
The role of bone marrow stromal cells in blood diseases and clinical significance as a crucial part of the hematopoietic microenvironment
Introduction
At first, in the bone marrow, another non-hematopoietic population of multipotential members distinguished from hematopoietic stems cells was discovered to be the progenitor of myeloid stroma, which was usually termed “bone marrow stromal cells” (BMSCs) or “marrow mesenchymal stem cells” (MSCs) (1). Nowadays, further studies have demonstrated that BMSCs are a heterogenous, pluripotential group which expresses markers of multiple systems, differentiates into various lineages (e.g., endothelial cells, fibroblasts, adipocytes, macrophages, osteocytes, muscle cells, neurons, etc.), and is actually widely distributed in many parts of the body more than bone marrow (2-4). This infers that BMSCs can not only traditionally generate skeletal and mesenchymal counterparts such as bone, cartilage and fat, but also possess a great potency evolving into or repairing other tissues (1,5,6).
When concerned about the hematopoietic system only, BMSCs mainly act as the progenitor of bone and marrow structure and simultaneously are indispensable to long-term maintain hematopoiesis since they could establish the hematopoietic microenvironment (HME) that mostly contains stromal elements and cytokines for hematopoietic stem cells (HSCs) residence and blood cells production (7-11). Therefore, in the blood system, BMSCs play roles as the founder of myeloid mesenchymal section and HME, as well as the sponsor of hematopoietic members.
Nevertheless, when blood diseases occur [e.g., hematopoietic failures like aplastic anemia (AA) or malignancies], BMSCs change to become indolent or corrupt, resulting in dysfunction in HME and finally cause aggravated situation and disruption of normal hematopoiesis (12,13). The disorder in BMSCs may also be regarded as an initiating agent of hematopoietic diseases (14). Thus, by exploring the function of BMSCs and transformations in illnesses, we could deeply understand mechanisms of disease development, and then search for novel potential targets, invent effective therapeutic protocols to control disease progression and obtain the best cure effect.
A brief discussion about features of BMSCs
BMSCs were firstly isolated as the progeny of the colony-forming-unit fibroblast (CFU-F) colonies that expanded when cultured single-cell suspensions of bone marrow in vitro and showed a spindle-shaped fibroblast-like morphology and an adherent habit (1,15-17). It is now clear that BMSCs form a complicated community with heterogeneous and pluripotential nature containing sorts of phenotypes, whereas no certain unique marker has been uncovered yet (18). Surface antigens of stroma-genic differentiation like CD10, CD13, CD29, CD44, CD49e, CD73, CD90, CD92, CD105, CD146, CD166, SSEA4, Stro-1 and intracellular proteins could be detected synthetically for the identification (15,19-22). Furthermore, these myeloid progenitor stem cells express molecules mainly found in other organs like neural ganglioside GD2, nucleostemin, cardiac-specific symbols, and thus might be considered as special markers (23,24).
More importantly, not just express various markers, BMSCs could directly differentiate into multiple lineages with surprising potency and plasticity such as fibroblasts, adipocytes, endothelial cells, macrophages, osteoblasts and osteoclasts in general, which together generate the bone marrow mesenchymal tissue and what’s more, could even differentiate into hepatocyte-like cells, nerve cells, cardiac phenotypes, muscle cells for injury repair (25-29) or support the regeneration of other tissues (23,30,31). Indeed, BMSCs are prone to home to sites more than bone marrow and are widespread in all parts of the body to function appropriately or pathologically, expressing lineage-related mRNA species, mediating liver fibrosis, targeting tumor stem cells, etc. (32-34).
For the hematopoietic system we focus on, BMSCs occupies an essential position in the formation of HME, or bone marrow microenvironment including each stromal cells, cytokines and extracellular matrix to maintain the normal hematopoiesis of HSCs, which owns a considerable transcriptomic profile (9,35). Data illustrates that microenvironmental niches usually refer to several parts consists of the endosteal niche (osteocytes), vascular niche (sinusoidal endothelium), and, more importantly, reticular niche consists of primitive BMSCs (CXCL12-abundant reticular cells and nestin-expressing cells) (36,37). In these niches, stromal cell types related to bone marrow tissues and extracellular matrix assembling in complicated networks, where HSCs can settle, proliferate, and mature, producing blood components through proximity to or intimate interaction with BMSCs and their derivatives (38-40). Evidence also suggests that factors synthesized by stromal cells like stem cell factor (SCF) from leptin receptor(+) cells, transforming growth factor-beta (TGF-β) from megakaryocytes, intercellular adhesion molecule-1 (ICAM-1), etc. are required to retain the propagation of HSCs (41-43).
However, in pathological conditions, HME experiences chaos in which BMSCs impede regulatory hematopoiesis and bring about the occurrence and progression of diseases, as elaborated below.
The role of BMSCs in benign hematopoietic disorders
AA is a kind of pancytopenia-bone marrow failure disease in which HSCs suffer injury by cross talking with the surrounding microenvironment and BMSCs (44). In AA, the clonogenic and proliferative competence of BMSCs is significantly attenuated and their effect to support hematopoiesis is slashed (45). Research has shown that high percentage of BMSCs from five children with severe aplastic anemia (SAA) stagnate in aberrant sub-G1 phase of cell cycle indicating a rising rate of apoptosis and they secrete anomalous increased cytokines of interleukin-6 (IL-6), interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), and interleukin-1β (IL-1β), which contribute to an abnormal microenvironment and immune dysfunction of peripheral blood mononuclear cell (PBMC) (46). Similar evidences arise that BMSCs of AA patients markedly express lower concentrations of macrophage inflammatory protein (MIP)-1α (P<0.001) and higher TNF-α (P<0.001), granulocyte colony-stimulating factor (G-CSF) (P<0.001) and stromal cell-derived factor (SDF)-1α (P<0.01) transcripts compared to the control (47). Another deficiency of downregulated CD106(vascular cell adhesion molecule-1, VCAM-1) gene expression through the NF-κB signaling pathway in vitro is also exhibited in BMSCs from AA patients, and thus capillary tube-like formation and vasculogenesis in vivo are attenuated (48). BMSCs that produce altered hematopoiesis regulatory molecules reveal a vital role in the pathogenesis of AA.
