
Rethinking internal quality control and external quality assessment for laboratory diagnostics of von Willebrand disease
Introduction
von Willebrand disease (VWD) is well recognised as the most common congenital bleeding disorder (1,2). Acquired forms of VWD named von Willebrand syndrome (AVWS) may also arise in a variety of disease states (3). Therefore, laboratory testing for both VWD and AVWS represents a key diagnostic activity of many hematology facilities performing advanced hemostasis diagnostics (1-4).
VWD and AVWS basically arise due to deficiency and/or defect(s) in an adhesive plasma protein called von Willebrand factor (VWF) (1,3). In turn, VWF represents a key element of primary hemostasis, and also contributes to secondary hemostasis (1,5). In summary, VWF binds to a number of other proteins, including platelet cell surface receptors [chiefly glycoprotein Ib (GPIb)], as well as sub-endothelial matrix components (especially collagen), and also to coagulation factor VIII (FVIII) (1). Binding of VWF to platelets (e.g., via GPIb) and to subendothelium (e.g., to collagen) permits the platelets to arrest at sites of vascular injury, and furthermore facilitates formation of platelet aggregates that eventually help to form a platelet plug to seal off the site of vascular injury. VWF binding to FVIII permits delivery of this coagulation factor to the site of vascular injury and thus helps facilitate secondary hemostasis (blood coagulation) (1,5,6). Plasma fibrinogen also becomes actively involved in this process, binding to platelets (chiefly via platelet GPII/IIIa, also called integrin αIIbβ3, but also via GPIb), and undergoes conversion to insoluble fibrin as a result of the coagulation (secondary hemostasis) process. Thereby, deficiency and/or defects in VWF, be these either congenital or acquired, leads to a bleeding diathesis in affected individuals.
Each molecule of VWF carries the same adhesive functionality (1). However, VWF forms into dimers of increasing size, and so the largest [high molecular weight (HMW)] forms possess the greatest overall adhesiveness, given they carry the largest number of adhesive sites in overall combination. It may be easier to think of VWF as a form of ‘sticky string’ that ties platelets to each other and also to the damaged endothelium. The larger the VWF ‘sticky string’, the more platelets that VWF can immobilise and conglomerate at the site of injury, and thus the larger and stronger the platelet plug. Thus, a deficiency of HMW VWF, even should overall VWF ‘quantity’ be in the normal range, can still lead to bleeding.
A wide variety of laboratory tests may be performed to investigate, diagnose or exclude VWD/AVWS, including screening assays, more complex functional assays, and molecular analysis (1,4). VWD testing currently incorporates a variety of processes, and potential methodologies, including enzyme linked immunosorbent assay (ELISA), latex immunoassay (LIA), other agglutination assays, chemiluminescence, and even coagulation-based testing (to measure FVIII). As the same testing procedures are used for VWD and AVWS, and both congenital and acquired forms are classified into the same disease ‘types’, subsequent detail in this review will pertain to both VWD and AVWS, and this can be simply referred to as ‘VWD diagnostics’ for plainness of message.
Internal quality control (IQC) and external quality assessment (EQA) are critical to ensuring the quality of all laboratory testing, including within VWD diagnostics (7). However, in VWD diagnostics, both IQC and EQA are made somewhat difficult by the nature and variety of the tests available, and the heterogeneity of VWD. To facilitate accurate diagnosis to assist patient management, VWD is classified into one of six types (8) (Table 1). Type 1 and 3 VWD represent quantitative deficiency (respectively partial and complete) of VWF, and any VWF present in type 1 VWD is essentially functionally ‘normal’. Qualitative defects in VWF are represented by type 2 VWD, of which there are four types. Type 2A VWD represents a form of VWD in which there is a deficiency of HMW VWF, either because of faulty production or increased clearance from blood circulation. Type 2B VWD describes a form of VWF that is functionally ‘over-active’, and binds to platelets ‘too well’, or ‘spontaneously’ without evident trigger of vascular damage. This means that VWF and platelets combine without any injury and are then cleared from circulation. Notably, as the HMW VWF forms are most adhesive, these bind best, thereby leading to loss of HMW VWF and also platelets, which sometimes manifests in patients as (mild) thrombocytopenia. Type 2N VWD reflects a form of VWF that fails to bind FVIII, and thus FVIII becomes ‘unprotected’ and is quickly degraded and removed from circulation. Thus, the characteristic feature of 2N VWD is a low level of plasma FVIII relative to VWF, and this therefore phenotypically mimics a haemophilia A. The final classification form of qualitative defect is represented within type 2M VWD, which describes qualitative defects of VWF not associated with loss of HMW or FVIII binding (i.e., qualitative defect(s) not otherwise characterised into 2A, 2B or 2N VWD).
