
The efficacy of HBsAg detection using electro-chemiluminescence immunoassay for blood donor screening in China
Introduction
One of the major risks of blood and blood component infusion is the presence of infectious diseases, especially hepatitis B virus (HBV), which causes inflammation of the liver and multiple organ damage (1-5). According to World Health Organization, approximately 257 million people were living with HBV infection, only 10.5% of whom (27 million) were aware of their infection (6). It is reported that hepatitis B complications, including cirrhosis and hepatocellular carcinoma, killed 880,000 people (7-11).
Main transmission routes of HBV include exposure to infected blood or blood products, mother-to-child, and sexual contact (1,3-5). HBsAg is a serological marker of acute and chronic HBV infection and a common marker for screening (1-4). The level of HBsAg not only indicates active hepatitis B infection, but also predicts clinical and therapeutic effects. Therefore, the current blood centers internationally require HBsAg testing for blood donors (1-3,5).
At present, only enzyme-linked immunosorbent assay (ELISA) method was permitted for HBsAg screening in blood donors in China. The advantages of ELISA methods include simple operation and low cost, and it is suitable for high throughput testing of samples. However, because ELISA uses an open detection system, the detection performance is easily affected by factors during the operation process, such as assay preparation, and culture time, etc. (12-14).
Chemiluminescence immunoassay (CLIA) has been used for HBsAg screening in blood donor around the world (15-20). Some studies have compared the sensitivity for HBsAg testing using CLIA and ELISA methods (21). However, the data for the efficacy of HBsAg screening using CLIA for blood donor in HBV infection high endemic region is rare. Currently, the prevalence of HBsAg is 5.4–6.8% in the Chinese population (22). In this study, the sensitivity and specificity of blood donor HBsAg testing, using electro-CLIA (ECLIA) and ELISA methods were analyzed and compared, with samples from regular Chinese blood donors.
Methods
Study design
All regular blood donors received a pre-donation screening, according to standard of blood donation in China. A rapid pre-donation testing for HBsAg was done according to manufacturer’s instruction (colloidal gold strip method, Intec Company, Xiamen, China), and positive result was deferred for donation. A total of 5,021 samples from regular blood donors were tested for HBsAg with ECLIA (Elecsys® HBsAg II, Roche-diagnostics, Mannheim, Germany; defined as Elecsys), a domestic ELISA (Intec Company, Xiamen, China, defined as ELISA 1) and an imported ELISA (Bio-Rad, Marne la Cocquette, France, defined as ELISA 2), respectively. The analytical sensitivity (limit of detection) by testing the WHO 2nd International standard NIBSC code 00/588 was estimated ≤0.1 IU/mL for Elecsys and <0.130 IU/mL for ELISA 2.
The study was approved by ethical committee at the Blood Center of Zhejiang Province (ZJB2018001), and conducted in full compliance with the principles of the Helsinki Declaration and national regulations.
Total precision analysis
The commercial quality control (QC) sample (0.2 IU/mL, Beijing Controls & Standards Co., Ltd, Beijing, China) was used to analyze the precision data of the 3 assays. The QC samples were routinely tested using the 3 assays as above described. About 55 QC tests were conducted for 5,021 sample testings. QC frequency was kept the same for both ECLIA and ELISAs. The stability of the assays was evaluated by calculating the total precision of the QC results.
Evaluate the repeatability of the methods
All initial screening reactive samples were retested in duplicate. If the results of both retests were negative, the sample was defined as negative. If one or both retests was reactive, the sample was defined as reactive. The repeatedly reactive rates were calculated to evaluate the repeatability of the methods.
Confirmed positive or negative results
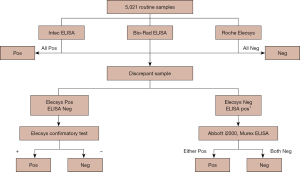
Results of the samples were classified as confirmed positive or negative, following the confirmatory algorithm (Figure 1). In the confirmation procedure, supplementary tests for HBsAg were performed with a CLIA (ARCHITECT HBsAg, Abbott, USA) and an ELISA method (Abbott Murex, Dartfort, UK) (Figure 1). In addition, nucleic acid amplification test was done for all samples, using Roche Cobas AmpliPrep with real-time PCR on the Cobas TaqMan analyzer (Roche Diagnostics, Mannheim, Germany). Referring to the confirmation results, the screening sensitivity and specificity of Elecsys and two ELISAs were calculated, respectively.
Results
The confirmed positive or negative results
A total of 5,021 samples were tested. Fourteen samples were tested consistently reactive by all 3 assays and 4,991 samples were consistently non-reactive. Discrepant results occurred in 16 samples. After all confirmation tests, 23 samples were confirmed positive and 4,998 were confirmed negative. The specificity and sensitivity of the 3 assays were summarized in Table 1.
