
The Joint Commission National Patient Safety Goals (NPSG) directing anticoagulation safety in the United States
Background
In 2002 the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) implemented initiatives for accredited institutions that provide health care services (hospitals, nursing care centers, medical centers, laboratory programs, etc.) for improving patient safety, which was enforced in January 2003 (1). The first six goals required these institutions to address and improved the identified short comings in patient misidentification, communications between caregivers, use of high-alert medications, reduction of surgical misadventures (e.g. wrong site, wrong patient, wrong procedure), use of drug infusion pumps, and effectiveness of clinical alarm systems. In 2005, JCAHO implemented a new NPSG, labeled as Goal #3c, designed to improve safety of medication use. In 2007, JCAHO was officially renamed The Joint Commission (TJC). In 2008, TJC adopted Goal #3e which was aimed to improve safety for those patients receiving anticoagulants. In 2009, the safety goals were renamed as National Patient Safety Goals (NPSG), with Goal 3e now being identified as NPSG.03.05.01. While the crux of NPSG.03.05.01 has remained, several modifications (additional requirements) have evolved since 2009, usually in concert with changes in achieving goals nationwide, identification of new safety concerns or identified concerns with newer anticoagulation options. Within institutions, select group of individuals will be charged with selected aspect of the Safety goals, which may include pharmacy to address medication, and the clinical laboratory for laboratory issues. The newly implemented 2019 NPSG.03.05.01 has eight different elements of performance (EP1 – EP8) (1,2). The purpose of this article is to review the NPSG.03.05.01 EPs and identify the pharmacy and laboratory relationships to assure patient safety during anticoagulant therapy. While laboratory services have their own specific NPSG (e.g., patient identification for collection blood samples), they will not be addressed. This document explores the eight EPs required by TJC program.
NPSG.03.05.01: EP1–8: what are they?
In 2019, six new and 2 revised EPs related to therapeutic anticoagulation safety were implemented (Table 1). The main emphasis of the 2019 NPSG.03.05.01 are protocols or guidelines using “evidence-based practice guidelines”, monitoring methods, reversal strategies, and include a new emphasis on the direct oral anticoagulants (DOACs) (1,2). TJC’s R3 report is a supplementary publication that provides a truncated version of the required EP (2). The R3 report informs the readership that guidance for the EP recommendations were based on input from the Technical Advisory Panel (TAP) and Standards Review Panel (SRP) (2). The rationale for each EP provides some insight to both panels’ thought processes, with references cited if additional information is sought. EP-4s has a focus on laboratory considerations for DOAC anticoagulation management although there is potential laboratory relationship for EP1, EP2, and EP3. EP5 addresses the process for managing adverse drug events (ADE) associated with anticoagulant therapy. The institution should consider approaches to identify potential benefits of selected laboratory measurements to guide management decisions and presence of any ADE triggers (e.g., INR >5.0 on warfarin, or tests suggesting presence of a DOAC) that would initiate follow-up through pharmacy or institutional safety committees. This could additionally fit into meeting components of EP-5.
Table 1
| Element of performance | Requirement |
|---|---|
| EP1 | The [hospital/organization] uses approved protocols and evidence-based practice guidelines for the initiation and maintenance of anticoagulant therapy that address medication selection; dosing, including adjustments for age and renal or liver function; drug-drug and drug-food interactions; and other risk factors as applicable |
| EP2 | The [hospital/organization] uses approved protocols and evidence-based practice guidelines for reversal of anticoagulation |
| EP3 | The hospital uses approved protocols and evidence-based practice guidelines for perioperative management of all patients on oral anticoagulants |
| Note: perioperative management may address the use of bridging medications, timing for stopping an anticoagulant, and timing and dosing for restarting an anticoagulant | |
| EP4 | The [hospital/organization] has a written policy addressing the need for baseline and ongoing laboratory tests to monitor and adjust anticoagulant therapy |
| Note: for all patients receiving warfarin therapy, use a current international normalized ratio (INR) to monitor and adjust dosage. For patients on a direct oral anticoagulant (DOAC), follow evidence-based practice guidelines regarding the need for laboratory testing | |
| EP5 | The [hospital/organization] addresses anticoagulation safety practices through the following: |
| Establishing a process to identify, respond to, and report adverse drug events, including adverse drug event outcomes | |
| Evaluating anticoagulation safety practices, taking actions to improve safety practices, and measuring the effectiveness of those actions in a time frame determined by the organization | |
| EP6 | The [hospital/organization] provides education to patients and families specific to the anticoagulant medication prescribed, including the following: |
| Adherence to medication dose and schedule | |
| Importance of follow-up appointments and laboratory testing (if applicable) | |
| Potential drug-drug and drug-food interactions | |
| The potential for adverse drug reactions | |
| EP7 | The [hospital/organization] uses only oral unit-dose products, prefilled syringes, or premixed infusion bags when these types of products are available |
| Note: for pediatric patients, prefilled syringe products should only be used if specifically designed for children | |
| EP8 | When heparin is administered intravenously and continuously, the [hospital/organization] uses programmable pumps to provide consistent and accurate dosing |
TJC, The Joint Commission.
