
Hepatitis E virus and blood products
Background
Hepatitis E virus (HEV) was previously thought to be restricted to resource-poor areas in Asia/Africa (1-3). In these settings, infection is largely caused by HEV genotypes (gt) 1 and 2, which are obligate human pathogens spread oro-faecally via contaminated drinking water. This usually results in a self-limiting acute hepatitis in young adults, but there is a mortality of up to 25% in pregnant women. Cases of acute hepatitis occur sporadically, but also occasionally in outbreaks involving hundreds or thousands of cases. The largest documented outbreak (HEV gt1) was in the late 1980’s in Xinjiang province China, with approximately 120,000 cases and over 700 deaths (4). Chronic infection has not been documented with gt1 or gt2.
Our understanding of HEV has changed completely in the last few years (1-3,5). We now know that HEV is also endemic in higher income countries, caused by HEV gt3 (Europe and Japan) and gt4 (Japan and China), which are mainly zoonotic infections with pigs as the primary reservoir. Zoonotic HEV is highly infectious to pigs producing asymptomatic carriage in pig herds worldwide. In Europe, the number of laboratory-confirmed cases of HEV infection (the vast majority of which are caused by locally acquired HEV gt3) has increased rapidly over the last ten years (6), and it is estimated that there at least two million human infections with HEV each year (5). In China the epidemiology of HEV infection has changed radically over the last twenty years (2,4,7). For reasons that are not completely understood, HEV gt1 is much less common than previously and the predominant genotype affecting humans in China is now zoonotic HEV gt4 (2,7). The incidence of laboratory-confirmed cases of HEV infection in China has increased in recent years (7). As in Europe with HEV gt3, acute hepatitis E is mostly seen in older males, chronic infection can occur, and asymptomatic infection is common. In humans most infections are asymptomatic, but 1–2% develop acute self-limiting hepatitis, and occasionally liver failure (8-10). Chronic infection occurs in the immunosuppressed, including patients undergoing treatment for haematological malignancy and 50–60% of organ transplant recipients exposed to HEV gt3 (11-15). Chronic infection also occurs in the immunosuppressed with HEV gt4 but is less well documented than with gt3. Chronic infections are usually clinically silent, but untreated can cause rapidly progressive cirrhosis (2,3,14,16). The most common route of infection is by consumption of infected pork meat products, but there is increasing evidence of transmission by blood products with some well-documented adverse outcomes in recipients, including death (17-23).
HEV and the blood supply
Because zoonotic HEV infection is very common, and so commonly asymptomatic, it is no surprise that HEV has found its way into the human blood supply. It is likely that we have been unknowingly infecting our recipients with HEV for many years, as transfusion transmitted infection is usually asymptomatic (20,21). Often the only clue to the diagnosis of transfusion transmitted infection is the finding of abnormal serum liver enzymes in recipients, which may be minor and/or transitory (or occasionally absent altogether) and sometimes occurring several weeks or months after transfusion. Transfusion transmitted infection has been described in an increasing number of countries in Europe and Japan (17-23).
Satake and colleagues described 19 cases of confirmed transfusion transmitted infection in Japan (20). Seventeen patients were infected with HEV gt3 and two patients with gt4. Eleven of these cases were identified by a ‘look-back’ exercise, as at the time of transfusion the possibility of hepatitis E infection was not considered. All donors had a serum alanine aminotransferase (ALT) <60 IU/L (the national cut-off acceptable donation in Japan). Transfusion transmitted infection was caused by a range of blood products, including red blood cells (n=10), platelet concentrates (n=6) and fresh frozen plasma (n=3). The total viral load of transfused components ranged from 3.6×104 to 5.3×106 IU and the overall infectivity of HEV contaminated blood was 50%. In two cases, the donor components contained anti-HEV IgG antibodies. Only two recipients had an ALT >1,000 IU/L, and in one case the ALT was normal. There was no relationship between viral load and ALT rise. Nine of the recipients were immunosuppressed, four (44%) of whom developed chronic infection with persistent viraemia for more than 3 months. Three of these cases required anti-viral therapy with ribavirin, which resulted in successful viral clearance in two.