Table 1
| VWD type | Description | Phenotypic presentation |
|---|---|---|
| 1 | Partial quantitative deficiency of VWF | Low levels of VWF, with VWF functional concordance [i.e., ratio of functional VWF/VWF:Ag approximates unity (typically >0.6)] |
| 2A | Decreased VWF-dependent platelet adhesion and a selective deficiency of high-molecular-weight (HMW) VWF multimers | Loss of HMW VWF. Usually low levels of VWF, with VWF functional discordance (i.e., ratios of RCo/Ag* and CB/Ag typically ≤0.6). |
| 2B | Increased affinity of VWF for platelet glycoprotein Ib | Low to normal levels of VWF, typically with VWF functional discordance (i.e., ratios of RCo/Ag* and CB/Ag generally ≤0.6), loss of HMW VWF and (mild) thrombocytopenia. Atypical cases may not show this pattern |
| 2M | Decreased VWF-dependent platelet adhesion without a selective deficiency of high-molecular-weight (HMW) VWF multimers | Low to normal levels of VWF, usually with VWF functional discordance detected by RCo/Ag* (generally ≤0.6), but CB/Ag ratios may be low or normal. HMW VWF present, but multimers may show other abnormalities |
| 2N | Markedly decreased binding affinity for factor VIII | Identified by VWF:FVIIIB assay, with low FVIIIB/VWF ratios |
| 3 | Virtually complete deficiency of VWF | Typically defined by VWF levels <2 U/dL and FVIII <10 U/dL |
Classification scheme derived and adapted from Sadler
The current review primarily looks at ‘VWD diagnostics’ (i.e., VWD and AVWS testing) from the perspective of IQC and EQA. The review considers standard approaches and also highlights some novel tactics to help ensure the accuracy and quality of this activity as applied to the heterogeneous methods used and also because of the heterogeneity of VWD.
What assays are used for investigating VWD and what are the problems associated with these tests?
Simplistically, VWD investigation aims to identify whether or not VWD/AVWS is present (i.e., diagnosis or exclusion). VWD testing involves measuring the level and activity of VWF, as well as associated activities. For example, FVIII testing is often performed in VWD diagnostics because VWF binds to FVIII, and thus the level of VWF is associated to the level of FVIII (i.e., the lower the VWF, the lower the FVIII). In addition, loss of FVIII represents an additional (secondary hemostasis) bleeding risk to patients that compounds the loss of VWF (representing chiefly a primary hemostasis bleeding risk). Finally, FVIII testing is important in terms of identifying 2N VWD or another bleeding disorder, haemophilia A. Typically, FVIII is assessed using one-stage clotting assays, and occasionally using chromogenic assays (9,10).
As another example of ‘associated testing’, performing platelet counts is also important in VWD diagnostics. Firstly, reduction in platelet counts represents another (primary hemostasis) bleeding risk to patients that compounds the loss of VWF (and FVIII). Secondly, a (mild) thrombocytopenia might point to type 2B VWD, or its ‘cousin’, platelet type VWD (PT-VWD) (11). The platelet count is typically normal in VWD subtypes other than type 2B and PT-VWD.
Nevertheless, most laboratory activity in VWD diagnostics is reflected by measuring VWF, both in terms of its ‘level’ [‘quantity’; achieved by assessing VWF antigen; VWF:Ag (4,12)] and VWF activity, with this being reflective of many functions (Table 2). Thus, there are assays that are able to measure VWF binding to platelets, especially to GPIb, or binding to collagen, or binding to FVIII. Binding of VWF to (platelet) GPIb is in turn reflected by a variety of assays, such as ristocetin cofactor [VWF:RCo (13); and other so-called ‘gain of function’ (GOF) assays (14,15) or GPIb-binding assays (e.g., VWF:GPIbM) (Table 2). Binding of VWF to collagen is assessed using a collagen binding assay [VWF:CB (16)], and binding of VWF to FVIII is assessed using a VWF:FVIII binding (VWF:FVIIIB) assay (17). The multimer distribution of VWF can be assessed by electrophoretic assays (18,19).
Table 2
| Abbreviation for assay | Description of assay | Comments |
|---|---|---|
| VWF:Ag | von Willebrand factor antigen | All assays that provide a quantitative level of VWF protein, be it by ELISA, LIA or other methodology |
| VWF:CB | von Willebrand factor collagen binding capacity | All assays that provide a quantitative level of VWF—collagen binding capacity, be it by ELISA or other methodology |
| VWF:RCo | von Willebrand factor ristocetin cofactor activity: all assays that use platelets and ristocetin | Historically, the only such assay type available was that based on platelet agglutination. This has changed with the advent of non-platelet-based methods |
| VWF:GPIbR | All assays that are based on the ristocetin-induced binding of von Willebrand factor to a recombinant wild type GPIb fragment | Essentially, a VWF:RCo assay that does not use platelets, and which currently comprises the IL Werfen VWF:RCo assays, as performed by either CLIA or LIA technology. These assays are essentially generate test results that are very similar to those generated using other ‘standard’ VWF:RCo assays that utilise platelets |
| VWF:GPIbM | All assays that are based on the spontaneous binding of von Willebrand factor to a gain-of-function mutant GPIb fragment | Essentially a GPIb binding assay that does not use platelets, and which currently comprises the Siemens Innovance VWF Ac assay (by LIA), as well as non-commercialised ELISA based assays. These assays essentially generate test results that are very similar to those generated using VWF:RCo assays, but do not use ristocetin in the assay |
| VWF:Ab | All assays that are based on the binding of a monoclonal antibody (MAB) to a von Willebrand factor A1 domain epitope | Essentially a VWF binding assay that utilizes a monoclonal antibody; this currently comprises the IL Werfen ‘VWF Activity’ assay (LIA), as well as ELISA based assays. These assays do not use ristocetin |
| VWF:FVIIIB | von Willebrand factor: factor VIII binding capacity | All assays that provide a quantitative level of VWF—factor VIII binding capacity, irrespective of specific methodology. Generally performed by ELISA |
Adapted from reference (
As mentioned earlier, these VWF assays may utilise a variety of methodologies, including ELISA (e.g., VWF:Ag, VWF:GPIbM, VWF:FVIIIB), LIA (e.g., VWF:Ag, VWF:GPIbM, VWF:RCo when performed as a VWF:GPIbR assay), chemiluminescence (e.g., VWF:Ag, VWF:CB, VWF:GPIbR), and platelet agglutination (VWF:RCo) (4,12-16).