Prior to anticoagulation related formulary decisions regarding anticoagulants or revisions in laboratory assay methods, the review should consider any safety issues and relevant laboratory testing. A laboratory consideration may include which test should be used is a selected situation, and describe limits of detection, whether they be lower limit of detection (LLOD) indicating drug under exposure, or upper limit of detection (ULOD) indicating drug over exposure. The laboratory should be able to equate the testing LLOD and ULOD to estimated or approximated drug levels (3). EP6 addresses patient education issues related to anticoagulation, whether it be oral (e.g., warfarin or DOACs) or subcutaneous (e.g., low molecular weight heparin, LMWH). While laboratory measurements may be required, especially with warfarin, the emphasis of EP6 is patient education. Each institution shall provide the patient or caregiver anticoagulant-specific education and should provide foreign-written instructions for patients which English is not their primary language. This may include educating the patient on need for laboratory draw and assessments as a component of their therapy. EP7 addresses the use of medication vessels of delivery, including syringes, oral unit-dose products, and recommends the use of “pre-products”, such as pre-filled syringes or pre-filled infusion bags. EP8 is strictly a pharmacy issue, as this requires the use of programmable pumps for assuring constant and accurate drug dosing and delivery. However, intergrading the anticoagulation dose (including infusion rate) and laboratory results into the electronic medical record and preferably allowing clinicians to see both within the same screen has many advantages in the assessment of therapy and fulfilling other EP targets.
NPSG.03.05.01 EP1—pharmacy + laboratory
The ambulatory healthcare program must use approved protocols and/or evidence-based practice guidelines for initiating and maintaining anticoagulant therapy. They should address drug selection (options including drug type but drug delivery such as parenteral or non-parenteral), dosing (predicated on patient-specific factors such as age, renal, and liver function) that can be individualized to each patient, concomitant drug interactions (4-6), potential food interactions (e.g., vitamin K intake on warfarin anticoagulation) (7,8), and additional risk factors (e.g., scoring models such as HAS-BLED) (9). Additionally, electronic (or equivalent) medication order sets should be considered, especially for those drugs that may require target range adjustments that adapt for specific patient care needs (adjusted targets for bleeding or thrombosis risks), changes in laboratory practice [e.g., heparin therapeutic range (HTR) change due to reagent changes], of a specific assay application to a unique anticoagulant (APTT target may be different between parenteral unfractioned heparin versus parenteral direct thrombin inhibitors). Protocols or guidelines should include specific guidance that address specific indications (e.g., venous thromboembolism, VTE), but allow flexibility and guidance for special populations (e.g., pediatric patients), specific management events (e.g., neuraxial anesthesia, surgery, trauma), and various anticoagulation reversal strategies (e.g., bleeding patient or urgent/emergent intervention required) that might occur depending on the situation.