Hewitt and colleagues prospectively studied 225,000 blood donors for HEV in southeast England (21). The donations were tested for HEV RNA in mini pools of 24 samples. Positive mini pools were deconstructed and tested individually. Seventy-nine donations (1 in 2,848) were found to contain HEV RNA (all gt3 when sequencing was possible) with a median viral load of 3,900 IU/L (range, 50 to 2.37×106). 71% of viraemic donations were anti-HEV IgM and IgG negative. 62/129 viraemic components were given to 60 recipients, of whom 42 were available for follow up. Eighteen (42%) of these recipients had evidence of transfusion transmitted HEV infection, 12 of whom were viraemic. HEV infection was transmitted by a range of blood components, including red blood cells, platelets, fresh frozen plasma, and granulocytes. Infection was more likely in components with higher viral loads and lower anti-HEV IgG levels and in higher volume components such as fresh frozen plasma and platelets. The lowest viral dose that resulted in infection was found to be 2×104 IU and 55% of donations that contained at least this dose transmitted infection (21,24). Eight of the infected recipients were immunocompetent. The serum ALT was mildly elevated in three of these cases (range, 42–375 IU/L), only one of whom developed mild clinical hepatitis. All cases seroconverted and cleared HEV without intervention. Ten of the infected recipients were immunosuppressed and, compared to the immunocompetent infected recipients, had more prolonged viraemia and delayed seroconversion. The serum ALT was elevated in six of these cases (range, 40–1,380 IU/L). Six cases had viraemia persisting for 3 months or more, so fulfilling the current definition of chronic infection. Three of these cases required intervention to achieve viral clearance: two patients had a reduction of immunosuppression; the other case required a prolonged course of ribavirin. One patient remained viraemic at a year following transfusion.
A recent study from northern Germany (22) showed that of 18,737 donors tested 23 (1 in 814) contained HEV RNA and was genotype 3 where sequencing was possible. Only two donors had mildly elevated serum ALT. One of the donors had a period of extended viraemia (four months). Fourteen HEV contaminated blood products were given to 12 immunosuppressed and two immunocompetent recipients. One recipient went on to develop fatal acute on chronic liver failure.
Blood donor screening
As a consequence of high rates of donor viraemia and adverse outcomes in some infected recipients, universal screening of blood donors has been introduced in several European countries (Table 1) (5,19,34). This includes Ireland [2016], UK (2016/7), Netherlands [2017] and Switzerland [2018]. The UK initially started ‘targeted’ screening in 2016, i.e., selective HEV testing of donors whose blood products were destined for certain high-risk groups, including the immunosuppressed. However, in 2017 this was changed to universal screening of all donors, partly due to logistical difficulties and cost of maintaining a double inventory of donations that had been screened and those that had not (35). The initial outcomes of the screening exercise have recently been published.
Table 1
| Country | Number of blood donors HEV RNA positive | Study and date | Current status of blood donor screening for HEV |
|---|---|---|---|
| Shanghai, China | 1:808 | Chen |
No screening |
| Denmark | 1:2330 | Harritshøj |
Screening has been deemed unnecessary |
| England | 1:2848 | Hewitt |
High-risk/Universal screening since 2016-17 |
| France | 1:2218 | Gallian |
Screening of donations for pooled plasma components 2013 |
| Universal Screening under consideration | |||
| Germany | 1:1200 | Vollmer |
Voluntary screening in some areas 2018 |
| Universal screening starts 2020 | |||
| Ireland | 1:4997 | O’Riordan |
Universal screening since 2016 |
| Japan (Hokkaido) | 1:8173 | Matsubayashi |
Universal screening since 2005 |
| Netherlands | 1:2671 | Slot |
Universal screening since 2017 |
| Poland | 1:2109 | Grabarczyk |
Screening under consideration |
| Scotland | 1:2481 | Thom |
Universal screening since 2017 |
HEV, hepatitis E virus.
In Scotland 94,302 donors (33) were tested for HEV in the first 15 months of the screening programme (2016/7). Donor samples were tested for HEV RNA in mini pools of 24, and those found positive were deconstructed to determine the infected donation(s). Thirty-eight donors were found to be viraemic (1 in 2,481); 29 (76%) were anti-HEV IgM and IgG negative and only nine (25%) were IgM positive. 71% of viraemic donors were male with a median age of 47 years (range, 21–69 years). Viraemia rates found during the screening programme were fivefold higher than had previously been documented (36) in 2011 (1 in 14,520). The reasons for this significant increase in viraemic donors are uncertain.
