
Characterization of high-purity, double virus inactivated and stable plasma-derived anti-haemophilic clotting factor-VIII/vWF complex
Introduction
Factor-VIII (F-VIII) is an important plasma glycoprotein present in blood and is involved in the blood coagulation cascade. It is a heterodimeric multidomain protein of approximately 280 KDa molecular weight consisting of a heavy and a light chain. It is synthesized as a 2,351 residue single chain precursor protein consisting of A1-A2-B-A3-C1-C2 domains. The non-covalent heterodimer contains a heavy chain (HCh) with a molecular weight between 90 to 200 KDa (A1-A2-B domains) and a light chain (LCh) with a molecular weight of 80 KDa (A3-C1-C2 domains) (1).
In humans, F-VIII is encoded by the F8 gene which is located on the X chromosome at position q28 and is involved in the intrinsic pathway of blood coagulation (2,3). F-VIII occurs in human plasma in very low concentrations of about 100–200 ng/mL corresponding to one unit of F-VIII per mL. Insufficient levels or total absence of this protein causes haemophilia A, an X-linked genetic bleeding disorder (4).
One way our immune system is designed to protect us from foreign things is by making antibodies. A person with haemophilia either produces no clotting factor (most cases of severe haemophilia) or an altered dysfunctional factor (most cases of mild/moderate haemophilia A [factor eight (F-VIII) deficiency] and haemophilia B [factor nine (F-IX) deficiency]. When such people are exposed to factor concentrates to replace the clotting factor (FVIII or FIX) that they are missing or have in an altered form, their immune system may see it as a foreign protein and develop neutralizing allo-antibodies called “inhibitors” against it. This then makes factor concentrate replacement ineffective for the treatment or prevention of bleeds. Inhibitor development is a much more common problem in people with haemophilia A than in those with haemophilia B (5).
The availability of F-VIII concentrates affects life span and lifestyle of people with haemophilia A (6). The effect of replacement therapy has improved significantly the life expectancy and quality of life of patients with haemophilia A in high income countries (7,8). Before the development of replacement products for haemophilia in the 1960s, there was little difference in haemophilia care between the developed world and the developing world (9), with inadequate treatment resulting in terrible pain, joint deformities, arthropathy, disabilities and death in childhood or early adult life (10,11). It has been estimated that 70– 80% of people with haemophilia globally, primarily in the developing world, receive inadequate or no treatment (12,13) because of unavailability and/or unaffordable factor concentrates and also due to inadequate analytical methods for diagnosis (12). Increasing the availability and use of F-VIII concentrates will improve the mortality and morbidity outcomes for people with haemophilia A (14).
The Annual Global Survey 2015 conducted by World Federation of Haemophilia reported 17,346 registered patients diagnosed with haemophilia, of which 14,508 patients were identified with Haemophilia A, 2,127 patients with Haemophilia B, 711 patients with Haemophilia type unknown in India. Survey also reported 483 patients with von Willebrand’s disease and 305 patients with bleeding disorders (World Federation of Haemophilia, 2016).
Maintenance of normal levels of F-VIII is dependent on its formation of a complex with von Willebrand Factor (vWF). F-VIII circulates as an inactive pro-cofactor in complex with vWF. Thrombin is the principal physiological activator of F-VIII. This protease cleaves the protein at sites in both the HCh and LCh. Activated F-VIII (F-VIIIa) is a heterodimer of 50, 43 and 73 KDa subunits which are required for procoagulant activity (15,16). When vWF is bound to F-VIII, a stable association of its HCh and LCh is maintained. vWF also protects F-VIII from cleavage by activated F-X and from protein C-catalyzed degradation (17,18).
Defects in the vWF molecule causes an inherited bleeding disorder, von Willebrand’s disease (vWD) which has a prevalence of 0.83% in general population (19). The simplified classification of vWD distinguishes between three groups: partial quantitative deficiency (type I), qualitative deficiency (type-2), and total quantitative deficiency (type-3). Several laboratory tests are required for type and subtype identification: F-VIII activity, vWF antigen (vWF:Ag), vWF Ristocetin cofactor activity (vWF:RCo), vWF collagen binding activity (vWF:CB). vWF:RCo and the ratio with vWF:Ag (vWF:RCo: vWF:Ag) are used worldwide as a routine methods to estimate vWF function and for vWD diagnosis (20).
Early efforts for management of both diseases i.e., Haemophilia A and vWD patients were based on cryoprecipitate administration which was hampered by inability to eliminate viruses and extremely large volumes required. High purity plasma-derived products are now the treatment of choice for Haemophilia A patients because of the minimal protein load associated with high specific activity of F-VIII. However, the high cost of the product makes it unaffordable and unavailable to the haemophilic patients especially in the developing countries.
In the developing countries, about 80% of people with haemophilia receive inadequate or no treatment either because of lack of awareness of the disease or lack of diagnosis or even due to unavailability and/or unaffordability of Factor concentrates (21). Thus, there is a strong need to develop a pdF-VIII/vWF complex concentrate, meeting the existing unmet medical needs and clinical demands in these regions for both Haemophilia A as well as vWF deficient patients.