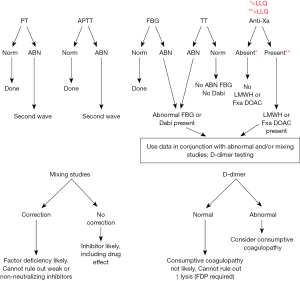
While most of the laboratory requirements will be specifically addressed for EP4, it is necessary for the laboratory and pharmacy to communicate and work with all clinical colleagues to determine how to provide services that may guide and optimize management or therapy. Drugs that are routinely monitored (such as unfractionated heparin (UFH) infusions or warfarin anticoagulation) will have laboratory monitoring requirements. However, the laboratory should be sensitive to how changes can impact protocols and guidelines such as rapid turnaround times, allowing priority in life threatening situations, or advance notification of reagent changes that would potentially result in therapeutic target changes. Usually, changes in the therapeutic target driven by assay reagents changes altering sensitivity would require adjustments in order sets that guide dosing based on laboratory results, and typically those require an approval from an oversight body within the institutions such as the Pharmacy and Therapeutics committee. Additionally, changes in electronic order sets would also require implementation via Information Technology (IT) services, which can create implementation delays. The laboratory should be aware of limitations of their methods, communicate it to clinicians utilizing assay results and provide guidance where necessary. The laboratory should also be aware of unexpected drug interactions that can alter test results [e.g., telavancin and INR prolongation (10) or DOACs on the anti-Xa for managing heparin (3,11)] or how other measurable factors that are abnormal may alter an assay [e.g., high triglyceride or total bilirubin on the anti-Xa assay (12)]. Based on the knowledge of their tests, the laboratory should provide guidance to clinicians regarding which tests would be suitable to either detect presence of an anticoagulant,, especially DOACs given the insensitivity of screening tests (prothrombin time, PT or activated partial thromboplastin time, APTT), or ability to quantitate how much anticoagulant effect is present (13-15) (Figure 1). The laboratory should also be aware of any food effect on coagulation tests that may be used to assess anti-thrombotics (anti-platelet drugs) effect such as cocoa (16,17) or polyphenols (18) or herbal supplements (19,20). Lastly, the laboratory must inform those responsible for anticoagulation or medication safety stewardship, pharmacy and clinical staff when it appears that tests are not being used appropriately. This would include, but not limited to, using insensitive tests to determine the pharmacodynamics of anticoagulants (e.g., oral anti-Xa DOACs and PT), and using tests that are inappropriate monitors of anticoagulation (APTT for warfarin therapy). However, the laboratory should engage with pharmacy and clinical staff to identify potential tests that can be used to guide management that may not be routinely used for that purpose (e.g., factor VIII or fibrinogen, FBG assay in a patient that is non-responding to escalating dose of IV UFH) (21).
NPSG.03.05.01 EP2—pharmacy + laboratory
The healthcare program must use an approved protocol using evidence-based practice guidelines for the reversal of anticoagulants, as well as managing any bleeding events attributed to anticoagulation. The policies should describe the specific reversal agent to a specific anticoagulant, as no universal reversal agent is currently available. Reversal strategies and related assay measurement strategies will be dependent on the anticoagulant involved. A grouping (PT, Anti-Xa and thrombin time) may be used to determine which anticoagulant class is present with it is unknown, and be expedited in the presence of urgent, life threatening bleeding to guide therapy. Reversal strategies and how laboratory measures are subsequently used to determine if full reversal of the anticoagulant (e.g., using an antidote specific to an anticoagulant), or other approaches (hemostatic agents) are meeting goals and when effects begin to dissipate. Examples include plasma sources (frozen plasma), blood products (platelets), drug antagonist (e.g., protamine sulfate), prothrombin complex concentrates (activated or nonactivated, three or four factor), recombinant factor VIIa, or drug specific antidote (e.g., idarucizumab). Institutions should consider whether electronic order sets for reversal agents would be beneficial for ease of use, uniformity in treatment, and expeditious antidote delivery.
The trigger for anticoagulation reversal may or may not be determined solely by laboratory results. For bleeding patients with known drug exposure, the treatment may be initiated prior to obtaining (or receiving) any laboratory value. The decision on whether to wait or treat before receiving a laboratory value is most likely dependent on the acute nature of the patient’s condition (e.g., urgency of bleeding event and site), with reversal treatment to begin immediately if the patient’s condition is critical (22-24). While reversal strategies may not be predicated on the “baseline” (pre-reversal treatment value) level, the type and/or duration of reversal treatment may be secondary to drug exposure. As such, laboratories from those sites that may receive samples from patients treated with DOACs, should have some methods in place to assist clinicians to rapidly assess (quantify) DOAC types (anti-IIa versus anti-Xa) and levels (24-26). Quantifying DOAC levels may assist in the determination of treatment duration (e.g., single dose or multiple doses), and if patient specific factors suggest that the anticoagulation regimen may result in excessive anticoagulant effects and continued bleeding risks (27).