Impaired function and defective proliferation of BMSCs exist in some more examples, such as immune thrombocytopenia (ITP) and thalassemia. BMSCs get regressive and senescent during ITP and exert less immunosuppression on T and B cells as well as induce regulatory DCs(regDCs) differentiated from CD34+ hematopoietic progenitor cells through Notch-1/Jagged-1 signaling pathway, which leads to over-activated autoimmunity and platelet destruction (49,50). Additionally, Crippa et al. (51) elucidate that BMSCs from β-thalassemia (BT) patients prove a diminished hematopoietic supportive capacity. The most primitive BMSCs pool suffers pauperization by ascending ROS production in vitro while a reduced frequency of BMSCs in vivo, is verified. The study also views a weakened antioxidative response and lacking expression of associated genes in BT-BMSCs.
In brief, during the occurrence of benign hematopoietic diseases, BMSCs usually appear to be hypofunction and serious dereliction of duty, bringing about or aggravating anemia and damage of other hemocytes.
The role of BMSCs in malignant hematopoietic tumors
In hematological malignancies, however, the bone marrow milieu could be a cradle or a shelter for hematopoietic tumor development, and BMSCs may turn into the evil crime culprit aiding tumor reproduction and metastasis. They secrete some biological or angiogenic factors, enhance tumor-related genes expression, closely interact with malignant cells, or through numerous methods in this microenvironment to induce the growth and progression of cancer and finally impede normal hematopoiesis (52-54) (Figure 1).
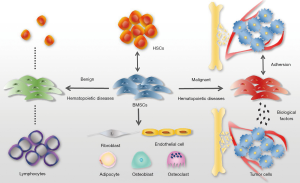
Multiple myelomas (MMs)
MM is an aberrant clonal-proliferation disease of plasma cells within the bone marrow, which causes extensive osteolytic destruction. BMSCs experience altered characterization when MM happens. The study of Arnulf et al. (55) noted that though regular phenotype, differentiation capacity, and long-term hematopoietic sponsor were retained, inferior potency suppressing T cell-reproduction and high concentration of IL-6 generation were found in BMSCs from MM patients. Moreover, Corre et al. (56) further discovered that expression property of 145 genes in BMSCs are distinct between MM and normal individuals, among which 46% might associate with the tumor-microenvironment crosstalk mainly increased IL-6 and growth and differentiation factor 15 (GDF15) levels. Other abnormal secretion profiles accounting for MM pathogenesis and progressions like IL-10, CD40/40L, VCAM1, ICAM-1, LFA-3, HO-1, HLA-DR, and HLA-ABC are also detected in MM-BMSCs (57).
MM is deemed to be a paradigm for exploring the interaction between tumor cells and the surrounding microenvironment (58,59). It has been emphasized repeatedly that the malignant MM cell generally communicates with its ecological niche(especially with BMSCs through close contact or biological factors like well-known IL-6, CXCR4 and RANKL which refers to receptor activator of nuclear factor-κB ligand) to get abnormal elevation, bone resorption, angiogenesis, drug resistance and could, in turn, inhibit the proper operation of BMSCs (60-62).
For the MM-BMSCs interaction, it’s noteworthy in recent research that adherence of malignant plasma cells to mesenchymal stem cells enhances tumor necrosis factor receptor-associated factor 6 (TRAF6) expression reciprocally by NF-κB activation, fuels spliced form of X-box binding protein-1 (XBP1s) overexpression and induces altered transcriptomic profile in vitro in BMSCs, which together promote osteoclastogenesis and the growth/survival of tumor cells (63-65). Immunosuppressive molecule B7-H1 on myeloma cells induced by IL-6 from BMSCs links to T-cell downregulation and aggressive MM cell characteristics (66), while increased levels of GDF15, B-cell-activating factor(BAFF) and the transcriptional repressor Gfi1 synthesized by BMSCs may give rise to chemoprotective effect for MM cells, poor survival for patients and inhibition of osteoblast differentiation (67-69). Besides, McNee et al. (70) imply a novel mechanism that citrullination of histone H3 arginine 26 in BMSCs from MM patients directly triggers the upregulation of IL-6 and thus incurs resistance to chemotherapy by MM cells. All of the exosomes derived from a normal donor, MM patient, and murine 5T33 BMSCs also favor tumor cell growth and drug resistance to bortezomib through several relevant survival pathways (e.g., c-Jun N-terminal kinase, p38, p53, and Akt) (71).
Meanwhile, study from patient bone marrow aspirates and C57BL/KaLwRij murine model of myeloma confirms that the plasma cell burden leads to a decrease in alkaline phosphatase osteoblasts and a rise in the incidence of STRO-1 (a stromal cell-related surface marker) positive BMSCs that express higher levels of plasma cell- and osteoclast-activating molecules (72). MM cell-produced CCL25 could, in turn, attract mesenchymal stromal cells and upregulated IL-6, IL-10, insulin growth factor-1, vascular endothelial growth factor (VEGF), and dickkopf homolog 1 expression in BMSCs promising tumor growth in vitro and in vivo (73).