In England 1,838,747 blood donors were tested for HEV in the first 22 months of the screening programme 2016-7 (35). 480 donations were found to contain HEV RNA (1 in 3,830) with a mean viral load of 883 IU/mL (range 1 to 3,230,000 IU/mL). All sequences belonged to gt3, except one which may represent a novel genotype. 66% of donors were unreactive for both anti-HEV IgM and IgG, and only 24% were reactive anti-HEV IgM. The rate of viraemia varied significantly throughout the study period with a peak of viraemic donors in the first few months of screening (0.69 per thousand donations), mainly due to increased rates of viraemia in younger donors <25 years of age. Following this unexplained increase, the viraemia rates settled to a constant baseline of around 0.2 per thousand donations, following a reduction in the excess numbers of viraemic younger donors. HEV viraemia was more common in male donors but there was no geographical clustering, with viraemic donors documented from all over the country. Of 334 viraemic donors where information was available 146 (44%) reported clinical symptoms before, at the time of, or following donation. Most commonly reported symptoms (in order of decreasing frequency) were fatigue, joint pain, general malaise and nausea. Only 8/334 (2%) complained of jaundice.
No case of transfusion transmitted HEV infection was reported during the donor screening period in either Scotland or England, which supports the notion that screening donors is an effective way of abolishing iatrogenic infection via blood products. However, transfusion-transmitted infection only accounts for a tiny minority of HEV infections, most of which are thought to be dietary. Modelling suggests that, at least in England, the annual risk of transfusion transmitted infection prior to donor screening exceeds the annual risk of dietary infection when more than 13 individual components are transfused (37). Despite this comparatively small risk of transfusion transmitted infection, a study from the Netherlands showed that the cost/benefit analysis of screening donors for HEV compares favourably to other existing screening procedures, e.g., for HBV, HIV and HCV (38). Transfusion transmitted HEV was estimated to cause 1 in 700 cases of hepatitis E in the general population, but 1 in 3.5 of chronic infections. Blood donor screening by PCR in mini pools of 24 was estimated to reduce the incidence of transfusion transmitted infection by 91% with a cost of Euro 310,000 per chronic case prevented (38).
HEV in blood donors in China
In a recently published study from Shanghai, 4,044 voluntary blood donors were tested for HEV (25). 19.8% and 1.1% were reactive to anti-HEV IgG and IgM respectively. Five of the donations were found to contain HEV RNA (1:808), four of which were gt4 (the fifth sample was too small for sequencing), two of whom were anti-HEV IgG negative. All viraemic donors had normal serum liver function tests. Previous studies (Figures 1,2) have shown that HEV RNA can be found in donors in many geographical locations across China (39,40). This meta-analysis showed that 58% were HEV gt1 and 42% gt4. However, some of the studies included in the meta-analysis reported data from 15 to 20 years ago, and so may not be representative of the current situation, given the recognised emergence of HEV gt4 as the predominant circulating genotype in China in recent years.
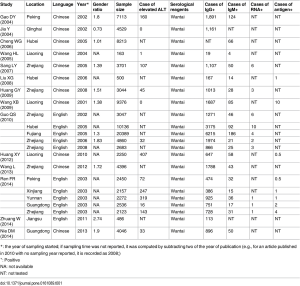
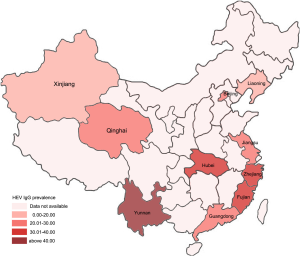
Transfusion transmitted infection has not yet been documented in China. However, in a recent report from Hong Kong, a deceased organ donor with normal liver blood tests and negative HEV serology, transmitted HEV gt4 to five organ recipients, including recipients of the heart, lung, both kidneys and liver (41). All five recipients developed chronic hepatitis, with evidence of progressive chronic liver disease in the liver transplant patient. Four patients required intervention with ribavirin to achieve viral clearance. The lung transplant patient died of unrelated causes. These observations underscore the potential iatrogenic hazard (particularly in the immunosuppressed) of using materials of human origin unknowingly infected with HEV gt4.
The above findings raise the issue of whether HEV donor screening should be considered in China. This will require a detailed cost-benefit analysis, during which the following points should be borne in mind:
- The clinical consequences of transfusion transmitted HEV are likely to be similar for HEV gt3 and gt4, but are best documented in HEV gt3 (20-22);
- HEV gt4 may result in a more severe hepatitis than gt3, with a poorer outcome (30);
- Proof of concept was established some time ago, as HEV screening was successfully introduced in blood donors in Hokkaido, Japan in 2005, an area where the predominant circulating HEV is gt4 (42);
- The incidence of HEV gt3 infection varies significantly within many countries in Europe (32,43,44). It is likely that the same also applies in China with HEV gt4, as existing data suggests that the incidence and prevalence of HEV in China also varies by geographical location (Figures 1,239,40). This means that the potential utility of donor screening is unlikely to be uniformly distributed throughout the country;
- In Europe, nucleic acid testing (NAT) is the screening tool of choice, as in viraemic donors HEV serology is often negative and liver blood tests normal (5,19,21,34).