For most tests of hemostasis, before patient samples can be tested, the testing process needs to be ‘controlled’ by use of IQC material that can identify whether or not the tests are working appropriately (7). Essentially, IQC chiefly assesses assay precision—i.e., assay reproducibility. IQC also helps to identify issues with sensitivity, for example low level analyte detection or failures therein. EQA alternatively represents a different activity, and largely assesses assay accuracy—i.e., assay ‘truth’.
IQC and EQA for most tests of hemostasis can typically be achieved using lyophilised or frozen plasma control material (7). In order for a laboratory to accept test results for a particular assay, IQC samples must yield test results that are within an acceptable pre-defined range of expected values. Typically, IQC is performed using commercially available (plasma) materials representing several ‘levels’ of the analyte to be controlled, usually meaning a normal sample to control test results around the normal range (or reference interval) and also a ‘pathological’ sample, to control test results above or below the normal range. For VWF testing, representing a ‘deficiency’, the ‘pathological’ control is one that yields values lower than the reference interval. In this way, IQC helps to ensure the quality of the tests that are performed.
Some recommendations around IQC and EQA in VWD diagnostics
To some extent, what specific IQC and EQA is performed for VWD investigation depends on the type of tests that are performed. At the very least, most manufacturers making VWF assays or assay kits include two levels of controls for use as IQC, with one representing a ‘normal’ control (i.e., within reference interval test values), and another reflective of ‘pathology’ (namely below reference interval test values). These manufacturer-provided controls should be included whenever performing laboratory testing using these manufacturer-provided assays/kits, and IQC should be run following manufacturer recommendations. Nevertheless, additional (non-manufacturer) controls can be recommended to be utilised in addition to the mandatory manufacturer controls (Table 3).
Table 3
| Recommended IQC | Description | Comments |
|---|---|---|
| Normal control | Normal plasma that contains normal levels of VWF for all test parameters | Easily obtained commercially. Usually included as part of a commercial method or kit. Helps control for assay issues at normal levels of VWF |
| Pathological (‘abnormal’) control | Sample that contains low levels of VWF for a particular test parameter. Essentially mimics a type 1 VWD sample | Easily obtained commercially. Usually included as part of a commercial method or kit. Can be easily created, usually as a dilution (e.g., 1:3) of a normal plasma sample. Helps control for assay issues at low levels of VWF |
| Type 2A-VWD-mimic sample | Sample that is deficient in high molecular weight VWF | Not easily obtained commercially. Not usually included as part of a commercial method or kit. Cryosupernatant may be available and suffice for particular assays. Some specialist manufacturers may be able to supply true type 2A or 2B VWD samples, although this may be expensive. Helps control assay combinations, in particular to define VWF activity/Ag ratios as ‘discordant’ (i.e., <0.6) |
| Type 3-VWD-mimic sample | Sample that is totally deficient in VWF | Not easily obtained commercially but is available from some specialist manufacturers/suppliers. Not usually included as part of a commercial method or kit. Some specialist manufacturers may be able to supply true type 3 VWD samples, although this may be expensive. Helps control VWF assays at their limit of detection (i.e., <5 U/dL), and can also help improve assays for their low VWF limit detection capacity |
| Manufacturer provided IQC | Typically provided for use within a particular assay, method, or kit | Essential. All manufacturer provided IQC should be used as specified by manufacturer. Helps to specifically control the test for which the IQC is included. May not facilitate control of other assays. Will not facilitate assessments of any change to a test or method after a change in lot (i.e., only applicable to particular lot of assay, method, or kit, as provided) |
| Non-manufacturer provided IQC | Not provided by specific manufacturer for use within their particular assay, method, or kit. Can represent material from another manufacturer/supplier, or a non-commercial material | Highly recommended. All manufacturer provided IQC represents limitations—essentially to control a particular assay or method, and typically only to control assays at normal and ‘low’ levels of VWF. Non-manufacturer specific IQC may help control other aspects of testing. For example, totally VWF deficient and HMW VWF deficient samples (as detailed above) respectively help control the limits of VWF detection and assay pairs (e.g., activity/Ag ratios). As a second example, non-manufacturer specific IQC can help control assay performance at time of batch lot changes |
For example, one additional control that can be recommended is a type 2A VWD-like sample (i.e., reduced in, or missing, HMW VWF). This is particularly useful to help assess the utility of combined procedures used to identify VWF functional defects (i.e., type 2 VWD). Thus, in type 2A VWD, representing a loss of HMW VWF, one would expect to find a reduced VWF:Ag, but also a much greater reduction in a VWF activity assay such as VWF:CB or VWF:RCo, and thus leading to low VWF:CB/Ag and VWF:RCo/Ag ratios (Table 4). Although each individual VWF assay would have their own (e.g., manufacturer provided) IQC to assess for normal and low values, a type 2A VWD-like control sample helps to control the VWF assay ‘pairs’ (e.g., VWF:Ag and VWF:CB, or VWF:Ag and VWF:RCo) in terms of identifying functional discordance via their combination as low assay ratios. Type 2A VWD-like controls are not normally provided by VWF test manufacturers. Laboratories may be able to use either previous 2A VWD patient samples, a pool of previous patient samples, or even cryosupernatant for such purpose. Cryosupernatant is often carried by blood-banks and is sometimes discarded due to ‘expiry’ or accidental non-use by clinicians’ post blood-bank issue. What-ever type 2A VWD-like control is used, it is best to have a decent pool and then to aliquot and freeze in smaller volumes to permit the use of the same control material across several assays (e.g., covering 6–12 months of VWF testing, including during cross over of different assay/kit lots).