With this goal, the present study was aimed to develop pdF-VIII/vWF complex by appropriately processing freshly frozen recovered plasma collected from blood banks across India, approved by Drug Controller of India. The final purified product obtained from optimized downstream processing has been well-characterized by various physicochemical, functional assays and compared to already available marketed products which were used as comparator, in order to demonstrate the quality of F-VIII produced from cryoprecipitate irrespective of its origin. Using downscaling models for S/D treatment and dry heat treatment, the virus safety of the final product was also validated. Additionally, the final product was also charged on stability at pre-defined conditions for different time-intervals and the stability data obtained has been discussed. The stability of the reconstituted product was also established at different time intervals. The said product yields a highly pure, biologically active, safe and affordable pdF-VIII/vWF product catering to the needs of the Indian population.
Methods
Materials
Freshly frozen recovered plasma was obtained in polyvinyl bags from Indian blood banks approved by Drug Controller General of India (DCGI) and registered with the National Aids Control Organization under the Government of India. Antibody testing for HIV-I, II and HCV was performed using Biorad and HBV kit from Difpro. Nucleic acid testing (NAT) of plasma was done for Hepatitis-B virus (HBV), Hepatitis-C virus (HCV) and Human Immunodeficiency virus-I (HIV- I) using standard USFDA approved kits, from Roche, USA.
F-VIII potency was analyzed by Chromogenic assay using Electrachrome F-VIII kit from Instrumentation Laboratory, USA.
One-stage potency assay was determined using coagulation based assay reagents from Instrumentation Laboratory, USA.
F-II, F-VII, F-X activity assays were also determined using reagents from Instrumentation Laboratories, USA.
Standard protein molecular weight marker for SDS-PAGE and Quick start Bradford 1X dye reagent for total protein estimation was procured from Biorad (USA). Anti-F8 antibody produced in rabbit purified immunoglobulin (Sigma) was used as primary antibody for detecting heavy chains of Factor VIII. Secondary antibody used was Goat anti-rabbit IgG HRP from (Abcam) against primary antibody for heavy chain. For detecting F-VIII light chains, primary antibody used was mouse Anti-F8 light chain (Merck) and secondary antibody used was Goat anti-mouse IgG HRP (Antibodies). 3, 3-diaminobenzidine (Biorad) was used as developing agent.
Bovine serum Albumin used for total protein estimation was from Sigma Aldrich Chemicals Pvt. Ltd. (USA).
High performance Size-exclusion column was from TOSOH Biosciences, Japan. Other chemicals required for preparing mobile phase-phosphate buffer saline were procured from Merck (Darmstadt, Germany).
Functional assays like vWF:RCo and vWF:Antigen assay kits were from Instrumentation laboratory, USA whereas vWF:CB ELISA kit was from Hyphen Biomed, France.
Virus validation study was performed in a GLP certified qualified laboratory.
Procedures
Antibody testing
Every unit of plasma used for purification of pdFactor-VIII/vWF (F-VIII) was tested for presence of antibodies against HIV-I, II, HBV and HCV and those units that tested negative for antibodies were further tested for the viral antigens by NAT. Plasma units which were tested positive were discarded as per standard procedures.
NAT
Every unit of Indian plasma used was further tested for HBV, HIV-I and HCV and all units that tested negative for the above viruses were pooled and used for the purification of pdF-VIII/vWF. This testing was performed in addition to the certification provided by different blood banks.
Manufacturing of Indigenous pdF-VIII/vWF
Manufacturing process
F-VIII is produced from human plasma of Indian origin. Plasma was snap frozen within 6 hours of donation, thawed in controlled environment (~9 hours) and cryo-separation was performed within 3hours of complete thawing. The cold insoluble fraction of plasma called cryoprecipitate obtained upon centrifugation of pooled plasma contains pdF-VIII/vWF along with other proteins. This cryoprecipitate was dissolved in WFI and treated with Aluminium Hydroxide for removal of prothrombin complex proteins. Fibrinogen was precipitated using PEG 4000. Appropriate concentrations of calcium chloride were maintained throughout the process. For virus inactivation, solvent/detergent was performed with 1% (w/v) Tween 80 and 0.3% (w/v) TnBP for 6h at ambient temperature. Anion Exchange chromatography resin (Fractogel EMD TMAE; Merck) was performed to capture and purify the pdF-VIII/vWF complex. The entire process from thawing of fresh frozen plasma to formulation was carried out in ~30 hours to ensure minimal/no loss in F-VIII activity. It was then formulated and lyophilized (250 IU/vial) using optimized lyophilization cycle. The lyophilized product was then subjected to terminal dry heat treatment at 100 °C for 30 minutes for virus inactivation. Different batches of the indigenous pdF-VIII/vWF drug product were characterized and compared with different marketed products derived from human plasma, procured from different manufacturers.
Characterization of indigenously manufactured pdF-VIII/vWF
Reconstitution of lyophilizates and its stability
The lyophilized pdF-VIII/vWF product was reconstituted using 10 mL of water for injection to achieve final concentration of 25 IU/mL. The vial content dissolved completely within 5 minutes of reconstitution. Samples were tested by various physicochemical and functional assays.
The reconstitution stability was evaluated with 3 batches at 5±3 °C for 48 hours. Activity was determined by chromogenic assay at each sampling time-point.
