
A review of 10 years of data from an external quality assurance program for antiphospholipid antibodies: no evidence for improved aCL and β2GPI assay standardization
Introduction
The antiphospholipid syndrome (APS) is characterized by the occurrence of vascular thromboses (arterial and/or venous) and/or pregnancy morbidity, in the presence of antiphospholipid antibodies (aPL) (1). Recently, there has been an expansion in the repertoire of novel antibodies that have been proposed to identify APS patients, including anti-prothrombin/phosphatidyl serine (aPT/PS) antibodies (2). Despite this, the mainstay of diagnostic laboratory testing and the only assays included in the latest 2006 APS Classification Criteria remains the identification of anticardiolipin (aCL) and anti-β2-glycoprotein I (aβ2GPI) antibodies, in addition to clot-based tests for lupus anticoagulant (LA) (1).
Previous attempts to ensure standardization across these assays, especially in regard to enzyme-linked immunosorbent assays (ELISAs) for aCL and aβ2GPI antibodies have included international workshops, Consensus Guidelines and the formation of Working Parties including the Australasian Anticardiolipin Working party and the College of American Pathologists Working Group (1,3-9). In addition to this, polyclonal IgG and IgM calibrators for aβ2GPI antibodies have recently been developed (10,11). Despite these initiatives, there remains ongoing issues with assay reproducibility and standardization (12-18). This variation limits the clinical utility of these assays.
The high degree of variation and subsequent requirement for interpretation of these assays are highlighted by results reported in External Quality Assurance (EQA) programs, as well as in previous publications using such data or other cross laboratory data (12-15,18). For more than 20 years the Royal College of Pathologists of Australasia Quality Assurance Programs (RCPAQAP) has been performing an EQA for IgG and IgM aCL and IgG aβ2GPI antibodies. Herein, we provide an updated review of data obtained as part of this program, which demonstrates ongoing variation in the reporting of aPL antibodies in the period 2009–2018, and thus indicates a limited impact of the current International Consensus Guidelines to improve the standardization of results reported for aCL and aβ2GPI over this time period.
Methods
For the IgG and IgM aCL and IgG aβ2GPI antibodies program, donor samples are collected from a single source (individual patient) with a clinical history consistent with the diagnosis of APS, or in the case of negative samples, collection is from patients with no history of autoimmune disease. Samples are stored at −80 °C before being aliquoted into 500 µL vials and shipped to participating laboratories, where they are stored at −20 °C before analysis. Approximately 70 laboratories participate in this program, with all reporting IgG aCL antibodies, an average of 54 reporting IgM aCL antibodies and 37 reporting IgG aβ2GPI antibodies. Results of each sample, including the methodology, kit and manufacturer were submitted to the RCPAQAP by participants through an online portal.
Each aPL EQA module consisted of 8 samples sent on an annual basis (80 samples over a 10-year period) to be tested throughout the year, and included a range of low to high samples and an average of one negative sample per year. Reported methodology, consensus of results against the target set by RCPAQAP, and the number of laboratories reporting semi-quantitative results were analysed over a ten-year period (2009–2018) to identify changes to laboratory testing and reporting procedures during this time.
Data was analysed using linear regression, one-way ANOVA and Kruskal-Wallis multiple comparison tests in Prism™ v 8.0 statistical software (GraphPad, San Diego, CA). A P value of <0.05 was considered significant.
Results
Changes in methodologies
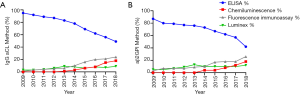
An analysis of results reported to the RCPAQAP between 2009 and 2018 demonstrated a marked shift in the methodologies used for the detection of IgG aCL (Figure 1A) and IgG aβ2GPI (Figure 1B). In 2009, ELISA methodology was used by >90% of participants; however, over the 10-year evaluation period, there has been a steady trend for laboratories to switch to non-ELISA based methodologies, in particular to chemiluminescence, fluorescence immunoassay and Luminex based techniques. Laboratories who also reported IgM aCL antibodies always used the same methodology for both IgG and IgM aCL assays.
Concordance of qualitative results
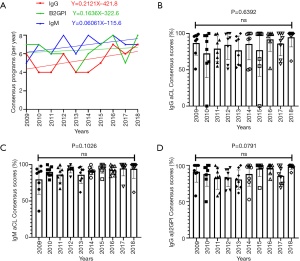
Consensus (as defined by ≥80% concordance in reporting “negative” or “positive” for a sample) did not significantly change across the 10-year period for all tests (IgG and IgM aCL and IgG aβ2GPI) when analysed using linear regression (Figure 2A). When concordance data for individual programs was analysed using a one-way ANOVA across the years 2009–2018, the results indicate no significant variation of consensus reporting across all tests and programs (Figure 2B,C,D), even when accounting for the change in methods (Figure 1) and proportion of qualitative reporting (Figure 3).