- NAT testing is very costly (38) and other less expensive alternatives could be considered. One possibility is HEV Ag testing, which has shown to be effective at detecting individuals with high viral loads capable of causing transfusion transmitted infection. It is less sensitive at detecting lower viral loads, but these may be insufficient to infect recipients (45).
- A vaccine developed in China for HEV (HEV 239) has been shown to be safe and efficacious against HEV gt4 (46). It is currently only licensed for use in China, where uptake has been low. The possibility of vaccinating Chinese blood donors with HEV239 prior to donation to reduce the amount of HEV contaminating the blood supply merits further study.
HEV in blood products: a ‘One Health’ approach
Eliminating HEV from the human blood supply will only have a small effect on the overall incidence of human infection >95% of which is dietary in origin (37). Tackling the emerging issue of HEV in countries with zoonotic HEV gt3 and 4 requires a ‘One Health’ approach, involving multiple agencies addressing the problem together (47). This includes food safety specialists who, at least in Europe, have been relatively slow in addressing the threat of HEV to human health. A good illustration of this, of interest to blood safety specialists, is the use of pig blood (obtained at the time of slaughter) products by the food industry. These products include porcine whole blood that has been spray dried; porcine plasma prepared as a liquid, powder, or frozen; porcine haemoglobin and various serum proteins. Batches of these preparations have recently been found to contain HEV RNA (48). In Europe, these preparations are used in a range of meat products (not just pork) in the human food-chain including as colourants and meat fillers. Pig-blood products are also widely used in animal feed (both domestic and farmed animals), including as a growth promoter in pig feed (49). The clinical consequences of these potentially high risk virological practices remain to be determined (49). However, some of the porcine blood products used in the food industry in Europe originate from China, which might explain the origin of HEV gt4 infections in humans in Europe that are increasingly recognised, including a cluster of cases in Rome, Italy (49,50). This underscores that the potential threat to human health of HEV in blood products should be considered in the broadest sense possible.
Conclusions
HEV is a pathogen of global significance. Recent observations have shown high rates of viraemia in blood donors in many countries, with adverse outcome in some recipients. This has led to the introduction of HEV screening in donors of several countries, mainly in Europe. HEV also appears to be a threat to the blood supply in China, and it would seem appropriate to consider donor screening. There are a number of possible approaches to mitigate the threat of HEV in the blood supply, which ideally should be considered within a ‘One Health’ framework.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: HR Dalton has had travel and accommodation costs and consultancy fees from Roche and Wantai; travel, accommodation and lecture fees from Merck, Gilead and GFE Blut GmBh; travel and accommodation fees from the Gates Foundation and Médecins Sans Frontières; and a grant from the British Medical Association.
Ethical Statement: The author is accountable for all aspects of the manuscript and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dalton HR, Bendall R, Ijaz S, et al. Hepatitis E: an emerging infection in developed countries. Lancet Infect Dis 2008;8:698-709. [Crossref] [PubMed]
- Kamar N, Bendall R, Legrand-Abravanel F, et al. Hepatitis E. Lancet 2012;379:2477-88. [Crossref] [PubMed]
- Kamar N, Izopet J, Pavio N, et al. Hepatitis E virus infection. Nat Rev Dis Primers 2017;3:17086. [Crossref] [PubMed]
