abnormal’ test samples, representing an armamentarium of ‘abnormal’ individuals, or else, the ‘construction’ of abnormal test samples covering a wide variety of potential defects. This is simply not feasible.
EQA for PFTs poses additional challenges. EQA would theoretically require collection of a huge amount of normal blood (to assess/control for normal PFT results) as well as much larger quantities of blood from various abnormal individuals, and/or samples be purpose constructed to reflect a wide variety of potential platelet function abnormalities. This material would then need to be transported to a multitude of EQA participating laboratories, within a few hours (to maintain platelet integrity) and to mitigate adverse effects on platelet function. This is simply a current logistic impossibility.
The (potential) solutions to IQC and EQA for PFTs—focus on the PFA-100/-200
Given the difficulty of IQC and EQA for PFTs (summarised in Table 2) as otherwise ‘classically defined’ for hemostasis tests, there is a need to think ‘outside the box’ and devise alternate strategies to control laboratory performed PFTs and thereby ensure the quality of such testing.
Table 2
| Platelets are easily activated and can be activated simply by the process of blood collection and/or transport |
| Once activated, the platelets can no longer be assessed for their activity or ‘function’ |
| Timeliness of PFTs—need to be performed within hours of blood collection |
| IQC requires collection of fresh whole blood/platelets on regular basis (e.g., daily, if PFTs performed daily)—this carries ethical and sustainable concerns |
| IQC typically involves assessment of ‘normal’ and ‘pathological/therapeutic’ regions of assay performance. Although the former can be controlled by collection of normal individuals, the latter would require collection of a wide range of ‘abnormal’ test samples, or else, the ‘construction’ of abnormal test samples covering a wide variety of potential defects—this is not feasible |
| EQA provides even greater challenges. EQA would theoretically require collection of a huge amount of normal blood (to assess/control for normal PFT results) as well as a much larger quantity of blood from abnormal individuals, and/or purpose constructed to reflect a wide variety of potential platelet function abnormalities, and then this blood transported to a multitude of participating laboratories within a few hours of collection and without adverse effect on platelet function. This is simply a current logistic impossibility |
The feasibility of alternate approaches to IQC and EQA for PFA-100/-200 testing has been explored by two EQA groups. The College of American Pathologists (CAP) reported on one potential strategy in 2007 (22). This approach utilised an inhibitor of platelet function to generate ‘pathological’ PFT results in the PFA-100 after addition of whole blood from a normal individual, as collected fresh on site by the participating laboratory. Thus, the EQA (CAP in this case) sent the laboratory a ‘wet-challenge’ tube, with this containing a platelet function antagonist, and the EQA participating laboratory then tested normal whole blood collected fresh on site on their PFA-100, either without any manipulation (to generate a normal PFA-100 EQA test sample result) and also repeating the test(s) after addition of this normal whole blood to the ‘wet-challenge’ tube (to generate a ‘pathological’ PFA-100 EQA test sample result). Test results obtained by participant laboratories were then compared with other laboratories in a peer-assessment process. CAP reported this produced a successful EQA for the PFA-100 (22), but inter-laboratory co-efficient of variation (CVs) produced for the wet-challenges were as high as 50%, as compared to about 20% for the normal (non-manipulated test sample), thereby potentially limiting the overall usefulness of the EQA. CAP recently published an update (23) to the original report (22), and the PFA-100 based program remains available more than a decade later in 2019 (https://www.cap.org/). Indeed, this EQA program now also has wet-challenges for ‘Platelet aggregation’ and ‘Helena Plateletworks’, according to the latest CAP catalogue (available at https://www.cap.org/). Moreover, the specific nature of these ‘wet-challenges’, although not identified in the catalogue, is elaborated on in the recent publication (23). It seems that essentially the same approach is used for all the platelet function options—PFA-100, Platelet aggregation, Helena Plateletworks and also PlateletMapping (23). Two specimen challenge tubes are sent with each platelet function survey. One tube contains saline, while the other contains tirofiban, a platelet GPIIb/IIIa inhibitor. If a normal donor is collected, the saline challenge should provide normal results. In contrast, tirofiban would simulate a severe platelet aggregation defect similar to homozygous GPIIb/IIIa deficiency (Glanzmann thrombasthenia). Thus, tirofiban would be expected to produce an abnormal result for all types of platelet function testing. Participating laboratories are instructed to pipette citrated whole blood from a normal donor into each of the challenge tubes and mix gently by inversion 8 to 10 times.