Table 1
| Assay | Confirmed positive (n=23) | Confirmed negative (n=4,998) | Result (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| Pos | Neg | Neg | Pos | Sensitivity (%) | Specificity (%) | |||
| ELISA 1 | 17 | 6 | 4,996 | 2 | 73.91 (51.59–89.77) | 99.96 (99.86–100.00) | ||
| ELISA 2 | 15 | 8 | 4,993 | 5 | 65.22 (42.73–83.62) | 99.90 (99.77–99.97) | ||
| Elecsys | 23 | 0 | 4,998 | 0 | 100.00 (85.18–100.00) | 100.00 (99.93–100.00) | ||
ELISA 1: HBsAg ELISA from Intec Company, China. ELISA 2: HBsAg ELISA from BIO-RAD Company, France. Elecsys: Elecsys® HBsAg II (ECLIA) from Roche Diagnostics, Germany.
The rate of the initial reactive and repeatedly reactive
The results of the initial reactive and repeatedly reactive are summarized in Table 2. The percentage of initial reactive samples that were tested repeatedly reactive for Elecsys was 100%.
Table 2
| Assay | INR | IR | RR | RR% in IR |
|---|---|---|---|---|
| ELISA1 | 4,999 | 22 | 19 | 86.36 |
| ELISA 2 | 4,999 | 22 | 20 | 90.91 |
| Elecsys | 4,998 | 23 | 23 | 100.00 |
ELISA 1: HBsAg ELISA from Intec Company, China. ELISA 2: HBsAg ELISA from BIO-RAD Company, France. Elecsys: Elecsys® HBsAg II (ECLIA) from Roche Diagnostics, Germany. IR, initially reactive; INR, initially nonreactive; RR, repeatedly reactive.
CV value of different assays
The coefficient of variation (CV%) of the total precision were 7.07%, 11.24% and 14.46%, respectively, for the 3 assays (Table 3).
Table 3
| Assay | Mean (COI) | SD | CV (%) |
|---|---|---|---|
| ELISA 1 (n=115) | 3.19 | 0.3589 | 11.24 |
| ELISA 2 (n=115) | 5.67 | 0.8193 | 14.46 |
| Elecsys (n=95) | 4.15 | 0.2936 | 7.07 |
ELISA 1: HBsAg ELISA from Intec Company, China. ELISA 2: HBsAg ELISA from BIO-RAD Company, France. Elecsys: Elecsys® HBsAg II (ECLIA) from Roche Diagnostics, Germany.
The discrepant results among 3 assays
In the study, discrepant results among the 3 assays occurred in 16 samples (Table 4). Two false positives and 6 false negatives were found in ELISA 1, while 5 false positives and 8 false negative were found in ELISA 2. Seven samples were HBV DNA positive among 9 samples with confirmed positive. One sample (ID 7) was negative in both ELISAs and HBV DNA negative, but reactive in ECLIA.
Table 4
| Sample ID | Elecsys | ELISA 1 | ELISA 2 | HBV DNA | Elecsys confirmation | Abbott i2000 | Murex ELISA | Confirmatory result |
|---|---|---|---|---|---|---|---|---|
| 1 | + | + | − | + | + | / | / | + |
| 2 | + | − | − | + | + | / | / | + |
| 3 | + | − | − | + | + | / | / | + |
| 4 | + | + | + | − | + | / | / | + |
| 5 | + | − | − | + | + | / | / | + |
| 6 | + | + | − | + | + | / | / | + |
| 7 | + | − | − | − | + | + | / | + |
| 8 | + | − | − | + | + | / | / | + |
| 9 | + | − | − | + | + | / | / | + |
| 10 | − | − | + | − | / | − | − | − |
| 11 | − | − | + | − | / | − | − | − |
| 12 | − | + | − | − | / | − | − | − |
| 13 | − | − | + | − | / | − | − | − |
| 14 | − | − | + | − | / | − | − | − |
| 15 | − | − | + | − | / | − | − | − |
| 16 | − | + | − | − | / | − | − | − |
“+” = positive; “−” = negative; “/” = untested. ELISA 1: HBsAg ELISA from Intec Company, China. ELISA 2: HBsAg ELISA from BIO-RAD Company, France. Elecsys: Elecsys® HBsAg II (ECLIA) from Roche Diagnostics, Germany.
Discussion
HBV is transmitted through direct exposure to infected blood or organic fluids. China is a HBV highly endemic country despite a nationwide vaccination program was launched in 1992 to reduce the burden of disease (22). HBsAg is the first serological marker to appear during the course of HBV infection and remains the first line of HBV screening in blood donors (3,4). However, HBsAg screening required an optimal analytical sensitivity to shorten the window period, commonly defined as the time between infection and detection of the viral antigen, and to enhance the ability to detect the smallest amount of HBsAg during the asymptomatic late stage of chronic infection (23).