Leukemia and lymphoma
Likewise, signatures of marrow stromal cells get changed when leukemia happens. The capacity of BMSCs from acute myeloid leukemia (AML) and acute lymphocytic leukemia (ALL) to maintain normal hematopoietic progenitor cells is markedly slashed compared to donors (74). Distinguished BMSCs protein expression characterizations are defined, and using RNA sequencing reveals transcriptional profiling with the deregulation of proteoglycans and adhesion factors in BMSCs with AML, whereas deregulated metabolic pathways and endocytosis in both DNA methylation and transcriptional features are uncovered by KEGG pathway enrichment analysis (75-77).
Like MM, leukemia-BMSCs usually favor tumor cell growth, invasion, drug resistance, and disease progression. In acute leukemia, Wnt and Notch signaling activation in malignant cells stimulated by BMSCs contributes to the protection, survival and chemoresistance of tumor (78,79), while in either chronic myeloid or lymphocytic leukemia (CML or CLL), BMSCs could induce mature DC (mDC) into another regulatory DC subset resulting in an immune-tolerance situation with less T cells and more Tregs proliferation through TGF-β1, and could long-term maintain CLL-cell existence due to increased soluble prosurvival factors such as CXCL12 when cocultured in 5% oxygen concentration (80,81). Furthermore, there is evidence that extracellular vesicles or exosomes play a fundamental role in crosstalk between BMSCs and leukemia cells. CML cells-derived exosome promotes BMSCs to produce IL-8 modulating tumor cell survival both in vitro and in vivo. In turn, extracellular vesicles from BMSCs prevent apoptosis and boost migration of leukemia cells as well (82,83).
Last but not least, according to several studies, BMSCs primarily act to protect non-Hodgkin’s lymphoma cells from apoptosis and can recruit follicular lymphoma cells supporting their survival (84). Conclusions depict that the protective effect may be attributed to the adhesion of malignant B cells to BMSCs through pro-survival adhesion molecule VLA-4, and ultimately the stimulation of either NF-κB pathway as well as related antiapoptotic proteins in lymphoma cells or BAFF expression in BMSCs (85-87).
Clinical significance of BMSCs in hematopoietic diseases
Transplantation and graft versus host disease (GVHD)
It has been reported that infusion with BMSCs in hematopoietic stem cell transplantation (HSCT) could repair the loss of stromal niche function and accelerate hematopoietic regeneration (88), and remarkable advantages of microenvironmental and hemopoietic compartments recovery within marrow are obtained via direct intra-bone marrow route rather than intravenous injection in mice (89-91). More importantly, the administration of BMSCs is thought to lower the incidence of GVHD following HSCT and even preserve graft-versus-leukemia activity (2,92,93). What is more, infusing BMSCs or BMSC-derived extracellular vesicles with unique microRNA property exerts great influence on the induction of T cell anergy, prevention of effector T cells differentiating from a naïve phenotype and maintenance of Treg population, which is superior to the conventional immunosuppressive regimen in curing severe acute GVHD refractory (94,95). The investigation also demonstrated that treating high grade acute GVHD with mesenchymal stromal cell end-products could achieve an overall survival of 71%±11% after a two-year follow up compared to 51.4%±9.0% in clinical statistics (96).
Potential targets of BMSCs in hematopoietic tumor treatment
Increasingly, mechanisms for tumor development correlated with BMSCs have been excavated, hence available targets could be applied to the therapeutic regimen. Experts have been working on to block the cross-talk between BMSCs and tumor cells; for example, bruton tyrosine kinase (Btk) inhibitor PCI-32765 suppresses MM cell growth induced by cocultured BMSCs and MM cell-mediated osteolysis both in vitro and in vivo (97). The pan-inhibitor of VEGF receptors, GW654652 as well as the VEGF receptor tyrosine kinase inhibitor PTK787/ZK222584, equally prevents the proliferation and migration of MM cells through inhibiting VEGF-triggered activation of downstream signaling factors, and IL-6 and VEGF secretion in tumor cells stimulated by the binding to BMSCs (98,99). Moreover, other agents like the inhibitor of oncogenic microRNA miR-21 in BMSCs, CXCR4 inhibitor AMD3100, arsenic trioxide, pentraxin 3 (PTX3) and sepantronium bromide (YM155) can not only disrupt stromal/plasma cell interaction and restrict tumor progression, but also improve the mesenchymal cell-mediated drug resistance and alleviate bone-resorbing activity in MM (100-104).
Additionally, in leukemia, targeting several pathways such as FGF2-FGFR1 and Wnt signaling which contributes to the leukemia-protective effect by BMSCs could get the disease relieved (78,105). Also, aiming at BMSCs-derived periostin that promotes CCL2 in B-ALL cells, together with platelet-derived growth factor receptors (PDGFRs) activated in CLL-BMSCs that is crucial for Akt stimulation, may reduce the tumor burden and an angiogenic switch to some extent (106,107).