Platelet function testing is then to be performed on these samples according to the standard procedure for each laboratory.
As a summary of their most recent report (23), and using proficiency testing data from 2012–2016, a total of 1,200 laboratories participated for PFA-100, with the coefficient variation (CV) of cartridge closure times for saline being was 22%. The CV for the tirofiban challenge was not reported. Nevertheless, 44,952 of 45,616 survey responses (99%) provided an interpretation, and 42,934 of 44,952 (96%) were correct. This indicated that the wet-challenge process for the PFA-100 worked as expected for the vast majority of participants. For optical platelet aggregation, 190 laboratories participated, and the CV for saline was 17%. Again, the CV for the tirofiban challenge was not reported. Nevertheless, 7,444 of 7,813 survey responses (95%) provided an interpretation, and 7,015 of 7,444 (94%) were correct. This again indicates that this wet-challenge process, this time for optical platelet aggregation, also worked as expected for the vast majority of participants. For PlateletWorks, 60 laboratories participated, and the CV was 3% to 11% (for saline challenge). Of 2,454 survey responses, 2,412 (98%) provided an interpretation, and 1,207 of 1,276 (95%) were correct for adenosine diphosphate (ADP) and 936 of 1,136 (82%) for collagen. For PlateletMapping, 200 laboratories participated; for ADP, 1,128 of 2,697 survey responses (42%) provided an interpretation, but only 927 of 1,128 (82%) were correct. For arachidonic acid, 1,139 of 2,604 survey responses (44%) provided an interpretation and 964 of 1,139 (85%) were correct. Thus, PlateletWorks using collagen and PlateletMapping showed worse interpretive accuracy than the other methods.
As a hemostasis advisor to the Royal College of Pathologists of Australasia (RCPA) hematology quality assurance program (QAP), the author developed an Australasian program for PFA-100 EQA in 2008, and the results of this EQA have since been reported in several publications (24-28). The premise of this EQA is similar to that of CAP, but alternate use of various (propriety) formulations to tirofiban (as used by CAP) has seemingly achieved a much tighter CV than that reported by CAP (22,23). A summary of the anticipated test patterns obtained for the PFA-100/-200 from the perspective of clinical scenarios is provided in Table 3, whereas the corresponding perspective of EQA is shown in Table 4.
Table 3
| C/Epi | C/ADP | ||
|---|---|---|---|
| Normal | Mildly Prolonged | Grossly Prolonged (or non-closure) | |
| Normal | Normal (mild defectb) | Rare event | Shouldn’t happen (repeat tests) |
| Mildly prolonged | Aspirin, mild defectb, mildly reduced hematocrit +/− platelet count | Mild defectb, mildly reduced hematocrit +/− platelet count | Shouldn’t happen (repeat tests)? Severe defectc |
| Grossly prolonged (or non-closure) | Aspirin | Moderate to severe defectc, reduced hematocrit +/− platelet count (aspirin) | Severe defectc, severely reduced hematocrit +/− platelet count, gross sample hemolysis |
a, table summarizes expected PFA-100/-200 test patterns for various clinical scenarios as may be encountered by laboratories undertaking PFA-100/200 testing. b, for example, mild type 1 von Willebrand disease, mild platelet dysfunction. c, for example, type 2A, 2B, 2M, or 3 von Willebrand disease, severe platelet dysfunction.