Protein concentration
Total protein was determined by the Bradford method with Bovine serum albumin as a standard. The sample concentration of pdF-VIII/vWF was determined by OD at 595 nm using standard curve.
SDS-PAGE and immunoblotting
Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) was carried out using 4–12% gradient separating gel under non-reducing conditions. Gel was run under constant voltage 200 volts for 45 minutes using Biorad miniprotean tetra cell assembly and was silver stained using in-house developed method.
Immunoblotting was performed by subsequent transfer of proteins onto nitrocellulose membranes, incubation with specific antibodies, washing and developing. Monoclonal antibodies were used for detection of heavy chains (Sigma) and light chains of F-VIII (Merck). Goat anti-rabbit IgG HRP (Abcam) and Goat anti-mouse IgG HRP (Antibodies) were used as secondary antibodies. 3, 3-diaminobenzidine (Biorad) was used for developing blot.
Protein purity by size-exclusion high performance liquid chromatography
Chromatographic separation was performed on TOSOH column 60 cm × 7.8 mm I.D, particle size 10 µ using phosphate buffer saline pH 7.0. Samples were normalized to 0.3 mg/mL in mobile phase, 100 µL of samples were injected, flow rate was 0.5 mL/min and absorbance was measured at 280 nm.
Potency analysis of pdF-VIII/vWF by chromogenic assay
Factor-VIII chromogenic assay was performed by Electrachrome F-VIII kit on automatic coagulometer (ACL Elite Pro) from Instrumentation laboratories as per the manufacturer’s instructions. For the calibration, the value of F-VIII in calibration plasma used was determined using WHO standard (07/316). Samples were diluted 50 fold in the kit buffer for analysis and the results obtained were multiplied with dilution factor to get final result.
Activity analysis of factor II, factor VII, factor X
The above clotting assays were performed on automatic coagulometer (ACL Elite Pro) from Instrumentation laboratories using (IL) reagents as per manufacturer’s instructions. For the assay calibration, the activities of F-II, F-VII and F-X in calibration plasma were determined using WHO standard (09/172). Drug product samples without dilution were analyzed to obtain the final result.
Von Willebrand Factor Collagen Binding assay (vWF:CB)
vWF:CB activity was determined by use of a commercial ELISA kit according to the manufacturer’s instructions. vWF calibrator provided in the kit was used for the calibration, which was calibrated against National Institute for Biological Standards and Control (NIBSC) standard and the assigned activity was established with respect to lot. Ratio of vWF:CB: vWF:Ag was also determined.
Von Willebrand Factor Ristocetin cofactor assay (vWF:RCo) and vWF: antigen assay
The ability of vWF to agglutinate fixed human platelets in the presence of ristocetin was determined using the vWF:RCo kit and vWF concentration using vWF:Ag kit according to the manufacturer’s instructions. For the calibration, the activities of vWF:RCo and vWF:Antigen in calibration plasma used were determined using WHO standard (07/316). The pdF-VIII/vWF samples were diluted 50 fold for vWF:RCo activity whereas 200 folds for vWF:Ag assay using kit buffer and were analyzed using automatic coagulometer. Ratio of vWF:RCo: vWF:Ag was also determined.
F-VIII antigen assay
F-VIII:Ag was quantified by an ELISA method using the Asserachrom VIIIC:Ag (Diagnostica Stago, Asnieres, France) commercial kit according to the manufacturer’s instructions.
F-VIII inhibitor assay
F-VIII inhibitor assay was performed using Technoclone inhibitor reagent kit (Bethesda units) and the assay was performed as per manufacturer’s instructions.
Circular Dichroism (CD) spectroscopy
CD spectra were recorded on a J-815 CD Spectrometer (Jasco) at 25 °C with a scanning speed of 20 nm/min, a response of 4 seconds and a bandwidth of 1.0 nm. Far-UV CD spectra were recorded at a wavelength range of 205–260 nm. The data presented were an accumulation of five scanning cycles and averaged to improve the signal quality. Protein spectra were corrected by a blank measurement performed prior to the analysis using buffer system applied.
Assessment of virus safety
The scaled-down model employed in the virus safety studies was validated to comply with the commercial scale process in all relevant parameters, and was implemented successfully. Virus validation study was performed using mainly two orthogonal steps viz. solvent detergent and dry heat treatment. In addition to this, aluminium hydroxide treatment step was also evaluated. The viruses selected for the study were as per ICHQ5A guideline (22). The product was spiked individually with the viruses viz. human immunodeficiency virus type 1 (HIV-1), pseudorabies virus (PRV), bovine viral diarrhoea virus (BVDV), hepatitis A virus (HAV), porcine parvovirus (PPV) as per the guidelines and tested for the robustness of the above mentioned steps for their inactivation and/or removal.
S/D treatment was highly effective for enveloped viruses like HIV-1, PRV, BVDV whereas both enveloped and non-enveloped viruses viz. HAV, PPV were investigated for evaluating aluminium hydroxide as well as dry heat treatment step. Cytotoxicity and interference tests were performed to determine necessary dilutions to avoid matrix effects. A validated assay system for endpoint titration and large-volume plating was used.