Table 4
| VWD type | VWF:Ag | VWF:RCo* | VWF:CB | FVIII:C | Multimers | RCo/Ag* | CB/Ag* | FVIII/VWF* | Comments/additional testing** |
|---|---|---|---|---|---|---|---|---|---|
| 1 | ↓ to ↓↓ | ↓ to ↓↓ | ↓ to ↓↓ | N to ↓↓ | Normal pattern but reduced intensity | Normal | Normal | Normal | VWF levels between ~30–50 U/dL will generally not be associated with VWF mutations and may be considered as representing ‘low’ VWF as a risk factor for bleeding. VWF levels below ~30 U/dL will often be associated with VWF mutations and can be considered as representing ‘true’ type 1 VWD |
| 2A | ↓ to ↓↓ | ↓↓ to ↓↓↓ | ↓↓ to ↓↓↓ | ↓ to ↓↓ | Loss of HMW VWF | Low | Low | Normal | 2A and 2B VWD can only be distinguished by means of RIPA. Platelet type (PT-) VWD phenotypically resembles 2B VWD; these can be distinguished by means of RIPA mixing studies, or by genetic analysis of VWF and/or platelet GPIb |
| 2B | N to ↓↓ | ↓ to ↓↓↓ | ↓ to ↓↓↓ | N to ↓↓ | Loss of HMW VWF | Low | Low | Normal | |
| 2N | N to ↓↓ | N to ↓↓ | N to ↓↓ | ↓↓ to ↓↓↓ | Normal pattern | Normal | Normal | Low | Phenotypically similar to haemophilia A; distinguish using VWF:FVIII binding assay or genetic analysis of FVIII and/or VWF |
| 2M | ↓ to ↓↓ | (↓ to ↓↓↓) | (↓ to ↓↓↓) | ↓ to ↓↓ | No loss of HMW VWF; some multimer defects may be present | Low (platelet binding defect) or normal (collagen binding defect) | Low (collagen binding defect) or normal (platelet binding defect) | Normal | 2A and 2M VWD can only be distinguished by comprehensive or composite panel testing, including VWF:Ag, VWF:RCo (or GPIb binding assay), plus VWF:CB or multimer analysis |
| 3 | ↓↓↓ (absent) | ↓↓↓ (absent) | ↓↓↓ (absent) | ↓↓↓ | No VWF present | NA | NA | NA | Type 3 VWD can only be identified when VWF tests are performed and these are sensitive to very low levels of VWF |
*, other VWF GPIb binding assays (‘VWF:GPIbR’, ‘VWF:GPIbM’) will provide results that will most closely match VWF:RCo. Low assay ratios are generally identified as ≤0.6), but the actual value may depend on the locally established cut-off, which may also be assay dependent; normal assay ratios are generally identified as >0.6), but again may depend on locally established cut-offs. **, units: U/mL, U/dL, %, IU/mL and IU/dL may alternatively be used as units for VWF and FVIII:C in various publications. Australia and the USA tend to use % or U/dL, but some hemophilia centres report FVIII in U/mL. Ag, antigen; CB, collagen binding; FVIII, factor VIIII; HMW, high molecular weight (VWF); GPIb, glycoprotein Ib (the platelet VWF receptor); N, normal; NA, not applicable; RCo, ristocetin cofactor; RIPA, ristocetin induced platelet aggregation; VWF, von Willebrand factor; VWD, von Willebrand disease.
Another useful IQC could be a type 3 VWD-like sample (i.e., plasma absent in VWF). This type of control is available commercially from specialist manufacturer/suppliers and is particularly useful to help assess the lower level of assay sensitivity (or limit of detection or quantification) of VWF on a test by test basis. If this IQC sample yields values close to 0 U/dL in VWF assays, then the laboratory is using assays with good low level VWF sensitivity. If, however, the sample yields values above 5 U/dL, then the assays are showing relatively poor low VWF level sensitivity, and this will compromise VWD diagnostics. For example, such assays will have limited utility in detection/identification/discrimination of types 1, 2 and 3 VWD when the patient’s true VWF level is below 15 U/dL. Such VWF-deficient IQC samples can also be used to improve the assay’s VWF sensitivity, for example by redefining assay calibration low-curves or sample dilutions to optimise assay performance (20).
Naturally, for specific VWF assays, additional controls would also be useful. For investigation of 2N VWD, for example, a type 2N VWD-like plasma is critical. This type of control may not be available commercially and would therefore likely need to be derived from a previously well-defined patient, or a pool of such patients. For performance of VWF multimers, a variety of controls could be employed, including normal, type 1 VWD-like, type 3 VWD-like and type 2A-VWD like, depending on the patient sample types being identified by the laboratory.
As mentioned, non-manufacturer IQC samples also help define any issues with changes in lots of reagents or tests or kits. Thus, although manufacturer IQC helps control assays on a per assay basis, once a change in lot occurs, this typically means a change in IQC lots as well—thus, any change in assay performance between lots cannot be assessed unless a non-manufacturer IQC is used across the lot change.