Table 4
| C/Epi | C/ADP | ||
|---|---|---|---|
| Normal | Mildly prolonged | Grossly prolonged (or non-closure) | |
| Normal | Normal | Rare event | Shouldn’t happen (repeat tests) |
| Mildly prolonged | Aspirin or mild defect | Mild defect | Shouldn’t happen (repeat tests)? Severe defect |
| Grossly prolonged (or non-closure) | Aspirin | Mild to severe defect | Severe defect |
a, table summarizes expected PFA-100/-200 interpretations for various test patterns as may be encountered by laboratories undertaking the PFA-100/200 EQA challenge. These potential scenarios are ‘stripped down’ from actual test practice shown in
A summary of the EQA material sent out by the RCPA QAP over the past 10 years is identified in Table 5, and the resultant outcomes summarised in Table 6. This EQA provides both positive (‘abnormal’ PFA) and ‘negative’ (‘normal’ PFA) wet challenges, and thus like CAP also provides an EQA wet-challenge for both normal and ‘pathological’ test results. Figure 1 shows data from Table 1 summarised according to challenge type, for main categories of ‘baseline’, ‘no additive challenge’, ‘mild/moderate defect’ challenges (various samples) and ‘severe defect’ challenges (various samples). Inter-laboratory CVs tend to be <20% for baseline test results and no additive ‘wet-challenges’ and are often <15% for ‘positive’ (severe defect’) challenges yielding grossly prolonged PFA test times (Table 6; Figure 1). CVs for ‘mild/moderate defect’ challenges tend to be higher, but are generally <30%, albeit representing quite a heterogeneous group of challenge samples. The EQA has been shown to be effective for both the PFA-100, and the newer PFA-200 model (not yet available in the USA). To date, a total of 47 separate EQA challenges have been distributed and undertaken by participants (Table 5), with results published for most challenges (24-28). A summary of data for baseline PFA test times, the negative ‘wet-challenges’, and some positive ‘wet-challenges’ is shown in Figure 2.
Table 5
| Year | Type of survey | Number of participants | Number of samples | Sample types/scenarios that samples designed to mimic |
|---|---|---|---|---|
| 2008 | Trial | 26 | 5 | Normal baseline CTs; mild defect; severe defect |
| 2009 | Trial | 26 | 6 | Normal baseline CTs; aspirin defect; mild defect; severe defect |
| 2010 | Formal EQA module | 47 | 4 | Normal baseline CTs; severe defect |
| 2011 | Formal EQA module | 47 | 4 | Normal baseline CTs; moderate defect; severe defect |
| 2012 | Formal EQA module | 49 | 4 | Normal baseline CTs; moderate defect; severe defect |
| 2013 | Formal EQA module | 50 | 4 | Normal baseline CTs; aspirin defect; severe defect |
| 2014 | Formal EQA module | 53 | 4 | Normal baseline CTs; severe defect |
| 2015 | Formal EQA module | 59 | 4 | Normal baseline CTs; moderate defect; severe defect |
| 2016 | Formal EQA module | 58 | 4 | Normal baseline CTs; moderate defect; severe defect |
| 2017 | Formal EQA module | 65 | 4 | Normal baseline CTs; moderate defect; severe defect |
| 2018 | Formal EQA module | 73 | 4 | Normal baseline CTs; severe defect |
| Total | 47 |
RCPAQAP, Royal College of Pathologists of Australasia Quality Assurance Program.