In addition to this, virus safety in the product was assessed for antibodies against HIV-I, II, HBV and HCV and were further tested for viral antigens viz. HBV, HIV-I and HCV by nucleic acid testing as per D & C act (23).
Stability studies
Stability study for pdF-VIII/vWF lyophilized product was performed according to the current guidelines of the International Conference on Harmonisation for stability testing. The product was put on stability under long-term conditions at +5±3 °C for 24 months and +25±2 °C (60%±5% relative humidity) for up to 6 months. Product was also evaluated under stress conditions +40±2 °C (75%±5% relative humidity) for 1 month.
Chromogenic activity, an indicative of functionality of F-VIII was tested during the release of every batch and at every stability time-point. Values at starting time point were set to 100%. For the following time points, chromogenic activities were calculated relative to the respective starting value. Mean values and standard deviations (SD) were calculated.
Results
Purification of human plasma derived F-VIII/vWF
The purification process of pdF-VIII/vWF from cryoprecipitate involved efficient steps in removing impurities. Aluminium hydroxide treatment effectively removed the plasma prothrombin complex proteins (Factor II, VII and X) to a major extent from cryoprecipitate. Fibrinogen was found to be precipitated by PEG treatment. The anion-exchange chromatography was also a crucial step in the purification of pdF-VIII/vWF.
Figure 1 exhibited the chromatographic elution profile of anion exchanger, Fractogel EMD TMAE. Solvent-detergent treated sample was loaded onto the column packed with anion-exchange resin wherein impurities were eluted with low salt concentration followed by increased salt concentration for pdF-VIII/vWF elution.
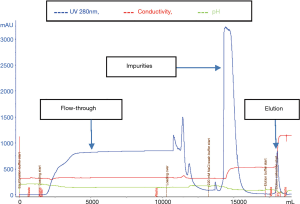
Factor II, factor VII and factor X activities:
As a part of product development of a therapeutic molecule from human plasma, we tested this indigenously developed pdF-VIII/vWF complex sample for factor II, factor VII and factor X which are considered as impurities co-eluting along with factor-VIII concentrates and could lead to F-VIII activation via the clotting cascade. Thrombin, activated Factor II is potentially harmful and had to be removed from the final product by chromatographic techniques (24). Results from Table 1 indicate negligible amounts of factor II, VII and X present in the drug product sample comparable to marketed products. Likewise, fibrinogen activity in the indigenously developed pdF-VIII/vWF batches were <0.35 mg/mL and were similar to commercially available marketed products (Table 1).
Table 1
| Samples | F-II activity (IU/mL) | F-VII activity (IU/mL) | F-X activity (IU/mL) |
|---|---|---|---|
| Marketed product A | 0.003 | 0.003 | 0.007 |
| Marketed product B | 0.002 | 0.004 | <0.016 |
| Marketed product C | 0.055 | 0.007 | 0.238 |
| pdF-VIII/vWF (indigenous) | 0.004* | 0.004* | 0.064* |
*, average values of three consistency pdF-VIII/vWF batches.
Specific activities and vWF content of pdF-VIII/vWF
This indigenously purified pdF-VIII/vWF was characterized by various physicochemical and functional assays in comparison with different marketed products. The manufacturing process yielded highly pure pdF-VIII/vWF drug product (F-VIIIC 25 IU/mL) with F-VIII yield of ~30% (~150 IU of F-VIII/L of plasma), specific activity of 60±20 IU F-VIII/mg of total protein which is comparable to marketed products (Table 2).
Table 2
| Sr. No. | Sample | Specific activity (IU/mg) | FVIII: C (IU/mL) | vWF:RCo activity (IU/mL) | vWF:Ag | vWF:RCo/vWF:Ag | vWF:CB activity (IU/mL) | vWF:CB/vWF:Ag | F-VIII Ag | F-VIIIC:F-VIII Ag |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Marketed product A | 50±10 | 25 | 5.66 | 8.22 | 0.69 | 5.32 | 0.65 | 26.94 | 0.93 |
| 2 | Marketed product B | 70±30 | 50 | 37.76 | 50.4 | 0.75 | 38.00 | 0.75 | 48.14 | 1.04 |
| 3 | Marketed product C | 22.00 | 50 | 27.56 | 52.6 | 0.52 | 26.07 | 0.50 | 55.60 | 0.90 |
| 4 | pdF-VIII/vWF (indigenous) | 60±20 | 25 | 28±4 | 34±4 | 0.87±0.1 | 26±2 | 0.79 ±0.1 | 24 ±0.9 | 1.05±0.04 |
In order to assess the quality of vWF, we determined the Ristocetin cofactor activity (vWF:RCo) and the degree of inactivation of vWF was measured by calculating the vWF:RCo/vWF:Ag ratio (25). The indigenously purified pdF-VIII/vWF showed a vWF:RCo/vWF:Ag ratio of 0.87±0.1 as all other marketed products (Table 2).
Another activity assay for vWF is the vWF:CBA ELISA, which is sensitive to the presence of high molecular weight multimers. The vWF:CB/vWF:Ag ratio in the indigenous plasma-derived product showed a value of 0.79±0.1 which was also compared to other marketed products (Table 2).