Naturally, EQA represents additional challenges to IQC. The role of EQA is to help address methodical issues that IQC cannot identify. As mentioned, IQC helps to control assay precision (or reproducibility) and other factors such as the previously mention limits of detection. EQA helps to assess assay accuracy or ‘truth’. An assay may be precise (give the same value on repeat testing), but unless the results are accurate the assay utility is compromised. EQA assesses the performance of a given laboratory against those of other laboratories using similar or different methodologies, and thereby identifies when a laboratory or method is performing well or not.
Given the heterogeneity of VWD, an EQA should ideally provide EQA material to cover the full range of VWD defects. Like IQC, most EQA programs would have no problem providing EQA samples that reflect normal or type 1 VWD-like samples. Indeed, these could probably be easily obtained from manufacturers of IQC samples, with the type 1 VWD-like sample potentially comprising a dilution of a normal sample. However, what is missing in most commercially supplied scenarios are type 2 VWD and type 3 VWD-like samples. Whilst a type 3-like sample can also be obtained from some specialised manufacturers/suppliers as a VWF-deficient plasma, type 2 VWD-like plasma plasmas are more difficult to obtain. As mentioned, cryosupernatant is sometimes available ex-blood bank ‘discard’. Only one or two specialist manufacturers/suppliers offer type 2-VWD plasmas, and these are very expensive and difficult to source, given they require VWD patient donations. An alternative to commercial supply is to ask VWD patients directly to donate their blood for EQA purposes. However, this may be difficult for many EQA programs due to the volume of material required for a single EQA despatch, or because of disconnections between the EQA programs and the patients/clinical/hospital facilities.
Potential solutions to restrictive supply of native samples for EQA?
Whilst patient samples inevitably make an invaluable contribution to EQA, and certainly should be sourced when they represent unique material for education of laboratory participants, there is also benefit to use of artificially generated material should this be feasible. This is also more ethically just and avoids unnecessarily collections of substantial volumes of blood from patients who otherwise express a bleeding disorder. Moreover, VWD-like material produced from normal plasma can be purpose constructed in the quantity required for EQA, and to the analyte specifications required for a particular EQA requirement.
As an advisor to the Royal College of Pathologists of Australasia (RCPA) hematology (hemostasis) quality assurance program (QAP), the author has in the past developed a range of VWD-mimic samples that have now been used in this EQA program, and the results of these EQA surveys have been reported in several publications (21-30). Indeed, a range of both type 1 VWD-like and type 2A-VWD like EQA samples have been produced, with one recent pairing of EQA samples expressing a similar level of VWF:Ag (~30 U/dL), but disparate activity based levels (30). Thus, the type 1-VWD-like plasma had levels of VWF:RCo, VWF:CB, VWF:GPIbM and other VWF activity levels that were similar to VWF:Ag (~30 U/dL), and thus showed functional concordance consistent with type 1 VWD (i.e., activity/antigen assay ratios >0.6). In contrast, the type 2A-VWD-like plasma had much lower levels of VWF:RCo, VWF:CB, VWF:GPIbM and other VWF activity levels, and thus showed functional concordance consistent with type 2A VWD (30) (i.e., activity/antigen assay ratios <0.6).
Some data from the RCPAQAP reflecting comparative findings between patient samples and artificially created samples is shown in Figures 1-3.
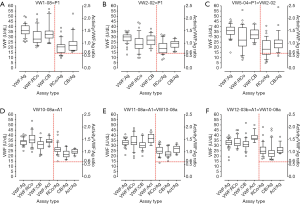
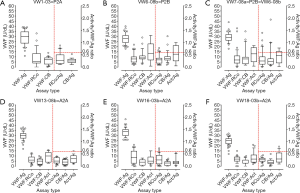
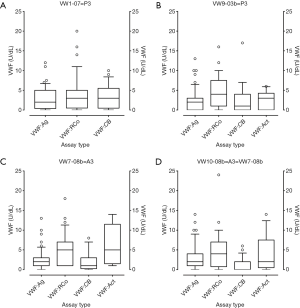
Figure 1 shows comparative findings for 3× type 1 VWD patient samples vs. one artificially created type 1 VWD-like plasma sample sent in three separate surveys. Several points can be highlighted. In both true patient samples (Figure 1A,B,C) and the artificially created samples (Figure 1D,E,F), VWF:Ag levels were similar to one another (~30 U/dL), and also similar to levels of VWF identified by activity assays (i.e., VWF:RCo, VWF:CB, and others). Thus, in all cases, VWF activity/Ag ratios were generally ‘normal’ (i.e., above 0.6). Most EQA laboratory participants report similar values to one another, as reflected by the generally narrow range of values reported, and this is the case with both true patient samples (Figure 1A,B,C) and artificially created samples (Figure 1D,E,F). On occasion, values reported are far outside the expected range of values—this is true for all sample types and reflects ‘errors’ in testing (either due to assay failure or technician failure). The values that are seen as dots on Figure 1 essential point to these situations, which in some cases reflect an assay yielding an incorrect value (assay problem) or the technician reporting the wrong value (e.g., transcription error). There are no more instances of such failures seen with artificial samples. That is, in an EQA setting, these samples essentially behave to all intended purposes like true patient samples. Also, of interest, the artificially generated samples are very stable—the three examples in Figure 1 represent the same EQA sample but separately dispatched in 2010, 2011, and 2012, and each survey yielded similar values and outcomes. Many other type 1 VWD-like artificially created samples have been produced for, and utilised by, the RCPAQAP, this author (21-30) (Table 5), with analogous conclusions drawn.