- Zhuang H, Cao XY, Liu CB, et al. Epidemiology of hepatitis E in China. Gastroenterol Jpn 1991;26:135-8. [Crossref] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on hepatitis E virus infection. J Hepatol 2018;68:1256-71. [Crossref] [PubMed]
- Adlhoch C, Avellon A, Baylis SA, et al. Hepatitis E virus: Assessment of the epidemiological situation in humans in Europe, 2014/15. J Clin Virol 2016;82:9-16. [Crossref] [PubMed]
- Ren X, Wu P, Wang L, et al. Changing Epidemiology of Hepatitis A and Hepatitis E Viruses in China, 1990 – 2014. Emerg Infect Dis 2017;23:276-9. [Crossref] [PubMed]
- Crossan CL, Simpson KJ, Craig DG, et al. Hepatitis E in patients with acute severe liver injury. World J Hepatol 2014;6:426-34. [Crossref] [PubMed]
- Dalton HR, Stableforth W, Thurairajah P, et al. Autochthonous hepatitis E in Southwest England: natural history, complications and seasonal variation, and hepatitis E virus IgG seroprevalence in blood donors, the elderly and patients with chronic liver disease. Eur J Gastroenterol Hepatol 2008;20:784-90. [Crossref] [PubMed]
- Blasco-Perrin H, Madden RG, Stanley A, et al. Hepatitis E virus in patients with decompensated chronic liver disease: a prospective UK/French study. Aliment Pharmacol Ther 2015;42:574-81. [Crossref] [PubMed]
- Kamar N, Selves J, Mansuy JM, et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med 2008;358:811-7. [Crossref] [PubMed]
- Kamar N, Mansuy JM, Cointault O, et al. Hepatitis E virus-related cirrhosis in kidney- and kidneypancreas-transplant recipients. Am J Transplant 2008;8:1744-8. [Crossref] [PubMed]
- Gérolami R, Moal V, Colson P. Chronic hepatitis E with cirrhosis in a kidney-transplant recipient. N Engl J Med 2008;358:859-60. [Crossref] [PubMed]
- Kamar N, Garrouste C, Haagsma EB, et al. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology 2011;140:1481-9. [Crossref] [PubMed]
- Versluis J, Pas SD, Agteresch HJ, et al. Hepatitis E virus: an underestimated opportunistic pathogen in recipients of allogeneic hematopoietic stem cell transplantation. Blood 2013;122:1079-86. [Crossref] [PubMed]
- Kamar N, Abravanel F, Selves J, et al. Influence of immunosuppressive therapy on the natural history of genotype 3 hepatitis-E virus infection after organ transplantation. Transplantation 2010;89:353-60. [Crossref] [PubMed]
- Colson P, Coze C, Gallian P, et al. Transfusion-associated hepatitis E, France. Emerg Infect Dis 2007;13:648-9. [Crossref] [PubMed]
- Riveiro-Barciela M, Sauleda S, Quer J, et al. Red blood cell transfusion-transmitted acute hepatitis E in an immunocompetent subject in Europe: a case report. Transfusion 2017;57:244-7. [Crossref] [PubMed]
- Domanović D, Tedder R, Blümel J, et al. Hepatitis E and blood donation safety in selected European countries: a shift to screening? Euro Surveill 2017;22:1-8. [Crossref] [PubMed]
- Satake M, Matsubayashi K, Hoshi Y, et al. Unique clinical courses of transfusion-transmitted hepatitis E in patients with immunosuppression. Transfusion 2017;57:280-8. [Crossref] [PubMed]
- Hewitt PE, Ijaz S, Brailsford SR, et al. Hepatitis E virus in blood components: a prevalence and transmission study in southeast England. Lancet 2014;384:1766-73. [Crossref] [PubMed]
- Westhölter D, Hiller J, Denzer U, et al. HEV-positive blood donations represent a relevant infection risk for immunosuppressed recipients. J Hepatol 2018;69:36-42. [Crossref] [PubMed]
- Petrik J, Lozano M, Seed CR, et al. Hepatitis E. Vox Sang 2016;110:93-130. [Crossref] [PubMed]
- Tedder RS, Tettmar KI, Brailsford SR, et al. Virology, serology, and demography of hepatitis E viremic blood donors in South East England. Transfusion 2016;56:1529-36. [Crossref] [PubMed]
- Chen X, Gong P, Wagner AL, et al. Identification of Hepatitis E Virus Subtype 4f in Blood Donors Shanghai, China. Virus Res 2019. [Epub ahead of print].