Protein activities to antigen (F-VIII C/F-VIII:Ag) were measured to evaluate the residual biological activity of the indigenous preparation of pdF-VIII/vWF. F-VIII preserved its activity in the final product (F-VIII C/F-VIII:Ag ~1) which is similar to that obtained in other marketed products (Table 2) (26).
Size-exclusion chromatography
Purity of the indigenously purified pdF-VIII/vWF was determined by High performance Size-exclusion chromatography technique (SE-HPLC). SE-HPLC was used to analyze protein samples for heterogeneity in terms of different molecular weights, for the presence of high molecular and low molecular weight species.
The chromatogram of marketed product A (without albumin) has a major peak of F-VIII/vWF complex along with one co-eluting peak and some low molecular weight species. The chromatographic profile of the marketed product A was comparable to the indigenously purified plasma-derived drug product (without Albumin). Marketed product C showed a different chromatographic profile as compared to other products (Figure 2A). Marketed product B containing albumin exhibited a chromatographic profile which matched with indigenously developed drug product spiked with albumin (Figure 2B).

SDS-PAGE and immunoblotting
Furthermore, the purity of indigenously developed pdF-VIII/vWF was also compared by SDS-PAGE analysis to already available and comparable marketed products used for reference (Figure 3). The SDS-PAGE was carried out under non-reducing condition which showed similar profiles as SE-HPLC (Figure 3A). The silver-stained gel of SDS-PAGE analysis of the indigenously developed pdF-VIII/vWF showed diffused bands in higher molecular weight region and distinct bands in the molecular weight region between 50–250 KDa. The F-VIII/vWF activity was predominantly found in higher molecular weight regions above 250 KDa. In addition to this, several bands were detected of which, bands corresponding to heavy chain domains ranged from 90–210 KDa and light chain was ~75 KDa.

The identity of the bands corresponding to heavy and light chain was confirmed by immunoblotting using monoclonal antibodies against F-VIII chains. Heavy chain bands corresponding to the A1-A2-B domain were visualized at molecular weights ranging from ~100–200 kDa along with full length protein at ~280 kDa whereas light chain bands (A3-C1-C2 domains) at ~75 kDa (Figure 3B,C).
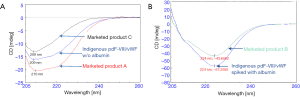
Far-UV CD spectroscopy
Far-UV CD spectroscopy results suggest that the indigenously developed pdF-VIII/vWF shared a similar structure with marketed products A and C (without albumin) (Figure 4A) and with marketed product B after spiking with albumin (Figure 4B).
Virus safety
Virus safety is a prerequisite for manufacturing of any biological, including antihemophilic factor VIII from human plasma. Besides providing the efficient plasma-derived concentrate of high purity, safety in terms of minimising the risk of viral transmission also has to be ensured. The safety of the product was ensured by testing the viral antigens by performing nucleic acid testing and testing of antibodies against given viral markers by ELISA (27). Additionally, virus validation study was performed in the dedicated orthogonal steps in the purification process including S/D treatment for 6 h at ambient temperatures, and dry heat treatment at 100 °C for 30 minutes. Further, aluminium hydroxide adsorption step was performed for 15 minutes at 25 °C for inactivating viruses that could potentially be present in the plasma. The S/D treatment used in the manufacturing process is known for its extremely fast inactivation kinetics, robustness, and superiority in terms of inactivating enveloped viruses based on the lipid membrane destroying properties of TnBP (solvent) and Tween-80 (detergent) exhibiting log reduction factors (LRFs) of ≥5.38 for BVDV, ≥5.20 for HIV-I, 5.79 for PRV (Table 3). Aluminium hydroxide adsorption step was evaluated for its potential in inactivating both enveloped as well as non-enveloped viruses and was found to be effective in pseudo rabies virus (PRV) removal (LRF 3.74). The dry heat treatment step performed after lyophilization was also used as synergistically effective step for inactivating both enveloped and non-enveloped viruses (28,29). With particular regards to the HAV, a terminal dry-heat treatment step (100 °C for 30 min), following lyophilization was developed to improve the virus safety of pdF-VIII/vWF product. The terminal dry-heat treatment after lyophilization was an effective process for inactivating all viruses. HAV, a non-enveloped virus exhibited a potential inactivation within 30 min of the dry-heat treatment with log reduction factor of 5.76. BVDV, an enveloped virus was potentially sensitive to the treatment (6.88 LRF). However, as expected, PPV was slightly resistant to the treatment. The log reduction factors achieved by dry-heat treatment were 6.88 for BVDV, 3.89 for HIV-I, 4.95 for PRV, 5.76 for HAV and 2.01 for PPV. This treatment is found to be effective even for the inactivation of non-enveloped viruses, which is resistant to solvent/detergent treatment (Table 3).