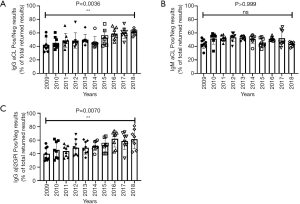
Reporting of semi-quantitative results
An interesting finding from this data was the significant increase in the proportion of laboratories returning qualitative results (i.e., positive or negative) as opposed to semi quantitative for IgG aCL (Figure 3A) (2009=42.5±10.5, 2018=61.4±4.1, P=0.0036) and IgG aβ2GPI antibodies (Figure 3C) (2009=39.9±10.0, 2018=61.6±14.0, P=0.007), indicating a noteworthy shift in results reporting. No significant change was noted for IgM aCL antibodies (Figure 3B) (2009=43.8±7.1, 2018=43.7±3.4, P>0.999); however, there were more “negative” samples for IgM aCL antibodies over the testing period, which influenced the number of semi-quantitative results reportable.
This correlates with the change in methods technologies (Figure 1) and is likely related to manufacturer recommendations, which often have not been validated for semi-quantitative reporting (as defined by the 2012 International Consensus Guidelines on Anticardiolipin and Anti-Beta2-Glycoprotein Testing) (3). In addition, semi-quantitative ranges have never been clearly defined for aβ2GPI testing, and therefore any definition of these ranges would be essentially on an arbitrary basis.
Discussion
We present data submitted to the RCPAQAP antiphospholipid antibody program over a 10-year period and demonstrate a sizeable shift in methodologies away from ELISA and towards chemiluminescence, fluorescence immunoassay and Luminex for both IgG and IgM aCL and IgG aβ2GPI antibodies (Figure 1). This shift introduces new challenges for standardization, including the introduction of new reporting units (such as chemiluminescent units or CUs), varied detection limits (including increases in the dynamic detection range for the newer methods compared with ELISA-based methods), non-linearity in some methodologies and multiple cut-off values for the detection of ‘positive’ samples. This shift away from a single method of antibody detection would be expected to lead to further disparities in assay repeatability and validity between methods, although intra-assay reproducibility with newer methods may be improved compared to historical ELISA assays.
Recent findings support the comparative performance of several aCL and IgG aβ2GPI antibody detection kits and their correlation with particular APS clinical manifestations (17). However, the results presented here do not demonstrate any significant improvement in the consensus obtained for representative samples for any aPL assay. Without the uniform adoption of a validated reference standard(s) that has also been proven to be transferable across different methodologies (i.e., on ELISA, chemiluminescence, fluorescence immunoassay and Luminex methodologies), by all manufacturers, it appears unlikely that improved consensus in aPL results will be achievable.
Amongst recommendations from the 2012 International Consensus Guidelines on Anticardiolipin and Anti-β2‐glycoprotein I Testing: Report from the 13th International Congress on Antiphospholipid Antibodies, was the endorsement that laboratories should use semi-quantitative (low/moderate/strong) reporting for positive results (3). This is in recognition of the high predictive value of strongly positive results (defined as >99th percentile of the reference population) and the low specificity of results close to assay cut-off values, especially for IgM assays (16,17). This analysis of EQA submissions demonstrates that more laboratories are moving away from reporting semi-quantitative results and instead reporting qualitative (positive/negative) determinations only (Figure 3). This is likely to be a consequence of laboratories aligning themselves with manufacturer’s recommendations for result reporting, as some of the newer aCL assays have not be validated for semi-quantitative reporting, along with the absence of defined semi-quantitative ranges for aβ2GPI results.
While the limitation of the analysis presented here is the relatively small number of patient samples (80 in total), a strength is the large number of laboratories reporting on each sample, and the fact that these results are representative of “real world” diagnostic findings. In addition, samples selected for the program included a large range of values across clinically important ranges.