Automated CLIAs/ECLIA using different platforms and methods are widely used for the detection of HBV in blood donor screening (15,24). A good correlation and high agreement were reported among different HBsAg CLIAs (24). The ECLIA (Elecsys) uses the streptavidin-biotin amplification system and the triple pyridinium to continuously obtain electrons provided by tripropylamine in the electric field (25,26). According to Huh (21), the analytical sensitivity, using WHO reference material and seroconversion panels, of HBsAg ELISAs and HBsAg CLIAs/ECLIA were variable and not related to the analytical methods. In our study, the limit of detection was up to 0.2 IU/mL for all assays, using HBsAg QC samples. However, the CV value was lower in Elecsys, which suggested that the variation for Elecsys was little. Interestingly, the detection ability of HBsAg in the blood donors were different among assays and most confirmed positive individuals were found with Elecsys. It suggested that the performance of HBsAg detection with ECLIA was better than that of HBsAg ELISAs in our study.
Accurate screening is of very high clinical importance (1-4). In our study, false negative or false positive were found in the ELISA methods. False negative results will cause potential health risks to blood or blood component receivers (27). According to a government public report, the total blood donation nationwide was nearly 15 million person-time in 2017, which was increased by 4.2% from the year before. However, it only counted about 1% of the total population, which was still lower than that of the developed countries (28). The high prevalence of HBV infection already restricted a large number of people from blood donation (22). False positive screening results will further reduce the number of available blood donors and hence, put a risk on the public health. It is crucial to choose a method with good sensitivity and specificity for HBsAg screening for blood donors, especially in HBV high endemic regions.
In conclusion, the clinical performance of Elecsys HBsAg II assay is sufficient for regular blood donor screening in the Chinese population. The high sensitivity of Elecsys HBsAg II assay can help identify HBV infected donors, while the improved specificity can reduce the donor deferral due to false positive screening result.
Acknowledgments
Source of support: Roche Diagnostics Shanghai Ltd. sponsored this study.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob.2019.12.02). Cunying Pu is a Roche employee. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by ethical committee at the Blood Center of Zhejiang Province (ZJB2018001), and conducted in full compliance with the principles of the Declaration of Helsinki (as revised in 2013). Individual informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dodd RY, Nguyen ML, Krysztof DE, et al. Blood donor testing for hepatitis B virus in the United States: is there a case for continuation of hepatitis B surface antigen detection? Transfusion 2018;58:2166-70. [Crossref] [PubMed]
- Degefa B, Gebreeyesus T, Gebremedhin Z, et al. Prevalence of hepatitis B virus, hepatitis C virus, and human immunodeficiency virus among blood donors of Mekelle blood bank, Northern Ethiopia: A three-year retrospective study. J Med Virol 2018;90:1724-9. [Crossref] [PubMed]
- Eko Mba JM, Bisseye C, Ntsame Ndong JM, et al. Prevalent hepatitis B surface antigen among first-time blood donors in Gabon. PLoS One 2018;13:e0194285 [Crossref] [PubMed]
- Zhang K, Liu Y, Chen R, et al. Antigenicity reduction contributes mostly to poor detectability of HBsAg by hepatitis B virus (HBV) S-gene mutants isolated from individuals with occult HBV infection. J Med Virol 2018;90:263-70. [Crossref] [PubMed]
- Saber HR, Tabatabaee SM, Abasian A, et al. Incidence and Residual Risk of HIV, HBV and HCV Infections Among Blood Donors in Tehran. Indian J Hematol Blood Transfus 2017;33:412-6. [Crossref] [PubMed]
- World Health Organization, Hepatitis B. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b
- Kamitsukasa H, Iri M, Tanaka A, et al. Spontaneous reactivation of hepatitis B virus (HBV) infection in patients with resolved or occult HBV infection. J Med Virol 2015;87:589-600. [Crossref] [PubMed]
- Christiansen KM, Mössner BK, Hansen JF, et al. Liver stiffness measurement among patients with chronic hepatitis B and C: results from a 5-year prospective study. PLoS One 2014;9:e111912 [Crossref] [PubMed]