Conclusions
We have summarized the features and actions of BMSCs in hematopoietic diseases. The conclusion is drawn that this kind of heterogeneous, stem-like lineage is infertile during benign anemia and rebel to collude with malignant cells and establish a distant metastasis when tumor occurs. Thus, treatment associated with BMSCs is common in transplantation and molecule-targeted therapy. However, it is still controversial if BMSCs act as a friend or a foe in oncogenesis, since they are also able to cross-present quite a few tumor antigens to overcome the immunological tolerance and express the suicide gene for anti-tumor remedy (108-111). Considering the tumor-homing ability and heterogeneity of BMSCs, the interaction of stromal and cancer cells is supposed to be complicated leading to diverse outcomes. There is an urgent need to clarify the cluster of BMSCs further, and only by creating certain solutions according to particular situations can we obtain an optimal therapeutic effect.
Acknowledgments
Funding: National Key Research and Development Program of China (2016YFC1302300); National Natural Science Foundation of China (81720108029, 81621004, 81490750, 81874226, 81803020); Guangdong Science and Technology Department (2016B030229004); Guangzhou Science Technology and Innovation Commission (201803040015).
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob.2019.12.03). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bianco P, Riminucci M, Gronthos S, et al. Bone marrow stromal stem cells: Nature, biology, and potential applications. Stem Cells 2001;19:180-92. [Crossref] [PubMed]
- Le Blanc K, Ringden O. Mesenchymal stem cells: properties and role in clinical bone marrow transplantation. Curr Opin Immunol 2006;18:586-91. [Crossref] [PubMed]
- Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002;418:41-9. [Crossref] [PubMed]
- Barbash IM, Chouraqui P, Baron J, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium - Feasibility, cell migration, and body distribution. Circulation 2003;108:863-8. [Crossref] [PubMed]
- Grove JE, Bruscia E, Krause DS. Plasticity of bone marrow-derived stem cells. Stem Cells 2004;22:487-500. [Crossref] [PubMed]
- Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurology 2002;1:92-100. [Crossref] [PubMed]
- Sacchetti B, Funari A, Michienzi S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 2007;131:324-36. [Crossref] [PubMed]
- Muguruma Y, Yahata T, Miyatake H, et al. Reconstitution of the functional human hematopoietic microenvironment derived from human mesenchymal stem cells in the murine bone marrow compartment. Blood 2006;107:1878-87. [Crossref] [PubMed]
- Shiozawa Y, Havens AM, Pienta KJ, et al. The bone marrow niche: habitat to hematopoietic and mesenchymal stem cells, and unwitting host to molecular parasites. Leukemia 2008;22:941-50. [Crossref] [PubMed]
- Anthony BA, Link DC. Regulation of hematopoietic stem cells by bone marrow stromal cells. Trends Immunol 2014;35:32-7. [Crossref] [PubMed]
- Méndez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010;466:829-34. [Crossref] [PubMed]
- McAllister SS, Weinberg RA. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat Cell Biol 2014;16:717-27. [Crossref] [PubMed]
- De Palma M, Venneri MA, Galli R, et al. Tie2 identifies a hematopoietic monocytes required for tumor lineage of proangiogenic vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 2005;8:211-26. [Crossref] [PubMed]
- Raaijmakers MH, Mukherjee S, Guo SQ, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature 2010;464:852-7. [Crossref] [PubMed]
- Mosna F, Sensebe L, Krampera M. Human Bone Marrow and Adipose Tissue Mesenchymal Stem Cells: A User's Guide. Stem Cells Dev 2010;19:1449-70. [Crossref] [PubMed]
- Jones E, McGonagle D. Human bone marrow mesenchymal stem cells in vivo. Rheumatology 2008;47:126-31. [Crossref] [PubMed]
- Charbord P. Bone Marrow Mesenchymal Stem Cells: Historical Overview and Concepts. Hum Gene Ther 2010;21:1045-56. [Crossref] [PubMed]
- Wolock SL, Krishnan I, Tenen DE, et al. Mapping Distinct Bone Marrow Niche Populations and Their Differentiation Paths. Cell Reports 2019;28:302-311.e5. [Crossref] [PubMed]
- Boxall SA, Jones E. Markers for Characterization of Bone Marrow Multipotential Stromal Cells. Stem Cells Int 2012;2012:975871 [Crossref] [PubMed]
- Siler U, Rousselle P, Muller CA, et al. Laminin gamma 2 chain as a stromal cell marker of the human bone marrow microenvironment. Br J Haematol 2002;119:212-20. [Crossref] [PubMed]
- Granéli C, Thorfve A, Ruetschi U, et al. Novel markers of osteogenic and adipogenic differentiation of human bone marrow stromal cells identified using a quantitative proteomics approach. Stem Cell Res 2014;12:153-65. [Crossref] [PubMed]
- Strioga M, Viswanathan S, Darinskas A, et al. Same or Not the Same? Comparison of Adipose Tissue-Derived Versus Bone Marrow-Derived Mesenchymal Stem and Stromal Cells. Stem Cells Dev 2012;21:2724-52. [Crossref] [PubMed]
- Rose RA, Jiang H, Wang X, et al. Bone Marrow-Derived Mesenchymal Stromal Cells Express Cardiac-Specific Markers, Retain the Stromal Phenotype, and Do Not Become Functional Cardiomyocytes In Vitro. Stem Cells 2008;26:2884-92. [Crossref] [PubMed]
- Martinez C, Hofmann TJ, Marino R, et al. Human bone marrow mesenchymal stromal cells express the neural ganglioside GD2: a novel surface marker for the identification of MSCs. Blood 2007;109:4245-8. [Crossref] [PubMed]