NPSG.03.05.01 EP3—pharmacy + laboratory
The ambulatory healthcare program must use an approved protocol using evidence-based practice guidelines for the management of anticoagulated patients in the perioperative period. These protocols should consider including the use of bridging medications, timing for stopping an anticoagulant, and the timing and dosing of restarting an anticoagulant. Common perioperative events to consider would be risk for bleeding during the procedures including plans when to hold the anticoagulant, need for bridging with a short acting anticoagulant, or risks for bleeding (including use of neuraxial anesthesia). If bridging anticoagulation is required, a strategy should be in place, especially when either bridge or primary anticoagulant will interfere or cross-react with traditional monitoring methods.
In addition to providing any routine anticoagulation assessment, the laboratory should have a strategy in place for assessing bridging therapy. The most problematic bridging therapy will be between DOACs and heparins. If a laboratory uses APTT for monitoring heparin infusions, then the interference of dabigatran and to a less degree rivaroxaban, will prolong the APTT prior to UFH infusion. As such, the APTT should not be used for monitoring UFH infusion for at least 24–48 hours, depending on last DOAC exposure. For dabigatran exposure, the alternative strategy for monitoring UFH would be heparin calibrated anti-Xa. If the institution uses anti-Xa to monitor UFH infusion, then any anti-Xa DOAC (rivaroxaban, apixaban, edoxaban, and betrixaban) will affect the result. Alternative strategies to consider in these patients would be either: (I) APTT with appropriate HTR; (II) UFH calibrated thrombin time; or (III) UFH calibrated anti-IIa assay (uncommon in US) (28).
NPSG.03.05.01 EP4—pharmacy + laboratory
The ambulatory healthcare program has a written policy describing the need and frequency of laboratory tests to monitor and adjust anticoagulation therapy. This broad spectrum of tests would include traditional monitors of anticoagulation (e.g., PT and APTT for warfarin and UFH monitoring, respectively), but also other assays that may alter the pharmacodynamics or pharmacokinetics of anticoagulants (e.g., assessing renal and/or liver function). Prior to initiation of an anticoagulant, baseline values should be obtained to ascertain whether screening assays appropriate (e.g., cannot use APTT to monitor UFH infusion if elevated due to a lupus anticoagulant, or anti-Xa if a DOAC was prescribed). For heparin infusion, timing of measurements after a bolus may need to be determined to reduce influence on adjusting the infusion rate. Episodic (2–3 days) assessment of hematocrit (assess for bleeding) and platelet count (to assess for heparin induced thrombocytopenia) is needed. Whether or not to measure DOACs is controversial (27,29,30), but there may be some utility in assessing levels in select populations (e.g., obese patients) (31). For warfarin monitoring, laboratory or point-of-care test (POCT) methods have been used with success, but differences between those methods prevent their interchangeable use. Reagent changes (PT or APTT) should be transparent to the end-users (clinicians, nurses, and pharmacists). However, as APTT methods are not standardized, there may be significant changes to UFH or DTI response with APTT reagent changes. As such, the pharmacy, if the stewards for clinicians handling anticoagulation, should be engaged with the laboratory to assure they are adequately informed of reagent changes. This includes being informed of specific date and time of reagent change implementation if therapeutic targets are different and are provided the appropriate data and data analysis to interpret independently in order to inquire or rebut any laboratory assessment or recommendation.