- Cheng J, Jiang SW, Zhou ZS, et al. Hepatic encephalomyelopathy: a complication following liver cirrhosis caused by Budd-Chiari syndrome and HBV. J Infect Dev Ctries 2014;8:551-3. [Crossref] [PubMed]
- Zhong S, Yeo W, Schroder C, et al. High hepatitis B virus (HBV) DNA viral load is an important risk factor for HBV reactivation in breast cancer patients undergoing cytotoxic chemotherapy. J Viral Hepat 2004;11:55-9. [Crossref] [PubMed]
- Lemoine M, Nayagam S, Thursz M. Viral hepatitis in resource-limited countries and access to antiviral therapies: current and future challenges. Future Virol 2013;8:371-80. [Crossref] [PubMed]
- Dhawan HK, Marwaha N, Sharma RR, et al. Anti-HBc screening in Indian blood donors: still an unresolved issue. World J Gastroenterol 2008;14:5327-30. [Crossref] [PubMed]
- Liu FP, Liu JC, Wang DW. Comparison of homemade and imported HbsAg ELISA kits on screening blood samples. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 2006;20:84-6. [PubMed]
- Hladik W, Kataaha P, Mermin J, et al. Prevalence and screening costs of hepatitis C virus among Ugandan blood donors. Trop Med Int Health 2006;11:951-4. [Crossref] [PubMed]
- Malm K, Kragsbjerg E, Andersson S. Performance of Liaison XL automated immunoassay platform for blood-borne infection screening on hepatitis B, hepatitis C, HIV 1/2, HTLV 1/2 and Treponema pallidum serological markers. Transfus Med 2015;25:101-5. [Crossref] [PubMed]
- Men SS, Shang FK, Han CH, et al. Analysis of the Detection Results of the Syphilis Specific Antibody in Blood Donors by Chemiluminescence Method and Enzyme Linked Immunosorbent Assay. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2017;25:226-30. [PubMed]
- Sommese L, Sabia C, Esposito A, et al. Comparison of performance of two Treponema pallidum automated chemiluminescent immunoassays in blood donors. Infect Dis (Lond) 2016;48:483-7. [Crossref] [PubMed]
- Ghani E, Rathore MA, Khan SA. Trends in human immunodeficiency virus seroprevalence in blood donors in northern Pakistan. Public Health 2016;131:71-4. [Crossref] [PubMed]
- Tiwari AK, Pandey PK, Dara RC, et al. Evaluation of a new serological test for syphilis based on chemiluminescence assay in a tertiary care hospital. Asian J Transfus Sci 2015;9:65-9. [Crossref] [PubMed]
- Chang CD, Cheng KY, Jiang LX, et al. Evaluation of a prototype Trypanosoma cruzi antibody assay with recombinant antigens on a fully automated chemiluminescence analyzer for blood donor screening. Transfusion 2006;46:1737-44. [Crossref] [PubMed]
- Huh HJ, Chae SL, Cha YJ. Comparison study with enzyme immunoassay and chemiluminescence immunoassay for hepatitis B virus surface antigen detection. Korean J Lab Med 2007;27:355-9. [Crossref] [PubMed]
- Zhang W, Ji Z, Wang L, et al. A meta-analysis of HBsAg-positive rate among general Chinese populations aged 1--59 years. Infect Dis (Lond) 2015;47:878-88. [Crossref] [PubMed]
- Candotti D, Laperche S, Hepatitis B. Virus Blood Screening: Need for Reappraisal of Blood Safety Measures? Front Med (Lausanne) 2018;5:29. [Crossref] [PubMed]
- Sommese L, Sabia C, Paolillo R, et al. Screening tests for hepatitis B virus, hepatitis C virus, and human immunodeficiency virus in blood donors: evaluation of two chemiluminescent immunoassay systems. Scand J Infect Dis 2014;46:660-4. [Crossref] [PubMed]
- Wang T, Li D, Yan K, et al. Performance evaluation of a new fourth-generation HIV Ag/Ab combination electrochemiluminescence immunoassay - evaluation of a new HIV assay. Int J STD AIDS 2014;25:267-72. [Crossref] [PubMed]
- Prusa AR, Hayde M, Unterasinger L, et al. Evaluation of the Roche Elecsys Toxo IgG and IgM electrochemiluminescence immunoassay for the detection of gestational Toxoplasma infection. Diagn Microbiol Infect Dis 2010;68:352-7. [Crossref] [PubMed]
- Kiely P, Stewart Y, Castro L. Analysis of voluntary blood donors with biologic false reactivity on chemiluminescent immunoassays and implications for donor management. Transfusion 2003;43:584-90. [Crossref] [PubMed]
- Shi L, Wang J, Liu Z, et al. Blood donor management in china. Transfus Med Hemother 2014;41:273-82. [Crossref] [PubMed]
Cite this article as: Wu Y, Ling X, Yu G, Zhu H, Hu W, Pu C, Zhu F. The efficacy of HBsAg detection using electro-chemiluminescence immunoassay for blood donor screening in China. Ann Blood 2019;4:30.