- Piryaei A, Valojerdi MR, Shahsavani M, et al. Differentiation of Bone Marrow-derived Mesenchymal Stem Cells into Hepatocyte-like Cells on Nanofibers and Their Transplantation into a Carbon Tetrachloride-Induced Liver Fibrosis Model. Stem Cell Rev Rep 2011;7:103-18. [Crossref] [PubMed]
- Wang L, Wang ZH, Shen CY, et al. Differentiation of human bone marrow mesenchymal stem cells grown in terpolyesters of 3-hydroxyalkanoates scaffolds into nerve cells. Biomaterials 2010;31:1691-8. [Crossref] [PubMed]
- Cai B, Li J, Wang J, et al. microRNA-124 Regulates Cardiomyocyte Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells Via Targeting STAT3 Signaling. Stem Cells 2012;30:1746-55. [Crossref] [PubMed]
- Dezawa M, Ishikawa H, Itokazu Y, et al. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science 2005;309:314-7. [Crossref] [PubMed]
- Li X, Yu X, Lin Q, et al. Bone marrow mesenchymal stem cells differentiate into functional cardiac phenotypes by cardiac microenvironment. J Mol Cell Cardiol 2007;42:295-303. [Crossref] [PubMed]
- Williams AR, Hatzistergos KE, Addicott B, et al. Enhanced Effect of Combining Human Cardiac Stem Cells and Bone Marrow Mesenchymal Stem Cells to Reduce Infarct Size and to Restore Cardiac Function After Myocardial Infarction. Circulation 2013;127:213-23. [Crossref] [PubMed]
- Sharma AK, Hota PV, Matoka DJ, et al. Urinary bladder smooth muscle regeneration utilizing bone marrow derived mesenchymal stem cell seeded elastomeric poly(1,8-octanediol-co-citrate) based thin films. Biomaterials 2010;31:6207-17. [Crossref] [PubMed]
- Li C, Kong Y, Wang H, et al. Homing of bone marrow mesenchymal stem cells mediated by sphingosine 1-phosphate contributes to liver fibrosis. J Hepatol 2009;50:1174-83. [Crossref] [PubMed]
- Direkze NC, Jeffery R, Hodivala-Dilke K, et al. Bone marrow-derived stromal cells express lineage-related messenger RNA species. Cancer Res 2006;66:1265-9. [Crossref] [PubMed]
- Shinojima N, Hossain A, Takezaki T, et al. TGF-beta Mediates Homing of Bone Marrow-Derived Human Mesenchymal Stem Cells to Glioma Stem Cells. Cancer Res 2013;73:2333-44. [Crossref] [PubMed]
- Tikhonova AN, Dolgalev I, Hu H, et al. The bone marrow microenvironment at single-cell resolution. Nature 2019;569:222-8. [Crossref] [PubMed]
- Nagasawa T, Omatsu Y, Sugiyama T. Control of hematopoietic stem cells by the bone marrow stromal niche: the role of reticular cells. Trends Immunol 2011;32:315-20. [Crossref] [PubMed]
- Ehninger A, Trumpp A. The bone marrow stem cell niche grows up: mesenchymal stem cells and macrophages move in. J Exp Med 2011;208:421-8. [Crossref] [PubMed]
- Chitteti BR, Cheng YH, Poteat B, et al. Impact of interactions of cellular components of the bone marrow microenvironment on hematopoietic stem and progenitor cell function. Blood 2010;115:3239-48. [Crossref] [PubMed]
- Hoffman CM, Calvi LM. Minireview: Complexity of Hematopoietic Stem Cell Regulation in the Bone Marrow Microenvironment. Mol Endocrinol 2014;28:1592-601. [Crossref] [PubMed]
- Gomariz A, Helbling PM, Isringhausen S, et al. Quantitative spatial analysis of haematopoiesis-regulating stromal cells in the bone marrow microenvironment by 3D microscopy. Nat Commun 2018;9:2532-46. [Crossref] [PubMed]
- Comazzetto S, Murphy MM, Berto S, et al. Restricted Hematopoietic Progenitors and Erythropoiesis Require SCF from Leptin Receptor plus Niche Cells in the Bone Marrow. Cell Stem Cell 2019;24:477-486.e6. [Crossref] [PubMed]
- Szade K, Gulati GS, Chan CKF, et al. Where Hematopoietic Stem Cells Live: The Bone Marrow Niche. Antioxid Redox Signal 2018;29:191-204. [Crossref] [PubMed]
- Liu YF, Zhang SY, Chen YY, et al. ICAM-1 Deficiency in the Bone Marrow Niche Impairs Quiescence and Repopulation of Hematopoietic Stem Cells. Stem Cell Reports 2018;11:258-73. [Crossref] [PubMed]
- Medinger M, Drexler B, Lengerke C, et al. Pathogenesis of Acquired Aplastic Anemia and the Role of the Bone Marrow Microenvironment. Front Oncol 2018;8:587-96. [Crossref] [PubMed]
- Hamzic E, Whiting K, Smith EG, et al. Characterization of bone marrow mesenchymal stromal cells in aplastic anaemia. Br J Haematol 2015;169:804-13. [Crossref] [PubMed]
- Chao YH, Lin CW, Pan HH, et al. Increased apoptosis and peripheral blood mononuclear cell suppression of bone marrow mesenchymal stem cells in severe aplastic anemia. Pediatr Blood Cancer 2018;65:e27247 [Crossref] [PubMed]
- Chaturvedi CP, Tripathy NK, Minocha E, et al. Altered Expression of Hematopoiesis Regulatory Molecules in Lipopolysaccharide-Induced Bone Marrow Mesenchymal Stem Cells of Patients with Aplastic Anemia. Stem Cells Int 2018;2018:6901761 [Crossref] [PubMed]
- Lu S, Ge M, Zheng Y, et al. CD106 is a novel mediator of bone marrow mesenchymal stem cells via NF-kappa B in the bone marrow failure of acquired aplastic anemia. Stem Cell Res Ther 2017;8:178-91. [Crossref] [PubMed]
- Xu LL, Fu HX, Zhang JM, et al. Impaired Function of Bone Marrow Mesenchymal Stem Cells from Immune Thrombocytopenia Patients in Inducing Regulatory Dendritic Cell Differentiation Through the Notch-1/Jagged-1 Signaling Pathway. Stem Cells Dev 2017;26:1648-61. [Crossref] [PubMed]
- Zhang D, Li H, Ma L, et al. Defective proliferation and the immunosuppressive function of bone marrow-derived mesenchymal stem cells in patients with primary immune thrombocytopenia. Journal of Thrombosis and Haemostasis 2013;11:300.