Table 5
| Year of sample despatch | True normal and VWD patient samples | Artificially created samples | Other | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Normal samples | Type 1 VWD | Type 2 VWD | Type 3 VWD | Normal | Type 1 VWD mimic | Type 2 VWD mimic | Type 3 VWD mimic | |||
| 2002 | 2 | 2 | 1 | 1 | 2 | |||||
| 2003 | 3 | 1 | 1 | 1 | 2 | |||||
| 2004 | 3 | 1 | ||||||||
| 2005 | 1 | 1 | 1 | 1 | ||||||
| 2006 | 1 | 3 | ||||||||
| 2007 | 1 | 2 | 1 | |||||||
| 2008 | 1 | 1 | 1 | 1 | ||||||
| 2009 | 1 | 1 | 2 | |||||||
| 2010 | 1 | 1 | 1 | 1 | ||||||
| 2011 | 1 | 1 | 2 | |||||||
| 2012 | 1 | 1 | 1 | 1 | ||||||
| 2013 | 1 | 2 | 1 | |||||||
| 2014 | 1 | 2 | 1 | |||||||
| 2015 | 1 | 1 | 1 | 1 | ||||||
| 2016 | 1 | 1 | 1 | 1 | ||||||
| 2017 | 2 | 2 | ||||||||
| 2018 | 1 | 1 | 1 | 1 | ||||||
RCPAQAP, Royal College of Pathologists of Australasia Quality Assurance Program; VWF, von Willebrand factor; EQA, external quality assessment; VWD, von Willebrand disease.
In similar vein, Figure 2 shows comparative findings for 3× type 2 VWD patient samples showing loss of HMW VWF vs. three different purpose created type 2A VWD-like plasma samples, with each sample sent out in a separate EQA survey. Several points can again be highlighted. In both true patient samples (Figure 2A,B,C) and the artificially created samples (Figure 2D,E,F), VWF:Ag levels were similar (~30 U/dL), and also similar to levels of VWF:Ag identified in type 1 VWD samples (either true patient or artificially created; Figure 1). However, in type 2 VWD showing loss of HMW VWF, VWF activity assays (i.e., VWF:RCo, VWF:CB, and others) yield much lower values due to this deficiency of HMW VWF. Thus, in all cases in Figure 2, VWF activity/Ag ratios were generally ‘abnormal’ (i.e., below 0.6). As per the case for type 1 VWD (Figure 1), most EQA laboratory participants report similar values to one another in type 2 VWD (Figure 2), as reflected by the generally narrow range of values reported, and this is again the case for both true patient samples (Figure 2A,B,C) and artificially created samples (Figure 2D,E,F). On occasion, values reported are far outside the expected range of values—this is true for all sample types, and again reflects ‘errors’ in testing (i.e., assay or technician failure; reflective of the dots shown in Figure 2). Again, there are no more instances of such failures seen with artificial samples (Figure 2D,E,F) compared to true patient samples (Figure 2A,B,C), and thus these samples essentially behave to all intended purposes like true patient samples in an EQA setting. Again, many other type 2A-VWD-like artificially created samples have been produced for, and utilised by, the RCPAQAP, this author (21-30) (Table 5), with analogous conclusions drawn.
Finally, Figure 3 shows comparative findings for 2× type 3 VWD patient samples vs. 2× artificial type 3 VWD-like samples, with each sent out in a separate EQA survey. Several points can again be highlighted. There is no VWF in these samples, and any test results reporting values of VWF reflect VWF assay sensitivity limitations around the lower range of detection (26). The same conclusions can be drawn for both true patient samples (Figure 3A,B) and artificially created samples (Figure 3C,D). Although there is no VWF in these samples, most participants report a low level of VWF, reflective of assay ‘noise’, and the reported values are similar for both true patient and artificial samples. Most EQA laboratory participants report values below 5 U/dL, irrespective of the VWF assay performed, but on occasion, values reported are above 5 U/dL and sometimes as high as 20–25 U/dL. This is true for all sample types—there are no more instances of such failures seen with artificial samples (Figure 3C,D) than in true patient samples (Figure 3A,B), and thus these samples essentially behave to all intended purposes like true patient samples in an EQA setting. Again, many other artificial type 3-VWD-like samples have been utilised by the RCPAQAP (21-30) (Table 5), with analogous conclusions drawn.
A summary of the survey samples sent by the RCPAQAP to EQA participants over the past 17 years is provided in Table 5. Although several interesting patient samples have been specifically utilised, artificially created samples have also been extensively used, and in general yield the same outcomes in terms of returned participant data, responses and ‘error rates’ (21-30). Thus, for all intended purposes, the artificially created samples provide near equivalence to true VWD samples in an EQA setting and can avoid the requirement to source VWD patient samples on all occasions. This is particular important considering the general disconnection of EQA programs with patients/their care givers, the scarcity of VWD sample supply, and ethical concerns of having to collect substantial volumes of blood from patients suffering from bleeding disorders.