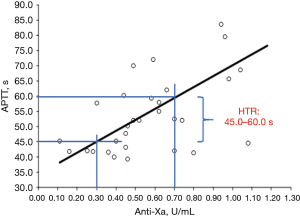
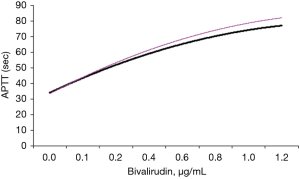
In addition to being able to provide hematology and chemistry values, the laboratory must be able to provide accurate assessment of anticoagulation. For warfarin, this would require the PT and internationalized ratio (INR), although just INR reporting would be suitable. If POCT methods are employed, they should be evaluated by the laboratory for imprecision and accuracy, prior to clinical use. Results need to be entered into the electronic record. The laboratory should be aware if their INR method is affected by lupus anticoagulants, lipoglycopeptide antibiotics, DOAC, DTIs and heparins (10-12,28,32-34). For UFH monitoring, the laboratory should use either an APTT or anti-Xa method using a heparin calibrator. For the APTT, the HTR is determined based on the correlation between anti-Xa and APTT values in patients treated with UFH (not prophylaxis). The intersection of the regression lines with 0.3–0.7 U/mL anti-Xa and corresponding APTTs is considered to be the HTR (35) (Figure 2). The HTR must be assessed with each new lot of APTT reagent using accepted practices (35,36). The laboratory must provide alternative strategies (e.g., anti-Xa or UFH calibrated thrombin time) if the baseline APTT is elevated prior to infusion. DTIs are parenteral anticoagulants (e.g., argatroban, bivalirudin) that require episodic monitoring using the APTT. The therapeutic target is often 1.5 to 2.5 times the baseline Aptt (37,38), but the sensitivity for each lot of APTT reagent may differ (Figure 3). The laboratory should provide the clinical staff comparison data for existing lot and new lot APTT responsiveness to DTIs using drug enriched plasma. As noted above, the need for DOAC measurement is controversial, but it is recommended that these drugs not be quantified using screening tests such as the PT or APTT. Additional limitations in the US being the current lack of Food and Drug Administration (FDA) approved methods (and drug specific calibrators) for quantifying the DAOC’s. Nonetheless, the current methods for measuring DOACs are rapid, linear, reproducible, and easy to adapt on coagulation analyzers (11,14,24-26,28). The laboratory should recommend trough DOAC collections, provide an expected trough range, and be able to respond to clinical inquiries about the level (26). Routinely collected random samples should be discouraged, but random samples may be collected in emergent situations (e.g., acute bleed, trauma, urgent/emergent invasive procedures, etc.). Antithrombotic therapy does not require routine measurements, but the laboratory should provide some method as to rapidly assess platelet function and guidance for result interpretation. It should be noted these rapid platelet function tests may be limited in sensitivity [e.g., PFA-100 analyzer and P2Y12 inhibitors (39)] and may not be suitable for assessing all anti-thrombotics. Viscoelastic measurements (e.g., thromboelastograph, TEG) may be suitable for some anti-thrombotic drug detection but appears to be overly sensitive to both P2Y12 and aspirin (40). Other drugs that may be infused may require assessment of efficacy, including thrombolytics and anti-thrombolytics. Efficacy of thrombolytic therapy is usually associated with rising D-dimer (as compared to pre-treatment); however, the thromboelastogram may be helpful as well. Anti-thrombolytics are used to decrease fibrinolysis but used most often to prolong clot stability. These drugs are often difficult to assess, but the TEG lysis parameter may be useful in assessing drug efficacy in a patient with fulminant fibrinolysis.
In addition to providing monitoring tools for clinicians, the laboratory should have some mechanism to rapidly assess those patients suspected with hypercoagulable disorders, acquired hemophilia, or heparin-induced thrombocytopenia (HIT). As HIT is associated with significant morbidity and mortality, the laboratory and clinical staff should create an algorithm for identifying patients suspected of HIT, incorporating pretest probability scoring and laboratory assay(s) (41-43). While the gold standard for HIT testing is considered to be the serotonin release assay, this method is cumbersome and requires radioactive labelled serotonin and method for assessing same. Rapid methods are available that can provide useful measurements in <15 min (41,44), or other methods, such as ELISA, can take a couple of hours. A clear strategy should exist between laboratory and clinical staff to: (I) identify patients suspected of HIT; (II) method for testing for HIT antibodies; and (III) plan for altering anticoagulation management. Additionally, in complex cases where UFH and DTI may be present, the laboratory should have a method for determining the prolongation of APTT is secondary to residual UFH or DTI. This can easily be achieved by performing concomitant (to APTT testing) thrombin time (TT) testing with and without protamine sulfate. As UFH and DTI both prolong the TT, only the protamine will neutralize the UFH. When the TT is no longer affected by protamine, then the prolongation of the concomitant APTT is solely secondary to DTI (assuming no other APTT related issues such as factor deficiency).