- Crippa S, Rossella V, Aprile A, et al. Bone marrow stromal cells from beta-thalassemia patients have impaired hematopoietic supportive capacity. J Clin Invest 2019;129:1566-80. [Crossref] [PubMed]
- Studeny M, Marini FC, Champlin RE, et al. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res 2002;62:3603-8. [PubMed]
- Quante M, Tu SP, Tomita H, et al. Bone Marrow-Derived Myofibroblasts Contribute to the Mesenchymal Stem Cell Niche and Promote Tumor Growth. Cancer Cell 2011;19:257-72. [Crossref] [PubMed]
- Askmyr M, Quach J, Purton LE. Effects of the bone marrow microenvironment on hematopoietic malignancy. Bone 2011;48:115-20. [Crossref] [PubMed]
- Arnulf B, Lecourt S, Soulier J, et al. Phenotypic and functional characterization of bone marrow mesenchymal stem cells derived from patients with multiple myeloma. Leukemia 2007;21:158-63. [Crossref] [PubMed]
- Corre J, Mahtouk K, Attal M, et al. Bone marrow mesenchymal stem cells are abnormal in multiple myeloma. Leukemia 2007;21:1079-88. [Crossref] [PubMed]
- André T, Najar M, Stamatopoulos B, et al. Immune impairments in multiple myeloma bone marrow mesenchymal stromal cells. Cancer Immunol Immunother 2015;64:213-24. [Crossref] [PubMed]
- Mitsiades CS, Mitsiades NS, Munshi NC, et al. The role of the bone microenvironment in the pathophysiology and therapeutic management of multiple myeloma: Interplay of growth factors, their receptors and stromal interactions. Eur J Cancer 2006;42:1564-73. [Crossref] [PubMed]
- Solimando AG, Da Via MC, Cicco S, et al. High-Risk Multiple Myeloma: Integrated Clinical and Omics Approach Dissects the Neoplastic Clone and the Tumor Microenvironment. J Clin Med 2019;8:997-1022. [Crossref] [PubMed]
- Shay G, Hazlehurst L, Lynch CC. Dissecting the multiple myeloma-bone microenvironment reveals new therapeutic opportunities. J Mol Med (Berl) 2016;94:21-35. [Crossref] [PubMed]
- Di Marzo L, Desantis V, Solimando AG, et al. Microenvironment drug resistance in multiple myeloma: emerging new players. Oncotarget 2016;7:60698-711. [Crossref] [PubMed]
- Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood 2006;107:1761-7. [Crossref] [PubMed]
- Morgan JJ, McAvera RM, Crawford LJ. TRAF6 Silencing Attenuates Multiple Myeloma Cell Adhesion to Bone Marrow Stromal Cells. Int J Mol Sci 2019;20:702-11. [Crossref] [PubMed]
- Xu G, Liu K, Anderson J, et al. Expression of XBP1s in bone marrow stromal cells is critical for myeloma cell growth and osteoclast formation. Blood 2012;119:4205-14. [Crossref] [PubMed]
- Garcia-Gomez A, De Las Rivas J, Ocio EM, et al. Transcriptomic profile induced in bone marrow mesenchymal stromal cells after interaction with multiple myeloma cells: implications in myeloma progression and myeloma bone disease. Oncotarget 2014;5:8284-305. [Crossref] [PubMed]
- Tamura H, Ishibashi M, Yamashita T, et al. Marrow stromal cells induce B7-H1 expression on myeloma cells, generating aggressive characteristics in multiple myeloma. Leukemia 2013;27:464-72. [Crossref] [PubMed]
- D'Souza S, del Prete D, Jin SQ, et al. Gfi1 expressed in bone marrow stromal cells is a novel osteoblast suppressor in patients with multiple myeloma bone disease. Blood 2011;118:6871-80. [Crossref] [PubMed]
- Corre J, Labat E, Espagnolle N, et al. Bioactivity and Prognostic Significance of Growth Differentiation Factor GDF15 Secreted by Bone Marrow Mesenchymal Stem Cells in Multiple Myeloma. Cancer Res 2012;72:1395-406. [Crossref] [PubMed]
- Tai YT, Li XF, Breitkreutz I, et al. Role of B-cell-activating factor in adhesion and growth of human multiple myeloma cells in the bone marrow microenvironment. Cancer Res 2006;66:6675-82. [Crossref] [PubMed]
- McNee G, Eales KL, Wei W, et al. Citrullination of histone H3 drives IL-6 production by bone marrow mesenchymal stem cells in MGUS and multiple myeloma. Leukemia 2017;31:373-81. [Crossref] [PubMed]
- Wang J, Hendrix A, Hernot S, et al. Bone marrow stromal cell-derived exosomes as communicators in drug resistance in multiple myeloma cells. Blood 2014;124:555-66. [Crossref] [PubMed]
- Noll JE, Williams SA, Tong CM, et al. Myeloma plasma cells alter the bone marrow microenvironment by stimulating the proliferation of mesenchymal stromal cells. Haematologica 2014;99:163-71. [Crossref] [PubMed]
- Xu S, Menu E, De Becker A, et al. Bone Marrow-Derived Mesenchymal Stromal Cells are Attracted by Multiple Myeloma Cell-Produced Chemokine CCL25 and Favor Myeloma Cell Growth in Vitro and In Vivo. Stem Cells 2012;30:266-79. [Crossref] [PubMed]