Conclusions
IQC and EQA for VWD testing and diagnosis remains one of the more challenging aspects of hemostasis practice but can be achieved with some clever thinking. In terms of EQA for VWD, the ability to create meaningful ‘wet-challenges’ to sustain ‘proficiency testing’ is made difficult because of the need to theoretically have access to significant volumes of plasma from a variety of VWD types. Although some limited true VWD patient material can be made available, it is the author’s view that for ethical reasons patient samples should only be used for high sample volume EQA when they represent unique educational opportunities, or where artificial samples cannot be specifically ‘manufactured’ for this purpose. This would only hold true at the moment for 2M and 2N VWD (31,32). However, for general EQA use, for types 1, 2A/2B (both reflecting loss of HMW VWF) and 3 VWD, artificially created plasmas seem to work just as effectively as true patient samples (21-30) (Figures 1-3). Moreover, artificially created samples would theoretically be available in greater volume—important in an EQA setting—and can be purpose constructed to match particular specifications [i.e., VWF:Ag of a particular specified level, with functional assays such as VWF:CB, VWF:RCo, etc., of the same (=‘type 1’) or of a lower level (=‘type 2’)]. Artificial samples can also be made to reflect pre-analytical issues in VWD testing (33,34), for example reflective of filtered plasma, or cold-stored whole blood. Irrespective, ‘thinking outside the box’ is sometimes required when initiating IQC and EQA for VWD testing.
Statement of limitations and disclaimer
The opinions expressed in this review are those of the author, and not necessarily those of NSW Health Pathology or of the RCPAQAP. This manuscript reflects findings from a single EQA provider, the RCPAQAP, and the opinions of other EQA organizations may differ. For findings from some of these other EQA organizations, readers are directed to additional publications (35-39).
Acknowledgments
This review includes some data derived as a result of an ongoing collaboration with the RCPAQAP. This data is presented in summary form, and/or may derive from prior studies in which the RCPAQAP and its staff contributed, either by provision of raw data, or by prior data analysis, or by other logistical support. This EQA program is therefore acknowledged as facilitating some of the work described in this paper. Several individuals within the RCPA can also be personally acknowledged, in particular Roslyn Bonar, who has recently retired from the workforce to concentrate on life and family.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Blood for the series “External Quality Assurance”. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/aob.2019.01.03). The series “External Quality Assurance” was commissioned by the editorial office without any funding or sponsorship. EJF served as an unpaid Guest Editor of the series. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dorgalaleh A, Tabibian S, Shiravand Y, et al. von Willebrand disease. In: Dorgalaleh A. editor. Congenital Bleeding Disorders – Diagnosis and Management. New York City: Springer International Publishing, 2018.
- Favaloro EJ. Preface to Special Issue: diagnosis and management of von Willebrand disease—diverse approaches to a global and common bleeding disorder. Ann Blood 2018;3:43. [Crossref]
- Federici AB, Budde U, Castaman G, et al. Current diagnostic and therapeutic approaches to patients with acquired von Willebrand syndrome: a 2013 update. Semin Thromb Hemost 2013;39:191-201. [Crossref] [PubMed]
- Favaloro EJ, Pasalic L, Curnow J. Laboratory tests used to help diagnose von Willebrand disease: an update. Pathology 2016;48:303-18. [Crossref] [PubMed]
- Bonar RA, Lippi G, Favaloro EJ. Overview of Hemostasis and Thrombosis and Contribution of Laboratory Testing to Diagnosis and Management of Hemostasis and Thrombosis Disorders. Methods Mol Biol 2017;1646:3-27. [Crossref] [PubMed]
- Lippi G, Favaloro EJ. Laboratory hemostasis: from biology to the bench. Clin Chem Lab Med 2018;56:1035-45. [Crossref] [PubMed]
- Bonar R, Favaloro EJ, Adcock D. Quality in coagulation and haemostasis testing. Biochemia Medica 2010;20:184-99. [Crossref]
- Sadler JE, Budde U, Eikenboom JC, et al. Update on the pathophysiology and classification of von Willebrand disease: a report of the Subcommittee on von Willebrand Factor. J Thromb Haemost 2006;4:2103-14. [Crossref] [PubMed]
- Duncan E, Rodgers S. One-Stage Factor VIII Assays. Methods Mol Biol 2017;1646:247-63. [Crossref] [PubMed]
- Rodgers S, Duncan E. Chromogenic Factor VIII Assays for Improved Diagnosis of Hemophilia A. Methods Mol Biol 2017;1646:265-76. [Crossref] [PubMed]
- Othman M, Kaur H, Favaloro EJSubcommittees on von Willebrand Disease and Platelet Physiology, et al. Platelet type von Willebrand disease and registry report: communication from the SSC of the ISTH. J Thromb Haemost 2016;14:411-4. [Crossref] [PubMed]
- Favaloro EJ, Mohammed S, Patzke J. Laboratory Testing for von Willebrand Factor Antigen (VWF:Ag). Methods Mol Biol 2017;1646:403-16. [Crossref] [PubMed]
- Mohammed S, Favaloro EJ. Laboratory Testing for von Willebrand Factor Ristocetin Cofactor (VWF:RCo). Methods Mol Biol 2017;1646:435-51. [Crossref] [PubMed]
- Patzke J, Favaloro EJ. Laboratory Testing for von Willebrand Factor Activity by Glycoprotein Ib Binding Assays (VWF:GPIb). Methods Mol Biol 2017;1646:453-60. [Crossref] [PubMed]