Lastly, both the clinical staff and laboratory staff need to reconfirm basic laboratory principles and their application to monitoring or measuring anticoagulant therapy. First, the PT and APTT have multiple functions (e.g., assessing factor deficiency, drug monitoring, replacement therapy, replacement efficacy) and thus they have the potential for having misleading results, secondary to preanalytical or physiological issues. On the other hand, tests such as anti-Xa or anti-IIa are designed specifically for measuring drug effect. While they can have some interferences or preanalytical biases, they are far less than those associated with the PT and APTT. Therefore, for anticoagulant monitoring, the more sensitive and specific tools we can use, the better for patient safety, although there may be insufficient clinical evidence for using these methods.
Conclusions
TJC is requiring all ambulatory healthcare programs to abide by the NPSG.03.05.01 to improve the safety to the patient undergoing anticoagulation. While the pharmacy and medication safety oversight committee may take on the leadership role in these NPSG, the laboratory is a crucial element and partner for facilitating safe and optimal use anticoagulants. With the increase use of DOACs, and the lack of laboratories performing tests to quantify these drugs is troublesome, given the relatively insensitivity of routine screening tests to these drugs (11,13,14,24). Based on the knowledge of their laboratory tests and anticoagulation, the laboratory must have mechanisms and plans in place for clinicians to use in order to identify patients at risk (e.g., unable to give history, stroke, trauma) for bleeding if any anticoagulant is on-board (15). As such, the laboratory must also take a leadership role respective to their testing, reference ranges, therapeutic ranges, and alternative strategies for monitoring patients when the routine tests are not viable options. Additionally, the laboratory should be constantly engaged with pharmacy and clinical services to determine their needs about assessing patients undergoing anticoagulant therapy. In contrast, clinicians using laboratory need to understand the laboratory test they utilize. Communication and understanding of what is unfolding at the patients care level is a critical component of providing the safe use of anticoagulants.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Emmanuel J. Favaloro) for the series “Anticoagulant and antithrombotic therapy: globally applied according to local geographical selection criteria” published in Annals of Blood. The article has undergone external peer review.
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/aob.2019.08.01). The series “Anticoagulant and antithrombotic therapy: globally applied according to local geographical selection criteria” was commissioned by the editorial office without any funding or sponsorship. Mr. Gosselin reports personal fees from Expert testimony for rivaroxaban and dabigatran testing, personal fees from Siemens Healthcare Diagnostics, personal fees from Diagnostica Grifols, personal fees from Machaon Diagnostic Laboratory, outside the submitted work. Dr. Roberts and Dr. Dager have nothing to disclose.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- The Joint Commission. Available online: https://www.jointcommission.org/facts_about_the_national_patient_safety_goals/, last accessed June 2, 2019.
- The Joint Commission R3 Report. Available online: https://www.jointcommission.org/standards_information/r3_report.aspx, last accessed, June 2, 2019.