Table 3
| Sr. No. | Target steps | Avg. Log 10 reduction factor (LRF) | |||||
|---|---|---|---|---|---|---|---|
| Enveloped viruses | Non-enveloped viruses | ||||||
| BVDV | HIV | PRV | HAV | PPV | |||
| 1 | S/D treatment (TnBP/Tween-80) | ≥5.38 | ≥5.20 | ≥5.79 | NA | NA | |
| 2 | Aluminium hydroxide step | NS | ≥1.25 | ≥3.74 | NS | NS | |
| 3 | Dry heat treatment | ≥6.88 | ≥3.89 | ≥4.95 | ≥5.76 | ≥2.01 | |
| Cumulative log reduction factor | ≥12.26 | ≥10.34 | ≥14.48 | ≥5.76 | ≥2.01 | ||
NA, not applicable; NS, no significant reduction.
In addition to the virus safeguarding steps in the manufacturing process of F-VIII, the overall safety strategy also includes selection of qualified donors, testing plasma donations and plasma pools for serological and viral markers (e.g., HIV, HBV and HCV) according to current guidelines for plasma-derived medicinal products (30). The fresh frozen plasma used for purification of F-VIII was tested and found negative for the blood borne viruses like HBV, HCV and HIV.
Stability studies
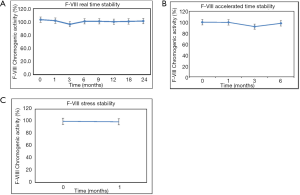
In order to demonstrate stability of this product, the functionality of the pdF-VIII/vWF product was evaluated with time in terms of F-VIII chromogenic activity. The long term stability data demonstrated a product, stable for more than 24 months at storage condition of 5±3 °C (Figure 5A) and 6 months under accelerated conditions (Figure 5B). The product was also found to be stable with no remarkable decrease in the F-VIII chromogenic activity with time at accelerated condition 25±2 °C for 6 months and stress condition of 40±2 °C for 1month (Figure 5C).
Discussion
Factor VIII is a plasma derived protein with a complex structure, the biological activity of which is easily lost. The chromatographic purification of FVIII/vWF complex is a crucial step in the preparation of a fully active product. It is really very important to find an ideal support that optimizes the chromatographic separation conditions. Loss of F-VIII may be caused by partial activation and non-specific interaction with the matrix. The use of agarose (DEAE sepharose, DEAE Macroprep) and polymer based support (Toyopearl DEAE) or tentacle support (Fractogel TMAE, Fractogel EMD TMAE, Fractogel EMD DEAE) should prevent the unwanted interactions. The choice of Fractogel EMD-TMAE as the best support tested was confirmed by final product characteristics. Fractogel TMAE resin exhibited high binding capacity, recovery and specific activity for pdF-VIII/vWF along with removal of major contaminants like fibrinogen, traces of F-II, F-VII and F-X and yielded a highly pure pdF-VIII/vWF.
The anti-haemophilic F-VIII fraction of plasma is an important plasma glycoprotein, deficiency of which causes Haemophilia A, an X-linked genetic bleeding disorder. It has a relatively short half-life in blood (approximately 12 h) (27). Plasma-derived F- VIII requires vWF as a carrier protein to protect itself from proteolytic degradation, thereby prolonging its half-life in circulation and efficiently localising itself at the site of the vascular injury.
Based on the current requirements for treatment of patients suffering from haemophilia A and vWD patients, a new generation FVIII-containing vWF concentrate has been developed which covers a very unique combination of high purity, nativity and appropriate FVIII:vWF ratios with two dedicated viral safety steps for ensuring pathogen safety exhibiting this formulation extremely safe for therapeutic use.
F-VIII in the final product retains its biological activity completely; in fact the ratio between F-VIII activity and antigen is about 1. In order to assess the quality of vWF, we measured the Ristocetin cofactor activity (vWF:RCo). The degree of inactivation of vWF can be measured by calculating the vWF:RCo/vWF:Ag ratio in normal plasma, the ratio is close to 1. A low ratio indicates that vWF has been denatured and is less active.
Most F-VIII/vWF complex exhibits low ratio vWF:RCo/vWF:Ag unlike our product with ratio of about 0.9 than in normal plasma which is close to 1. This proves that vWF is functionally active. Another activity assay for vWF is the vWF:CBA test. Our product shows lower vWF:CBA/vWF:Ag ratio of about 0.8 which is higher than most of the plasma derived marketed products compared.
Products with low F-VIII/vWF ratio (≤0.77) have been successfully administered in vWD patients and concentrates with these ratios can be easily used for treatment for vWD (31). The biological activity in the final product was preserved by exhibiting F-VIII C/F-VIII:Ag ~1 which is similar to that obtained in marketed products.
Furthermore, analytical size exclusion chromatography showed that the full length pdF-VIII/vWF molecule has a heterogeneous population with a paramount peak containing the F-VIII/vWF complex along with the smaller late eluting peaks with higher retention times corresponding to low molecular weights below the vWF associated F-VIII.
Inhibitors present a significant management challenge for people with haemophilia. FVIII inhibitors bind to functional epitopes that are most commonly found in the A2, C1, and C2 domains of the factor protein. This binding interferes with the function of infused FVIII. These inhibitor antibodies are most frequently formed at an early stage of therapy and are capable of blocking F-VIII activity. The indigenously developed pdFVIII/vWF product has no inhibitors.