Table 6
| Year and wet challenge sample identitya | Scenario that sample designed to mimic | Target PFA-100/200 CTsb | Median CTs | CVs (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| C/ADP (s) | C/Epi (s) | C/ADP (s) | C/Epi (s) | C/ADP | C/Epi | ||||
| 2008-baseline | Normal baseline CTs | Normal | Normal | 81 | 111 | 14.3 | 18.5 | ||
| 2008-2 | Mild defect | ~150–200 | ~200 | 141 | 170 | 27.8 | 17.3 | ||
| 2008-3 | Severe defect | >250 | >250 | 301 | 301 | 13.3 | 15.1 | ||
| 2008-4 | Mild defect | ~150–200 | ~200 | 170 | 210 | 22.0 | 23.7 | ||
| 2008-5 | Severe defect | >250 | >250 | 301 | 301 | 14.8 | 14.6 | ||
| 2009-Baseline ‘a’ | Normal baseline CTs | Normal | Normal | 97 | 125 | 18.0 | 19.1 | ||
| 2009-1a | Aspirin effect | Normal | >250 | 95 | 301 | 16.4 | 27.1 | ||
| 2009-2a | Severe defect | >250 | >250 | 296 | 301 | 14.7 | 11.6 | ||
| 2009-3a | Severe defect | >250 | >250 | 301 | 301 | 4.4 | 12.6 | ||
| 2009-Baseline ‘b’ | Normal baseline CTs | Normal | Normal | 93 | 107 | 15.4 | 18.8 | ||
| 2009-1b | Mild defect | ~150–200 | ~200 | 167 | 175 | 29.5 | 29.5 | ||
| 2009-2b | Severe defect | >250 | >250 | 291 | 301 | 10.9 | 13.9 | ||
| 2009-3b | Severe defect | >250 | >250 | 301 | 301 | 3.9 | 0.6 | ||
| 2010 Dispatch 1 baseline | Normal baseline CTs | Normal | Normal | 84 | 119 | 15.4 | 14.1 | ||
| 2010 PF10-03a | Severe defect | >250 | >250 | 301 | 301 | 19.4 | 12.0 | ||
| 2010 PF10-03b | Normal (no additive tube) | Normal | Normal | 100 | 130 | 18.4 | 15.2 | ||
| 2010 Dispatch 2 baseline | Normal baseline CTs | Normal | Normal | 86 | 115 | 15.1 | 16.6 | ||
| 2010 PF10-08a | Severe defect | >250 | >250 | 301 | 301 | 4.6 | 12.4 | ||
| 2010 PF10-08b | Normal (no additive tube) | Normal | Normal | 99 | 130 | 18.9 | 13.5 | ||
| 2011 Dispatch 1 baseline | Normal baseline CTs | Normal | Normal | 87 | 118 | 15.8 | 20.5 | ||
| 2011 PF11-03a | Normal (no additive tube) | Normal | Normal | 94 | 130 | 17.9 | 18.1 | ||
| 2011 PF11-03b | Severe defect | >250 | >250 | 301 | 301 | 17.4 | 14.6 | ||
| 2011 Dispatch 2 baseline | Normal baseline CTs | Normal | Normal | 89 | 130 | 14.2 | 14.1 | ||
| 2011 PF11-08a | Moderate/severe defect | >200 | >200 | 223 | 301 | 23.0 | 12.0 | ||
| 2011 PF11-08b | Severe defect | >250 | >250 | 301 | 301 | 7.3 | 5.1 | ||
| 2012 Dispatch 1 baseline | Normal baseline CTs | Normal | Normal | 84 | 119 | 14.2 | 15.6 | ||
| 2012 PF12-03a | Normal (no additive tube) | Normal | Normal | 96 | 132 | 19.9 | 17.3 | ||
| 2012 PF12-03b | Moderate/severe defect | >200 | >200 | 214 | 301 | 29.5 | 16.6 | ||
| 2012 Dispatch 2 baseline | Normal baseline CTs | Normal | Normal | 83 | 116 | 15.7 | 15.0 | ||
| 2012 PF12-08a | Moderate/severe defect | >200 | >200 | 214 | 301 | 29.4 | 16.4 | ||
| 2012 PF12-08b | Severe defect | >250 | >250 | 301 | 301 | 13.5 | 15.2 | ||
| 2013 Dispatch 1 baseline | Normal Baseline CTs | Normal | Normal | 88 | 118 | 17.5 | 19.0 | ||
| 2013 PF13-03a | Severe defect | >250 | >250 | 301 | 301 | 15.9 | 11.4 | ||
| 2013 PF13-03b | Aspirin effect |
Normal | >250 |
95 |
|
24.6 |
|
||
| 2013 Dispatch 2 baseline | Normal baseline CTs | Normal | Normal | 86 | 120 | 15.4 | 15.9 | ||
| 2013 PF13-08a | Normal (no additive tube) | Normal | Normal | 93 | 130 | 25.7 | 23.1 | ||
| 2013 PF13-08b | Severe defect | >250 | >250 | 301 | 301 | 15.5 | 8.2 | ||