Conclusions
This review of EQA data of IgG/IgM aCL and IgG aβ2GPI antibodies demonstrates that despite concerted efforts by a number of international groups to improve standardization across these assays, there is no evidence to support that these efforts has translated to improvements in the consistency of results from diagnostic laboratories enrolled in the RCPAQAP antiphospholipid program.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Blood for the series “External Quality Assurance”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/aob.2019.10.01). The series “External Quality Assurance” was commissioned by the editorial office without any funding or sponsorship. The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the declaration of Helsinki (as revised in 2013). The institutional ethical approval and individual informed consent were waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006;4:295-306. [Crossref] [PubMed]
- Jaskowski TD, Wilson AR, Hill HR, et al. Autoantibodies against phosphatidylserine, prothrombin and phosphatidylserine–prothrombin complex: identical or distinct diagnostic tools for antiphospholipid syndrome? Clin Chim Acta 2009;410:19-24. [Crossref] [PubMed]
- Lakos G, Favaloro EJ, Harris EN, et al. International consensus guidelines on anticardiolipin and anti–β2‐glycoprotein I testing: Report from the 13th International Congress on Antiphospholipid Antibodies. Arthritis Rheum 2012;64:1-10. [Crossref] [PubMed]
- Pierangeli SS, Harris EN. A protocol for determination of anticardiolipin antibodies by ELISA. Nat Protoc 2008;3:840-8. [Crossref] [PubMed]
- Wong RC, Gillis D, Adelstein S, et al. Consensus guidelines on anti-cardiolipin antibody testing and reporting. Pathology 2004;36:63-8. [Crossref] [PubMed]
- Wong RC, Favaloro EJ, Adelstein S, et al. Consensus guidelines on anti-2 glycoprotein I testing and reporting. Pathology 2008;40:58-63. [Crossref] [PubMed]
- Wong RC, Adelstein S, Gillis D, Favaloro EJ. Development of consensus guidelines for anticardiolipin and lupus anticoagulant testing. Semin Thromb Hemost 2005;31:39-48. [Crossref] [PubMed]
- Tincani A, Allegri F, Balestrieri G, et al. Minimal requirements for antiphospholipid antibodies ELISAs proposed by the European Forum on Antiphospholipid Antibodies. Thromb Res 2004;114:553-8. [Crossref] [PubMed]
- Reber G, Tincani A, Sanmarco M, et al. Proposals for the measurement of anti-2glycoprotein I antibodies: Standardization Group of the European Forum on Antiphospholipid Antibodies. J Thromb Haemost 2004;2:1860-2. [Crossref] [PubMed]
- Willis R, Grossi C, Orietta Borghi M, et al. International standards for IgG and IgM anti-β2glycoprotein antibody measurement. Lupus 2014;23:1317-9. [Crossref] [PubMed]
- Pierangeli SS, Favaloro EJ, Lakos G, et al. Standards and reference materials for the anticardiolipin and anti-β(2)glycoprotein I assays: A report of recommendations from the APL Task Force at the 13th International Congress on Antiphospholipid Antibodies. Clin Chim Acta 2012;413:358-60. [Crossref] [PubMed]
- Favaloro EJ, Silvestrini R, Mohammed A. Clinical Utility of Anticardiolipin Antibody Assays: High inter-laboratory variation and limited consensus by participants of external Quality Assurance Programs signals a cautious approach. Pathology 1999;31:142-7. [Crossref] [PubMed]
- Favaloro EJ, Silvestrini R. Assessing the utility of anticardiolipin antibody assays: A cautious approach is suggested by high variation and limited consensus in multi-laboratory testing. Am J Clin Path 2002;118:548-57. [Crossref] [PubMed]
- Favaloro EJ, Wong R, Silvestrini R, et al. A multi-laboratory peer-assessment quality assurance program based evaluation of anti-cardiolipin antibody, and beta-2-glycoprotein-1 antibody, testing. Semin Thromb Hemost 2005;31:73-84. [Crossref] [PubMed]
- Favaloro EJ, Wheatland L, Jovanovich S, et al. Internal quality control and external quality assurance in testing for antiphospholipid antibodies: Part I – anti-cardiolipin and anti-β2-glycoprotein I antibodies. Semin Thromb Hemost 2012;38:390-403. [Crossref] [PubMed]
- Montaruli B, De Luna E, Mengozzi G, et al. Anti-cardiolipin and anti-β2-glycoprotein I antibodies: normal reference ranges in northwestern Italy. Lupus 2012;21:799-801. [Crossref] [PubMed]
- Willis R, Pierangeli SS, Jaskowski TD, et al. Performance characteristics of commercial immunoassays for the detection of IgG and IgM antibodies to β2 glycoprotein I and an initial assessment of newly developed reference materials for assay calibration. Am J Clin Path 2016;145:796-805. [Crossref] [PubMed]
- Wong R, Favaloro E, Pollock W, et al. A multi-centre evaluation of the intra-assay and inter-assay variation of commercial and in-house anti-cardiolipin antibody assays. Pathology 2004;36:182-92. [Crossref] [PubMed]
Cite this article as: Wienholt LA, Richardson A, Wong RC, Chapman K, Lee FJ. A review of 10 years of data from an external quality assurance program for antiphospholipid antibodies: no evidence for improved aCL and β2GPI assay standardization. Ann Blood 2019;4:27.