- Sorokina T, Shipounova I, Bigildeev A, et al. The ability of multipotent mesenchymal stromal cells from the bone marrow of patients with leukemia to maintain normal hematopoietic progenitor cells. Eur J Haematol 2016;97:245-52. [Crossref] [PubMed]
- von der Heide EK, Neumann M, Vosberg S, et al. Molecular alterations in bone marrow mesenchymal stromal cells derived from acute myeloid leukemia patients. Leukemia 2017;31:1069-78. [Crossref] [PubMed]
- Kornblau SM, Ruvolo PP, Wang RY, et al. Distinct protein signatures of acute myeloid leukemia bone marrow-derived stromal cells are prognostic for patient survival. Haematologica 2018;103:810-21. [Crossref] [PubMed]
- Huang JC, Basu SK, Zhao X, et al. Mesenchymal stromal cells derived from acute myeloid leukemia bone marrow exhibit aberrant cytogenetics and cytokine elaboration. Blood Cancer J 2015;5:e302 [Crossref] [PubMed]
- Yang Y, Mallampati S, Sun BH, et al. Wnt pathway contributes to the protection by bone marrow stromal cells of acute lymphoblastic leukemia cells and is a potential therapeutic target. Cancer Lett 2013;333:9-17. [Crossref] [PubMed]
- Takam Kamga P, Bassi G, Cassaro A, et al. Notch signalling drives bone marrow stromal cell-mediated chemoresistance in acute myeloid leukemia. Oncotarget 2016;7:21713-27. [PubMed]
- Zhao ZG, Xu W, Sun L, et al. The characteristics and immunoregulatory functions of regulatory dendritic cells induced by mesenchymal stem cells derived from bone marrow of patient with chronic myeloid leukaemia. Eur J Cancer 2012;48:1884-95. [Crossref] [PubMed]
- Fecteau JF, Messmer D, Zhang SP, et al. Impact of oxygen concentration on growth of mesenchymal stromal cells from the marrow of patients with chronic lymphocytic leukemia. Blood 2013;121:971-4. [Crossref] [PubMed]
- Crompot E, Van Damme M, Pieters K, et al. Extracellular vesicles of bone marrow stromal cells rescue chronic lymphocytic leukemia B cells from apoptosis, enhance their migration and induce gene expression modifications. Haematologica 2017;102:1594-604. [Crossref] [PubMed]
- Corrado C, Raimondo S, Saieva L, et al. Exosome-mediated crosstalk between chronic myelogenous leukemia cells and human bone marrow stromal cells triggers an Interleukin 8-dependent survival of leukemia cells. Cancer Lett 2014;348:71-6. [Crossref] [PubMed]
- Amé-Thomas P, Maby-El Hajjami H, Monvoisin C, et al. Human mesenchymal stem cells isolated from bone marrow and lymphoid organs support tumor B-cell growth: role of stromal cells in follicular lymphoma pathogenesis. Blood 2007;109:693-702. [Crossref] [PubMed]
- Mraz M, Zent CS, Church AK, et al. Bone marrow stromal cells protect lymphoma B-cells from rituximab-induced apoptosis and targeting integrin alpha-4-beta-1 (VLA-4) with natalizumab can overcome this resistance. Br J Haematol 2011;155:53-64. [Crossref] [PubMed]
- Lwin T, Hazlehurst LA, Li Z, et al. Bone marrow stromal cells prevent apoptosis of lymphoma cells by upregulation of anti-apoptotic proteins associated with activation of NF-kappa B (RelB/p52) in non-Hodgkin's lymphoma cells. Leukemia 2007;21:1521-31. [Crossref] [PubMed]
- Lwin T, Crespo LA, Wu A, et al. Lymphoma cell adhesion-induced expression of B cell-activating factor of the TNF family in bone marrow stromal cells protects non-Hodgkin's B lymphoma cells from apoptosis. Leukemia 2009;23:170-7. [Crossref] [PubMed]
- Le Blanc K, Samuelsson H, Gustafsson B, et al. Transplantation of mesenchymal stem cells to enhance engraftment of hematopoietic stem cells. Leukemia 2007;21:1733-8. [Crossref] [PubMed]
- Li Q, Hisha H, Yasumizu R, et al. Analyses of very early hemopoietic regeneration after bone marrow transplantation: Comparison of intravenous and intrabone marrow routes. Stem Cells 2007;25:1186-94. [Crossref] [PubMed]
- Abbuehl JP, Tatarova Z, Held W, et al. Long-Term Engraftment of Primary Bone Marrow Stromal Cells Repairs Niche Damage and Improves Hematopoietic Stem Cell Transplantation. Cell Stem Cell 2017;21:241-255.e6. [Crossref] [PubMed]
- Ikehara S. The Future of Stem Cell Transplantation in Autoimmune Disease. Clin Rev Allergy Immunol 2010;38:292-7. [Crossref] [PubMed]
- Krampera M, Pasini A, Pizzolo G, et al. Regenerative and immunomodulatory potential of mesenchymal stem cells. Curr Opin Pharmacol 2006;6:435-41. [Crossref] [PubMed]