| 2014 Dispatch 1 baseline | Normal baseline CTs | Normal | Normal | 84 | 116 | 15.9 | 16.1 | ||
| 2014 PF14-03a | Normal (no additive tube) | Normal | Normal | 95 | 123 | 20.0 | 22.6 | ||
| 2014 PF14-03b | Severe defect | >250 | >250 | 301 | 301 | 17.1 | 14.9 | ||
| 2014 Dispatch 2 baseline | Normal baseline CTs | Normal | Normal | 83 | 130 | 15.1 | 15.3 | ||
| 2014 PF14-08a | Severe defect | >250 | >250 | 301 | 301 | 19.1 | 10.2 | ||
| 2014 PF14-08b | Moderate/severe defect | >200 | >200 | 301 | 301 | 9.8 | 10.1 | ||
| 2015 Dispatch 1 Baseline | Normal baseline CTs | Normal | Normal | 88 | 119 | 17.5 | 18.0 | ||
| 2015 PF15-03a | Moderate/severe defect | >200 | >200 | 301 | 301 | 16.9 | 5.4 | ||
| 2015 PF15-03b | Normal (no additive tube) | Normal | Normal | 98 | 132 | 26.2 | 29.8 | ||
| 2015 Dispatch 2 baseline | Normal baseline CTs | Normal | Normal | 87 | 117 | 16.6 | 15.5 | ||
| 2015 PF15-08a | Severe defect | >250 | >250 | 301 | 301 | 16.2 | 13.2 | ||
| 2015 PF15-08b | Normal (no additive tube) | Normal | Normal | 97 | 127 | 22.1 | 24.3 | ||
| 2016 Dispatch 1 baseline | Normal baseline CTs | Normal | Normal | 83 | 116 | 12.6 | 15.7 | ||
| 2016 PF16-03a | Severe defect | >250 | >250 | 301 | 301 | 9.1 | 12.8 | ||
| 2016 PF16-03b | Normal (no additive tube) | Normal | Normal | 94 | 122 | 18.8 | 25.2 | ||
| 2016 Dispatch 2 baseline | Normal baseline CTs | Normal | Normal | 86 | 113 | 17.9 | 21.6 | ||
| 2016 PF16-08a | Normal (no additive tube) | Normal | Normal | 97 | 129 | 22.1 | 24.5 | ||
| 2016 PF16-08b | Moderate/severe defect | >200 | >200 | 301 | 301 | 23.0 | 13.1 | ||
| 2017 Dispatch 1 baseline | Normal baseline CTs | Normal | Normal | 84 | 116.5 | 16.7 | 17.0 | ||
| 2017 PF17-03a | Moderate/severe defect | >200 | >200 | 301 | 301 | 15.7 | 11.8 | ||
| 2017 PF17-03b | Normal (no additive tube) | Normal | Normal | 95 | 125 | 19.2 | 29.1 | ||
| 2017 Dispatch 2 baseline | Normal Baseline CTs | Normal | Normal | 85 | 118 | 15.2 | 16.7 | ||
| 2017 PF17-08a | Normal (no additive tube) | Normal | Normal | 97 | 125 | 18.9 | 18.2 | ||
| 2017 PF17-08b | Severe defect | >250 | >250 | 301 | 301 | 15.6 | 13.3 | ||
| 2018 - Dispatch 1 baseline | Normal baseline CTs | Normal | Normal | 82 | 117 | 19.3 | 16.9 | ||
| 2018 PF18-03a | Severe defect | >250 | >250 | 301 | 301 | 5.9 | 8.5 | ||
| 2018 PF18-03b | Normal (no additive tube) | Normal | Normal | 94 | 121 | 18.2 | 18.6 | ||
| 2018 Dispatch 2 baseline | Normal baseline CTs | Normal | Normal | 84 | 118 | 19.5 | 19.7 | ||
| 2018 PF18-08a | Normal (no additive tube) | Normal | Normal | 95 | 125 | 19.4 | 23.1 | ||
| 2018 PF18-08b | Severe defect | >250 | >250 | 301 | 301 | 8.0 | 7.6 | ||
a, five challenge samples dispatched in 2008; data for one sample showing stability issues omitted. Six challenge samples dispatched to the same laboratories in the trial 2009 exercise, but testing was split into two sets of three samples. The formal PFA-100 external quality assessment (EQA) module began in 2010, where two samples were dispatched to 42 participants in March and another two samples to 47 participants in August. A similar dispatch process has been used thereafter. Some similarly or identically formulated challenge samples were dispatched in different exercises to help assess