- Gosselin RC, Francart SJ, Hawes EM, et al. Heparin-Calibrated Chromogenic Anti-Xa Activity Measurements in Patients Receiving Rivaroxaban: Can This Test Be Used to Quantify Drug Level? Ann Pharmacother 2015;49:777-83. [Crossref] [PubMed]
- Smythe MA, Fanikos J, Gulseth MP, et al. Rivaroxaban: practical considerations for ensuring safety and efficacy. Pharmacotherapy 2013;33:1223-45. [Crossref] [PubMed]
- Carpenter M, Berry H, Pelletier AL. Clinically Relevant Drug-Drug Interactions in Primary Care. Am Fam Physician 2019;99:558-64. [PubMed]
- Nguyen T, Wong E. Evaluating and assessing dabigatran drug interactions. Consult Pharm 2012;27:509-12. [Crossref] [PubMed]
- Yamreudeewong W, Henann NE, Fazio A, et al. Drug-food interactions in clinical practice. J Fam Pract 1995;40:376-84. [PubMed]
- Booth SL, Centurelli MA. Vitamin K. a practical guide to the dietary management of patients on warfarin. Nutr Rev 1999;57:288-96. [Crossref] [PubMed]
- Zhu W, He W, Guo L, et al. The HAS-BLED Score for Predicting Major Bleeding Risk in Anticoagulated Patients With Atrial Fibrillation: A Systematic Review and Meta-analysis. Clin Cardiol 2015;38:555-61. [Crossref] [PubMed]
- Gosselin R, Dager W, Roberts A, et al. Effect of telavancin (Vibativ) on routine coagulation test results. Am J Clin Pathol 2011;136:848-54. [Crossref] [PubMed]
- Gosselin RC, Gosselin R, Douxfils J, et al. Clinical Pearls: Laboratory assessments of Direct Oral Anticoagulants (DOACs). Hamostaseologie 2017; [Epub ahead of print]. [PubMed]
- Vandiver JW, Vondracek TG. Antifactor Xa levels versus activated partial thromboplastin time for monitoring unfractionated heparin. Pharmacotherapy 2012;32:546-58. [Crossref] [PubMed]
- Adcock DM, Gosselin RC. The danger of relying on the APTT and PT in patients on DOAC therapy, a potential patient safety issue. Int J Lab Hematol 2017;39:37-40. [Crossref] [PubMed]
- Douxfils J, Gosselin RC. Laboratory Assessment of Direct Oral Anticoagulants. Semin Thromb Hemost 2017;43:277-90. [Crossref] [PubMed]
- Gosselin RC, Adcock DM. The laboratory's 2015 perspective on direct oral anticoagulant testing. J Thromb Haemost 2016;14:886-93. [Crossref] [PubMed]
- Pearson DA, Paglieroni TG, Rein D, et al. The effects of flavanol-rich cocoa and aspirin on ex vivo platelet function. Thromb Res 2002;106:191-7. [Crossref] [PubMed]
- Paglieroni TG, Janatpour K, Gosselin R, et al. Platelet function abnormalities in qualified whole-blood donors: effects of medication and recent food intake. Vox Sang 2004;86:48-53. [Crossref] [PubMed]
- Rein D, Paglieroni TG, Pearson DA, et al. Cocoa and wine polyphenols modulate platelet activation and function. J Nutr 2000;130:2120S-6S. [Crossref] [PubMed]
- Tabeshpour J, Hashemzaei M, Sahebkar A. The regulatory role of curcumin on platelet functions. J Cell Biochem 2018;119:8713-22. [Crossref] [PubMed]
- de Pascual-Teresa S, Moreno DA, García-Viguera C. Flavanols and anthocyanins in cardiovascular health: a review of current evidence. Int J Mol Sci 2010;11:1679-703. [Crossref] [PubMed]
- Thota R, Ganti AK, Subbiah S. Apparent heparin resistance in a patient with infective endocarditis secondary to elevated factor VIII levels. J Thromb Thrombolysis 2012;34:132-4. [Crossref] [PubMed]
- Dager WE, Roberts AJ, Nishijima DK. Effect of low and moderate dose FEIBA to reverse major bleeding in patients on direct oral anticoagulants. Thromb Res 2019;173:71-6. [Crossref] [PubMed]
- Dager W, Hellwig T. Current knowledge on assessing the effects of and managing bleeding and urgent procedures with direct oral anticoagulants. Am J Health Syst Pharm 2016;73:S14-26. [Crossref] [PubMed]
- Gosselin RC, Adcock DM, Bates SM, et al. International Council for Standardization in Haematology (ICSH) Recommendations for Laboratory Measurement of Direct Oral Anticoagulants. Thromb Haemost 2018;118:437-50. [Crossref] [PubMed]