The size exclusion chromatographic profiles of indigenous purified product was comparable to marketed products with and without albumin expect for marketed product C which exhibited as different profile. This clearly indicates that the indigenously purified full length pdF-VIII/vWF is of high purity and homogeneity as its profile perfectly matched with marketed product A and with marketed product B, when spiked with albumin. Similar studies were also carried out from cryo-paste obtained from other geographical locations and both types of raw materials, used for the development of pdF-VIII/vWF concentrate showed identical results (data not shown).
In addition to this, SDS-PAGE purity profile of the indigenously developed pdF-VIII/vWF product exactly matched with the purity profile obtained with marketed product A and were also comparable to that of other such products, used for reference.
Far-UV CD spectroscopy results indicate that pdF-VIII/vWF predominantly exists in β-sheet secondary structure, which is in full agreement with the three-dimensional structure of F-VIII (32,33). The indigenously developed pdF-VIII/vWF product matches the commercially available marketed products both structurally as well as functionally.
Viral safety of human plasma derived therapeutic protein products has been the primary concern for a long time and therefore thorough virus validation needs to be performed to make these products virologically safe for human patients. Precautions against viral transmission was ensured at all the stages including the selection of plasma donors, screening of donations and plasma pool, and quality control measurements of the final product. Effective viral reduction obtained via a combination of S/D treatment, aluminium hydroxide precipitation and dry heat treatment during the production process was validated according to current international guidelines (34). The cumulative log reduction factors obtained by these process steps for enveloped and non-enveloped viruses were between ≥10.34 to ≥14.48 and ≥2.01 to ≥5.76 respectively. The total capacity of virus inactivation by these dedicated steps revealed the effectiveness of these steps in virus inactivation thus confirming the product safety from unknown and unpredicted viruses. This indigenously purified pdF-VIII/vWF product was found to be stable at 5± 3 °C for 24 months and can also be stored at 25±2 °C for 6 months within its total shelf life.
Conclusions
The Indigenously manufactured pdF-VIII/vWF complex processed from freshly frozen recovered plasma obtained from approved Indian blood banks will be able to clinically manage both haemophilia A and vWF deficient patients with a yield of about 150 IU of F-VIII/L of plasma with a specific activity of 60±20 IU F-VIII/mg of total protein. This is in comparison with other comparable marketed products. The virus validated product with dedicated orthogonal steps proved to be safe by global standards. This drug product also matched other commercially available products, both structurally and functionally and met all regulatory requirements as per Indian Pharmacopeia and European Medicines Evaluation Agency defining its proven quality and efficacy.
Hence, we conclude that there was no difference in the functional or structural properties of pdF-VIII/vWF complex developed either from cryoprecipitate of freshly frozen and appropriately processed recovered plasma obtained from blood banks across India or cryoprecipitate imported from other plasma product manufacturers from diverse geographical regions (data not shown). The product was also comparable to other commercially available formulations that were used for comparative analysis.
The extensive characterization of indigenously manufactured pdF-VIII/vWF and other commercially available products gives confidence on the safety, efficacy and biological activity of the product, irrespective of its origin or site of manufacturing. Virus validated product is available at affordable cost which meets quality attributes. This also implies that the starting material, when processed with adequate screening and by implementing appropriate unit operations in downstream purification, along with efficient processing speed, can be suitable to develop a quality preparation of F-VIII/vWF concentrate with the required regulatory standards which can be easily available to patients suffering from Haemophilia A and/or von Willebrand disease.
Acknowledgments
We would like to thank the management of Intas Pharmaceuticals Ltd for providing us the opportunity and resources to execute this work.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob.2019.12.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Freshly frozen recovered plasma was obtained in polyvinyl bags from Indian blood banks approved by Drug Controller General of India (DCGI) and registered with the National Aids Control Organization under the Government of India. This study was conducted in accordance with the declaration of Helsinki (as revised in 2013). The institutional ethical approval and individual informed consent were waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Timperio AM, Gevi F, Grazzini G, et al. Comparison among plasma-derived clotting factor Factor VIII by using monodimensional gel electrophoresis and Mass spectrometry. Blood Transfus 2010;8:s98-s104. [PubMed]