reproducibility of the system. ‘Baseline’ data represents data within each exercise using native whole blood prior to test challenges. b, ‘normal’ means CTs within the normal reference range. c, challenge PF13-03b was designed as an aspirin-challenge, and despite acceptable homogeneity testing, showed unacceptable stability findings, and lead to a failed EQA challenge. All other challenges were essentially deemed to be successful challenges. Similar sample sets are identified by scenario (e.g., all challenges that represent no additive, or all challenges that mimic severe defect. Identical sample sets are those that comprise the same challenge material sent in different surveys; viz: 2008-3 & 2009-3a; 2008-4 & 2009-1b; 2008-5 & 2009-3b; PF10-08a & PF11-08b & PF12-08b; PF14-03b & PF14-08a; PF14-08b & PF15-03a; PF15-08a & PF16-03a; PF17-08b & PF17-03a. RCPAQAP, Royal College of Pathologists of Australasia Quality Assurance Program; CTs, closure times; CVs, coefficient of variation; C/ADP, collagen/ADP; C/Epi, collagen/epinephrine.
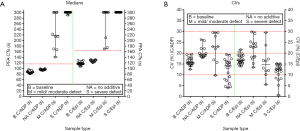
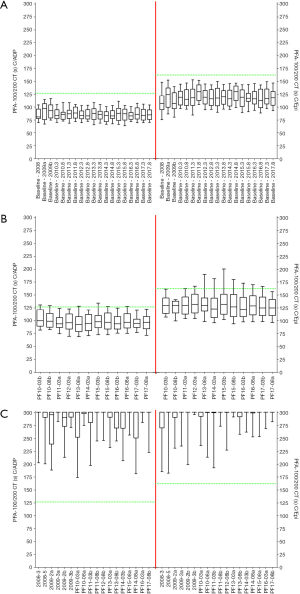
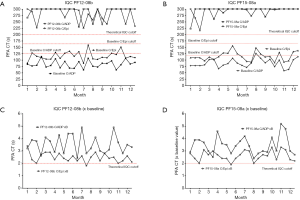
The PFA EQA has also been shown to be potentially useful in the setting of IQC (26-28). For the PFA-100/-200, the manufacturer only suggests performance of a normal test sample (normal fresh donor) with each change in lot of test cartridges, or after any major instrument maintenance, as IQC. The EQA ‘wet-challenges’ may be therefore potentially utilised to also provide some reassurance of PFA functionality around the ‘abnormal’ test region, and test data can be depicted in Levy-Jennings graphs (25-28) (samples shown in Figure 3).
Other EQA solutions for PFTs
Another EQA provider, ECAT (External Quality Control of Diagnostic Assays and Tests; http://www.ecat.nl/) in partnership with NASCOLA (North American Specialized Coagulation Laboratory Association; https://www.nascola.com/) offer a variety of ‘electronic’ surveys to support laboratories involved in PFTs. These comprise: (I) Post Analytical Platelet Function EQA (electronic survey); (II) Platelet Dense Granule exercise (electronic survey); (III) case studies on bleeding disorders (distribution separately from the regular surveys); (IV) pre- and post-analytical electronic surveys in haemostasis. Some publication around these exercises are available for the interested reader (29-32).
Conclusions
IQC and EQA for PFTs remains challenging, but some support is available from a variety of sources, especially in relation to EQA. Wet-challenges are so far limited to two EQA providers, RCPAQAP and CAP, although ECAT expects to also offer the same challenges as available to RCPAQAP participants in the near future. In terms of EQA for PFTs, the main alternative to ‘wet-challenges’ comes in the form of educational support, for example by electronic surveys, and typically ‘post-analytical’ (meaning that results of PFTs are provided the EQA to participants for their interpretation – and then these interpretations are then relayed to the EQA for review and assessment/peer comparison/reporting). Irrespective, ‘thinking outside the box’ is a critical requirement when contemplating IQC and EQA for PFTs, since standard hemostasis approaches simply remain infeasible (Table 2).