- Favaloro EJ, Mohammed S. Towards improved diagnosis of von Willebrand disease: comparative evaluations of several automated von Willebrand factor antigen and activity assays. Thromb Res 2014;134:1292-300. [Crossref] [PubMed]
- Favaloro EJ, Mohammed S. Laboratory Testing for von Willebrand Factor Collagen Binding (VWF:CB). Methods Mol Biol 2017;1646:417-33. [Crossref] [PubMed]
- Mohammed S, Favaloro EJ. Laboratory Testing for von Willebrand Factor: Factor VIII Binding (for 2N VWD). Methods Mol Biol 2017;1646:461-72. [Crossref] [PubMed]
- Oliver S, Lau KK, Chapman K, et al. Laboratory Testing for Von Willebrand Factor Multimers. Methods Mol Biol 2017;1646:495-511. [Crossref] [PubMed]
- Favaloro EJ, Oliver S. Evaluation of a new commercial von Willebrand factor multimer assay. Haemophilia 2017;23:e373-7. [Crossref] [PubMed]
- Favaloro EJ, Mohammed S, McDonald J. Validation of improved performance characteristics for the automated von Willebrand factor ristocetin cofactor activity assay. J Thromb Haemost 2010;8:2842-4. [Crossref] [PubMed]
- Favaloro EJ, Smith J, Petinos P, et al. Laboratory testing, diagnosis, and management of von Willebrand disease. Current practice in Australasia. RCPA Quality Assurance Program in Haematology Scientific Haemostasis Advisory Panel. Am J Clin Pathol 1999;112:712-9. [Crossref] [PubMed]
- Favaloro EJ, Bonar R, Sioufi J, et al. Laboratory diagnosis of von Willebrand Disorder: Current practice in the Southern Hemisphere. Am J Clin Pathol 2003;119:882-93. [Crossref] [PubMed]
- Favaloro EJ, Bonar R, Kershaw G, et al. Laboratory diagnosis of von Willebrand Disorder: Quality and diagnostic improvements driven by peer review in a multi-laboratory test process. Haemophilia 2004;10:232-42. [Crossref] [PubMed]
- Favaloro EJ, Bonar R, Kershaw G, et al. Laboratory diagnosis of von Willebrand Disorder: Use of multiple functional assays reduces diagnostic error rates. Lab Hematol 2005;11:91-7. [Crossref] [PubMed]
- Favaloro EJ, Bonar R, Kershaw G, et al. Reducing errors in identification of von Willebrand disease: The experience of the Royal college of Pathologists of Australasia Quality Assurance Program. Semin Thromb Hemost 2006;32:505-13. [Crossref] [PubMed]
- Favaloro EJ, Bonar R, Marsden K, et al. Lower limit of assay sensitivity: an under-recognised and significant problem in von Willebrand disease identification and classification. Clin Lab Sci 2008;21:178-83. [PubMed]
- Favaloro EJ, Bonar R, Favaloro J, et al. Diagnosis and management of von Willebrand disease in Australia. Semin Thromb Hemost 2011;37:542-54. [Crossref] [PubMed]
- Favaloro EJ, Bonar RA, Meiring M, et al. Evaluating errors in the laboratory identification of von Willebrand disease in the real world. Thromb Res 2014;134:393-403. [Crossref] [PubMed]
- Favaloro EJ, Bonar R, Chapman K, et al. Differential sensitivity of von Willebrand factor 'activity' assays to large and small VWF molecular weight forms: a cross-laboratory study comparing ristocetin cofactor, collagen binding and monoclonal antibody based assays. J Thromb Haemost 2012;10:1043-54. [Crossref] [PubMed]
- Favaloro EJ, Bonar R, Hollestelle MJ, et al. Differential sensitivity of von Willebrand factor activity assays to reduced VWF molecular weight forms: a large international cross-laboratory study. Thromb Res 2018;166:96-105. [Crossref] [PubMed]
- Favaloro EJ, Thom J, Baker R, et al. Assessment of current diagnostic practice and efficacy in testing for von Willebrand's disorder: Results from the second Australasian multi-laboratory survey. Blood Coagulation Fibrinolysis 2000;11:729-37. [Crossref] [PubMed]
- Favaloro EJ, Bonar RA, Mohammed S, et al. Type 2M von Willebrand disease – more often misidentified than correctly identified. Haemophilia 2016;22:e145-55. [Crossref] [PubMed]
- Lippi G, Favaloro EJ. Preanalytical Issues in Hemostasis and Thrombosis Testing. Methods Mol Biol 2017;1646:29-42. [Crossref] [PubMed]
- Favaloro EJ, Lippi G. Pre-analytical issues that may cause misdiagnosis in haemophilia and von Willebrand disease. Haemophilia 2018;24:198-210. [Crossref] [PubMed]
- Meijer P, Haverkate F. An external quality assessment program for von Willebrand factor laboratory analysis: an overview from the European concerted action on thrombosis and disabilities foundation. Semin Thromb Hemost 2006;32:485-91. [Crossref] [PubMed]
- Kitchen S, Jennings I, Woods TA, et al. Laboratory tests for measurement of von Willebrand factor show poor agreement among different centers: results from the United Kingdom National External Quality Assessment Scheme for Blood Coagulation. Semin Thromb Hemost 2006;32:492-8. [Crossref] [PubMed]
- Hayes TE, Brandt JT, Chandler WL, et al. External peer review quality assurance testing in von Willebrand disease: the recent experience of the United States College of American Pathologists proficiency testing program. Semin Thromb Hemost 2006;32:499-504. [Crossref] [PubMed]
- Chandler WL, Peerschke EI, Castellone D, et al. von Willebrand Factor Assay Proficiency Testing, the North American Specialized Coagulation Laboratory Association Experience. Am J Clin Pathol 2011;135:862-9. [Crossref] [PubMed]
- Ledford-Kraemer MR. Analysis of von Willebrand Factor (VWF) Structure by Multimer Analysis. Am J Hematol 2010;85:510-4. [Crossref] [PubMed]
Cite this article as: Favaloro EJ. Rethinking internal quality control and external quality assessment for laboratory diagnostics of von Willebrand disease. Ann Blood 2019;4:4.