- Auletta JJ, Eid SK, Wuttisarnwattana P, et al. Human Mesenchymal Stromal Cells Attenuate Graft-Versus-Host Disease and Maintain Graft-Versus-Leukemia Activity Following Experimental Allogeneic Bone Marrow Transplantation. Stem Cells 2015;33:601-14. [Crossref] [PubMed]
- Fujii S, Miura Y, Fujishiro A, et al. Graft-Versus-Host Disease Amelioration by Human Bone Marrow Mesenchymal Stromal/Stem Cell-Derived Extracellular Vesicles Is Associated with Peripheral Preservation of Naive T Cell Populations. Stem Cells 2018;36:434-45. [Crossref] [PubMed]
- Zhao L, Chen SQ, Yang PX, et al. The role of mesenchymal stem cells in hematopoietic stem cell transplantation: prevention and treatment of graft-versus-host disease. Stem Cell Res Ther 2019;10:182-94. [Crossref] [PubMed]
- Kuçi Z, Bonig H, Kreyenberg H, et al. Mesenchymal stromal cells from pooled mononuclear cells of multiple bone marrow donors as rescue therapy in pediatric severe steroid-refractory graft-versus-host disease: a multicenter survey. Haematologica 2016;101:985-94. [Crossref] [PubMed]
- Tai YT, Chang BY, Kong SY, et al. Bruton tyrosine kinase inhibition is a novel therapeutic strategy targeting tumor in the bone marrow microenvironment in multiple myeloma. Blood 2012;120:1877-87. [Crossref] [PubMed]
- Podar K, Catley LP, Tai YT, et al. GW654652, the pan-inhibitor of VEGF receptors, blocks the growth and migration of multiple myeloma cells in the bone marrow microenvironment. Blood 2004;103:3474-9. [Crossref] [PubMed]
- Lin B, Podar K, Gupta D, et al. The vascular endothelial growth factor receptor tyrosine kinase inhibitor PTK787/ZK222584 inhibits growth and migration of multiple myeloma cells in the bone marrow microenvironment. Cancer Res 2002;62:5019-26. [PubMed]
- Pitari MR, Rossi M, Amodio N, et al. Inhibition of miR-21 restores RANKL/OPG ratio in multiple myeloma-derived bone marrow stromal cells and impairs the resorbing activity of mature osteoclasts. Oncotarget 2015;6:27343-58. [Crossref] [PubMed]
- Azab AK, Runnels JM, Pitsillides C, et al. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood 2009;113:4341-51. [Crossref] [PubMed]
- Hayashi T, Hideshima T, Akiyama M, et al. Arsenic trioxide inhibits growth of human multiple myeloma cells in the bone marrow microenvironment. Mol Cancer Ther 2002;1:851-60. [PubMed]
- Basile A, Moschetta M, Ditonno P, et al. Pentraxin 3 (PTX3) inhibits plasma cell/stromal cell cross-talk in the bone marrow of multiple myeloma patients. J Pathol 2013;229:87-98. [Crossref] [PubMed]
- de Haart SJ, Holthof L, Noort WA, et al. Sepantronium bromide (YM155) improves daratumumab-mediated cellular lysis of multiple myeloma cells by abrogation of bone marrow stromal cell-induced resistance. Haematologica 2016;101:e339-e342. [Crossref] [PubMed]
- Javidi-Sharifi N, Martinez J, English I, et al. FGF2-FGFR1 signaling regulates release of Leukemia-Protective exosomes from bone marrow stromal cells. Elife 2019;8:e40033-e55. [Crossref] [PubMed]
- Ma Z, Zhao X, Deng M, et al. Bone Marrow Mesenchymal Stromal Cell-Derived Periostin Promotes B-ALL Progression by Modulating CCL2 in Leukemia Cells. Cell Rep 2019;26:1533-1543.e4. [Crossref] [PubMed]
- Ding W, Knox TR, Tschumper RC, et al. Platelet-derived growth factor (PDGF)-PDGF receptor interaction activates bone marrow-derived mesenchymal stromal cells derived from chronic lymphocytic leukemia: implications for an angiogenic switch. Blood 2010;116:2984-93. [Crossref] [PubMed]
- Xu S, De Veirman K, De Becker A, et al. Mesenchymal stem cells in multiple myeloma: a therapeutical tool or target? Leukemia 2018;32:1500-14. [Crossref] [PubMed]
- Spiotto MT, Yu P, Rowley DA, et al. Increasing tumor antigen expression overcomes "ignorance" to solid tumors via crosspresentation by bone marrow-derived stromal cells. Immunity 2002;17:737-47. [Crossref] [PubMed]
- Lee WYW, Zhang T, Lau CPY, et al. Immortalized human fetal bone marrow-derived mesenchymal stromal cell expressing suicide gene for anti-tumor therapy in vitro and in vivo. Cytotherapy 2013;15:1484-97. [Crossref] [PubMed]
- Bergfeld SA, DeClerck YA. Bone marrow-derived mesenchymal stem cells and the tumor microenvironment. Cancer Metastasis Rev 2010;29:249-61. [Crossref] [PubMed]
Cite this article as: Liang X, Song E. The role of bone marrow stromal cells in blood diseases and clinical significance as a crucial part of the hematopoietic microenvironment. Ann Blood 2020;5:2.