Acknowledgments
This review includes some data derived as a result of an ongoing collaboration with the RCPAQAP. This data is presented in summary form, and/or may derive from prior studies in which the RCPAQAP and its staff contributed, either by provision of raw data, or by prior data analysis, or by other logistical support. This EQA program is therefore acknowledged as facilitating some of the work described in this paper. Several individuals within the RCPA can also be personally acknowledged, in particular Roslyn Bonar, who has recently retired from the workforce to concentrate on life and family.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Blood for the series “External Quality Assurance”. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/aob.2019.01.02). The series “External Quality Assurance” was commissioned by the editorial office without any funding or sponsorship. EJF served as an unpaid Guest Editor of the series. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclaimer: The opinions expressed in this review are those of the author, and not necessarily those of NSW Health Pathology or of the RCPAQAP. This manuscript reflects findings from a single EQA provider, the RCPAQAP, and the opinions of other EQA organizations may differ.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gremmel T, Frelinger AL 3rd, Michelson AD. Platelet Physiology. Semin Thromb Hemost 2016;42:191-204. [Crossref] [PubMed]
- Hvas AM. Platelet Function in Thrombosis and Hemostasis. Semin Thromb Hemost 2016;42:183-4. [Crossref] [PubMed]
- Gresele P, Bury L, Falcinelli E. Inherited Platelet Function Disorders: Algorithms for Phenotypic and Genetic Investigation. Semin Thromb Hemost 2016;42:292-305. [Crossref] [PubMed]
- Harrison P, Mackie I, Mumford A, et al. British Committee for Standards in Haematology. Guidelines for the laboratory investigation of heritable disorders of platelet function. Br J Haematol 2011;155:30-44. [Crossref] [PubMed]
- Favaloro EJ, Lippi G, Franchini M. Contemporary platelet function testing. Clin Chem Lab Med 2010;48:579-98. [Crossref] [PubMed]
- Lordkipanidzé M. Platelet Function Tests. Semin Thromb Hemost 2016;42:258-67. [Crossref] [PubMed]
- Lassila R. Platelet Function Tests in Bleeding Disorders. Semin Thromb Hemost 2016;42:185-90. [Crossref] [PubMed]
- Fritsma GA, McGlasson DL. Whole Blood Platelet Aggregometry. Methods Mol Biol 2017;1646:333-47. [Crossref] [PubMed]
- Hvas AM, Favaloro EJ. Platelet Function Analyzed by Light Transmission Aggregometry. Methods Mol Biol 2017;1646:321-31. [Crossref] [PubMed]
- Favaloro EJ. Clinical utility of closure times using the Platelet Function Analyzer (PFA)-100/200. Am J Hematol 2017;92:398-404. [Crossref] [PubMed]
- Ramström S, Södergren AL, Tynngård N, Lindahl TL. Platelet Function Determined by Flow Cytometry: New Perspectives? Semin Thromb Hemost 2016;42:268-81. [Crossref] [PubMed]
- Cai H, Mullier F, Frotscher B, et al. Usefulness of Flow Cytometric Mepacrine Uptake/Release Combined with CD63 Assay in Diagnosis of Patients with Suspected Platelet Dense Granule Disorder. Semin Thromb Hemost 2016;42:282-91. [Crossref] [PubMed]
- Pasalic L. Assessment of Platelet Function in Whole Blood by Flow Cytometry. Methods Mol Biol 2017;1646:349-67. [Crossref] [PubMed]
- Gross L, Aradi D, Sibbing D. Platelet Function Testing in Patients on Antiplatelet Medications. Semin Thromb Hemost 2016;42:306-20. [Crossref] [PubMed]
- Bonar RA, Lippi G, Favaloro EJ. Overview of Hemostasis and Thrombosis and Contribution of Laboratory Testing to Diagnosis and Management of Hemostasis and Thrombosis Disorders. Methods Mol Biol 2017;1646:3-27. [Crossref] [PubMed]