- Tripodi A. To measure or not to measure direct oral anticoagulants before surgery or invasive procedures J Thromb Haemost 2016;14:2559-61. reply. [Crossref] [PubMed]
- Tripodi A, Marongiu F, Moia M, et al. The vexed question of whether or not to measure levels of direct oral anticoagulants before surgery or invasive procedures. Intern Emerg Med 2018;13:1029-36. [Crossref] [PubMed]
- Tripodi A, Ageno W, Ciaccio M, et al. Position Paper on laboratory testing for patients on direct oral anticoagulants. A Consensus Document from the SISET, FCSA, SIBioC and SIPMeL. Blood Transfus 2018;16:462-70. [PubMed]
- Gosselin RC, Adcock DM, Douxfils J. An update on laboratory assessment for direct oral anticoagulants (DOACs). Int J Lab Hematol 2019;41:33-39. [Crossref] [PubMed]
- Samuelson BT, Cuker A, Siegal DM, et al. Laboratory Assessment of the Anticoagulant Activity of Direct Oral Anticoagulants: A Systematic Review. Chest 2017;151:127-38. [Crossref] [PubMed]
- Witt DM, Nieuwlaat R, Clark NP, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv 2018;2:3257-91. [Crossref] [PubMed]
- Moll S, Crona DJ, Martin K. Direct oral anticoagulants in extremely obese patients: OK to use? Res Pract Thromb Haemost 2018;3:152-5. [Crossref] [PubMed]
- Dager WE, Gosselin RC, Kitchen S, et al. Dabigatran effects on the international normalized ratio, activated partial thromboplastin time, thrombin time, and fibrinogen: a multicenter, in vitro study. Ann Pharmacother 2012;46:1627-36. [Crossref] [PubMed]
- Gosselin RC, King JH, Janatpur KA, et al. Effects of pentasaccharide (fondaparinux) and direct thrombin inhibitors on coagulation testing. Arch Pathol Lab Med 2004;128:1142-5. [PubMed]
- Gosselin RC, Dager WE, King JH, et al. Effect of direct thrombin inhibitors, bivalirudin, lepirudin, and argatroban, on prothrombin time and INR values. Am J Clin Pathol 2004;121:593-9. [Crossref] [PubMed]
- Marlar RA, Clement B, Gausman J. Activated Partial Thromboplastin Time Monitoring of Unfractionated Heparin Therapy: Issues and Recommendations. Semin Thromb Hemost 2017;43:253-60. [PubMed]
- Olson JD, Arkin CF, Brandt JT, et al. College of American Pathologists Conference XXXI on laboratory monitoring of anticoagulant therapy: laboratory monitoring of unfractionated heparin therapy. Arch Pathol Lab Med 1998;122:782-98. [PubMed]
- Argatroban prescribing information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/020883s014lbl.pdf, last accessed June 2, 2019.
- Bivalirudin (Angiomax) prescribing information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/020873s037lbl.pdf, last accessed June 2, 2019.
- Lordkipanidzé M, Pharand C, Nguyen TA, et al. Comparison of four tests to assess inhibition of platelet function by clopidogrel in stable coronary artery disease patients. Eur Heart J 2008;29:2877-85. [Crossref] [PubMed]
- Gosselin RC, Estacio EE, Song JY, et al. Verifying the performance characteristics of the TEG5000 thromboelastogram in the clinical laboratory. Int J Lab Hematol 2016;38:183-92. [Crossref] [PubMed]
- Warkentin TE. Laboratory diagnosis of heparin-induced thrombocytopenia. Int J Lab Hematol 2019;41:15-25. [Crossref] [PubMed]
- Cuker A, Arepally GM, Chong BH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv 2018;2:3360-92. [Crossref] [PubMed]
- Warkentin TE. Clinical picture of heparin-induced thrombocytopenia (HIT) and its differentiation from non-HIT thrombocytopenia. Thromb Haemost 2016;116:813-22. [Crossref] [PubMed]
- Warkentin TE, Sheppard JI, Linkins LA, et al. High sensitivity and specificity of an automated IgG-specific chemiluminescence immunoassay for diagnosis of HIT. Blood 2018;132:1345-9. [Crossref] [PubMed]
Cite this article as: Gosselin RC, Roberts AJ, Dager WE. The Joint Commission National Patient Safety Goals (NPSG) directing anticoagulation safety in the United States. Ann Blood 2019;4:21.