- Harritshøj LH, Holm DK, Saekmose SG, et al. Low transfusion transmission of hepatitis E among 25,637 single-donation, nucleic acid-tested blood donors. Transfusion 2016;56:2225-32. [Crossref] [PubMed]
- Gallian P, Lhomme S, Piquet Y, et al. Hepatitis E virus infections in blood donors, France. Emerg Infect Dis 2014;20:1914-7. [Crossref] [PubMed]
- Vollmer T, Diekmann J, Johne R, et al. Novel approach for detection of hepatitis E virus infection in German blood donors. J Clin Microbiol 2012;50:2708-13. [Crossref] [PubMed]
- O'Riordan J, Boland F, Williams P, et al. Hepatitis E virus infection in the Irish blood donor population. Transfusion 2016;56:2868-76. [Crossref] [PubMed]
- Takahashi M, Okamoto H. Features of hepatitis E virus infection in humans and animals in Japan. Hepatol Res 2014;44:43-58. [Crossref] [PubMed]
- Slot E, Hogema B, Riezebos-Brilman A, et al. Silent hepatitis E virus infection in Dutch blood donors, 2011 to 2012. Euro Surveill 2013;18: [Crossref] [PubMed]
- Grabarczyk P, Sulkowska E, Gdowska J, et al. Molecular and serological infection marker screening in blood donors indicates high endemicity of hepatitis E virus in Poland. Transfusion 2018;58:1245-53. [Crossref] [PubMed]
- Thom K, Gilhooly P, McGowan K, et al. Hepatitis E virus (HEV) in Scotland: evidence of recent increase in viral circulation in humans. Euro Surveill 2018;23: [Crossref] [PubMed]
- Boland F, Martinez A, Pomeroy L, et al. Blood donor screening for HEV in the European Union. Transfus Med Hemother 2019;46:95-103. [Crossref] [PubMed]
- Harvala H, Hewitt PE, Reynolds C, et al. Hepatitis E virus in blood donors in England, 2016 to 2017: from selective to universal screening. Euro Surveill 2019;24: [Crossref] [PubMed]
- Cleland A, Smith L, Crossan C, et al. HEV seroprevalence and HEV RNA presence in plasma minipools of Scottish blood donors. Vox Sang 2013;105:283-9. [Crossref] [PubMed]
- Tedder RS, Ijaz S, Kitchen A, et al. Hepatitis E risks: pigs or blood-that is the question. Transfusion 2017;57:267-72. [Crossref] [PubMed]
- de Vos AS, Janssen MP, Zaaijer HL, et al. Cost-effectiveness of the screening of blood donations for hepatitis E virus in the Netherlands. Transfusion 2017;57:258-66. [Crossref] [PubMed]
- Wang M, Fu P, Yin Y, et al. Acute, Recent and Past HEV Infection among Voluntary Blood Donors in China: A Systematic Review and Meta-Analysis. PLoS One 2016;11:e0161089 [Crossref] [PubMed]
- Zhang L, Jiao S, Yang Z, et al. Prevalence of hepatitis E virus infection among blood donors in mainland China: a meta-analysis. Transfusion 2017;57:248-57. [Crossref] [PubMed]
- Sridhar S, Cheng VCC, Wong SC, et al. Donor-Derived Genotype 4 Hepatitis E Virus Infection, Hong Kong, China, 2018. Emerg Infect Dis 2019;25:425-33. [Crossref] [PubMed]
- Matsubayashi K, Sakata H, Ikeda H. Hepatitis E infection and blood transfusion in Japan. ISBT Science Series 2011;6:242-6. [Crossref]
- Mansuy JM, Gallian P, Dimeglio C, et al. A nationwide survey of hepatitis E viral infection in French blood donors. Hepatology 2016;63:1145-54. [Crossref] [PubMed]
- Spada E, Pupella S, Pisani G, et al. A nationwide retrospective study on prevalence of hepatitis E virus infection in Italian blood donors. Blood Transfus 2018;16:413-21. [PubMed]
- Zhang F, Li X, Li Z, et al. Detection of HEV antigen as a novel marker for the diagnosis of hepatitis E. J Med Virol 2006;78:1441-8. [Crossref] [PubMed]
- Zhu FC, Zhang J, Zhang XF, et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet 2010;376:895-902. [Crossref] [PubMed]
- Mulder AC, Kroneman A, Franz E, et al. HEVnet: a One Health, collaborative, interdisciplinary network and sequence data repository for enhanced hepatitis E virus molecular typing, characterisation and epidemiological investigations. Euro Surveill 2019;24: [Crossref] [PubMed]
- Boxman IL, Jansen CCC, Hägele G, et al. Porcine Blood Used as Ingredient in Meat Productions May Serve as a Vehicle for Hepatitis E Virus Transmission. Int J Food Microbiol 2017;257:225-31. [Crossref] [PubMed]
- Dalton HR. Hepatitis E virus; pigs might fly. Cambridge Scholars, 2019, Newcastle-upon-Tyne, UK. ISBN; 1-5275-2352-7.
- Bouamra Y, Gerolami R, Arzouni JP, et al. Emergence of autochthonous infections with hepatitis E virus of genotype 4 in Europe. Intervirology 2014;57:43-8. [Crossref] [PubMed]
Cite this article as: Dalton HR. Hepatitis E virus and blood products. Ann Blood 2019;4:12.