- Lippi G, Favaloro EJ. Laboratory hemostasis: from biology to the bench. Clin Chem Lab Med 2018;56:1035-45. [Crossref] [PubMed]
- Lippi G, Favaloro EJ. Preanalytical Issues in Hemostasis and Thrombosis Testing. Methods Mol Biol 2017;1646:29-42. [Crossref] [PubMed]
- Favaloro EJ. Optimizing the Verification of Mean Normal Prothrombin Time (MNPT) and International Sensitivity Index (ISI) for Accurate Conversion of Prothrombin Time (PT) to International Normalized Ratio (INR). Methods Mol Biol 2017;1646:59-74. [Crossref] [PubMed]
- Kershaw G. Performance of Activated Partial Thromboplastin Time (APTT): Determining Reagent Sensitivity to Factor Deficiencies, Heparin, and Lupus Anticoagulants. Methods Mol Biol 2017;1646:75-83. [Crossref] [PubMed]
- Bonar R, Favaloro EJ, Adcock D. Quality in coagulation and haemostasis testing. Biochemia Medica 2010;20:184-99. [Crossref]
- Favaloro EJ. Internal Quality Control and External Quality Assurance of Platelet Function Tests. Semin Thromb Hemost 2009;35:139-49. [Crossref] [PubMed]
- Cunningham MT, Brandt JT, Chandler WL, et al. Quality assurance in hemostasis: the perspective from the College of American Pathologists proficiency testing program. Semin Thromb Hemost 2007;33:250-8. [Crossref] [PubMed]
- Chandler WL, Brown AF, Chen D, et al. External Quality Assurance of Platelet Function Assays Results of the College of American Pathologists Proficiency Testing Program. Arch Pathol Lab Med 2018; [Crossref]
- Favaloro EJ, Bonar R. External quality assurance for the PFA-100. J Thromb Haemost 2011;9:878-80. [Crossref] [PubMed]
- Favaloro EJ, Bonar R. Proficiency testing/External quality assurance for the PFA-100. Clin Chem Lab Med 2012;50:1393-401. [Crossref] [PubMed]
- Favaloro EJ. Time for a conceptual shift in assessment of internal quality control for whole blood or cell based testing systems? An evaluation using platelet function and the PFA-100 as case example. Clin Chem Lab Med 2013;51:767-74. [Crossref] [PubMed]
- Favaloro EJ, Bonar R. External Quality Assessment/Proficiency Testing and Internal Quality Control for the PFA-100 and PFA-200: An Update. Semin Thromb Hemost 2014;40:239-53. [Crossref] [PubMed]
- Favaloro EJ, Bonar R. An update on quality control for the PFA-100/PFA-200. Platelets 2018;29:622-7. [Crossref] [PubMed]
- Hayward CP, Moffat KA, Spitzer E, et al. NASCOLA Working Group on Platelet Dense Granule Deficiency. Results of an external proficiency testing exercise on platelet dense-granule deficiency testing by whole mount electron microscopy. Am J Clin Pathol 2009;131:671-5. [Crossref] [PubMed]
- Hayward CP, Moffat KA, Raby A, et al. Development of North American Consensus Guidelines for Medical Laboratories That Perform and Interpret Platelet Function Testing Using Light Transmission Aggregometry. Am J Clin Pathol 2010;134:955-63. [Crossref] [PubMed]
- Hayward CP, Moffat KA, Plumhoff E, et al. External quality assessment of platelet disorder investigations: results of international surveys on diagnostic tests for dense granule deficiency and platelet aggregometry interpretation. Semin Thromb Hemost 2012;38:622-31. [Crossref] [PubMed]
- Chen D, Uhl CB, Bryant SC, et al. Diagnostic Laboratory Standardization and Validation of Platelet Transmission Electron Microscopy. Platelets 2018;29:574-82. [Crossref] [PubMed]
Cite this article as: Favaloro EJ. Novel approaches to quality control and external quality assessment for platelet function testing with a focus on the platelet function analyser (PFA-100 and PFA-200). Ann Blood 2019;4:3.