- Toole JJ, Knopf JL, Wozney JM, et al. Molecular cloning of a cDNA encoding human anti-haemophilic factor. Nature 1984;312:342-7. [Crossref] [PubMed]
- Truett MA, Blacher R, Burke RL, et al. Characterization of polypeptide composition of human factor VIII:C and the nucleotide sequence and expression of the human kidney cDNA. DNA 1985;4:333-49. [Crossref] [PubMed]
- Foster PA, Zimmerman TS. Factor VIII structure and function. Blood Rev 1989;3:180-91. [Crossref] [PubMed]
- World Federation of Haemophilia (WFH). Inhibitors in haemophilia: A primer 2018. 5th edition. Available online: http://www1.wfh.org/publication/files/pdf-1122.pdf
- Evatt BL. Demographics of haemophilia in developing countries. Semin Thromb Hemost 2005;31:489-94. [Crossref] [PubMed]
- Aledort LM. Unsolved problems in haemophilia. Haemophilia 1998;4:341-5. [Crossref] [PubMed]
- Lee CA. Towards achieving global haemophilia care-World Federation of Haemophilia programmes. Haemophilia 1998;4:463-73. [Crossref] [PubMed]
- Isarangkura P. Haemophilia care in the developing world: benchmarking for excellence. Haemophilia 2002;8:205-10. [Crossref] [PubMed]
- Ikkala E, Helske T, Myllylä G, et al. Changes in the life expectancy of patients with severe haemophilia A in Finland in 1930-79. Br J Haematol 1982;52:7-12. [Crossref] [PubMed]
- Srivastava A, Chuansumrit A, Chandy M, et al. Management of haemophilia in the developing world. Haemophilia 1998;4:474-80. [Crossref] [PubMed]
- Jones P. Haemophilia: a global challenge. Haemophilia 1995;1:11-3. [Crossref] [PubMed]
- O’Mahony B, Black C. Expanding haemophilia care in developing countries. Semin Thromb Hemost 2005;31:561-8. [Crossref] [PubMed]
- Stonebraker JS, Brooker M, Amand RE, et al. A study of reported factor VIII use around the world. Haemophilia 2010;16:33-46. [Crossref] [PubMed]
- Saenko EL, Scandella DJ. The acidic region of the factor VIII light chain and the C2 domain together form the high affinity binding site for von willebrand factor. J Biol Chem 1997;272:18007-14. [Crossref] [PubMed]
- Wise RJ, Dorner AJ, Krone M, et al. The role of von Willebrand factor multimers and propeptide cleavage in the binding and stabilization of factor VIII. J Biol Chem 1991;266:21948-55. [PubMed]
- Koppelman SJ, Koedam JA, Wijnen M, et al. Von Willebrand factor as a regulator of intrinsic factor X activation. J Lab Clin Med 1994;123:585-93. [PubMed]
- Fay PJ, Coumans JV, Walker FJ. Von Willebrand factor mediates protection of factor VIII from activated protein C-catalyzed inactivation. J Biol Chem 1991;266:2172-77. [PubMed]
- Kallas A, Talpsep T. The von Willebrand factor collagen-binding activity assay: clinical application. Ann Hematol 2001;80:466-71. [Crossref] [PubMed]
- Geisen U, Zieger B, Lea N, et al. Comparison of Von Willebrand factor (vWF) activity vWF:Ac with vWF Ristocetin cofactor activity vWF:RCo. Thromb Res 2014;134:246-50. [Crossref] [PubMed]
- Bird A, Isarangkura P, Almagro D, et al. Factor concentrates for haemophilia in the developing world. Haemophilia 1998;4:481-85. [Crossref] [PubMed]
- Available online: http://www.gmp-compliance.org/guidemgr/files/MEDIA425.pdf
- Available online: http://www.fardodisha.gov.in/sites/default/files/pdfs/Drugs%20%26%20Cosmetic%20Act_0.pdf
- Clifton JG, Huang F, Kovac S, et al. Proteomic Characterization of Plasma -derived clotting factor VIII- Von Willebrand Factor concentrates. Electrophoresis 2009;30:3636-46. [Crossref] [PubMed]
- Metzner HJ, Hermentin P, Cuesta-Linker T, et al. Characterization of Factor VIII/ von Willebrand Factor concentrates using a modified method of von Willebrand factor multimer analysis. Haemophilia 1998;4:25-32. [Crossref] [PubMed]
- Trossaërt M, Regnault V, Siguad M, et al. Mild Haemophilia A swith Factor VIII assay discrepancy using thrombin generation assay to assess the bleeding phenotype. J Thromb Haemost 2008;6:486-93. [Crossref] [PubMed]
- Tiede A. Half-life extended factor VIII for the treatment of haemophilia A. J Thromb Haemost 2015;13:S176-S179. [Crossref] [PubMed]
- Kim IS, Yoong WC, Yong K, et al. Dry-heat treatment process for enhancing viral safety of an Antihemophilic Factor VIII concentrate prepared from Human Plasma. J Microbiol Biotechnol 2008;18:997-1003. [PubMed]
- Stadler M, Gruber G, Kannicht C, et al. Characterisation of a novel high purity, double virus inactivated von Willebrand Factor and Factor VIII concentrate (Wilate). Biologicals 2006;34:281-8. [Crossref] [PubMed]
-
European directory for the Quality of Medicines (EDQM) 2013 . - Mori F, Nardini I, Rossi P, et al. Progress in large-scale purification of factor VIII/von willebrand factor concentrates using ion-exchange chromatography. Vox Sang 2008;95:298-307. [Crossref] [PubMed]
- Schmidbauer S, Witzel R, Robbel L, et al. Physicochemical characterisation of RF-VIII-single chain, a novel recombinant single- chain Factor VIII. Thromb Res 2015;136:388-95. [Crossref] [PubMed]
- Ngo JC, Huang M, Roth DA, et al. Crystal structure of human Factor VIII: Implications for the formation of the Factor IXa-Factor VIIIa complex. Structure 2008;16:597-606. [Crossref] [PubMed]
- Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/note-guidance-plasma-derived-medicinal-products_en.pdf
Cite this article as: Dolia S, Pawar A, Bardhan S, Patel V, Verma S, Ray S. Characterization of high-purity, double virus inactivated and stable plasma-derived anti-haemophilic clotting factor-VIII/vWF complex. Ann Blood 2019;4:31.


