
Tim-3 expression on t cells is correlated with liver inflammation, fibrosis and virological characteristics in treatment-naïve chronic hepatitis B patients: a cross-sectional study
Introduction
Although a prophylactic vaccine is available, chronic hepatitis B (CHB) remains a significant global health problem, affecting more than 240 million people. A majority of hepatitis B virus (HBV) infections eventually progress to end-stage liver diseases including cirrhosis, hepatocellular carcinoma (HCC) as well as liver failure (1).
HBV is a non-cytotoxic hepatotropic virus. The liver cell inflammation is not caused by the direct attack of the virus, but mediated by HBV induced chronic immune-mediated inflammatory response. It also mainly relies on the immune response to clear the virus, in which T cell immunity plays an important role (2-4). In acute HBV infection, the innate and adaptive immunity of the host can produce a large number of antiviral cytokines and maintain sufficient functional T cells to clear the HBV virus. While during chronic HBV infection, the immune system fails to produce enough antiviral cytokines for HBV clearing, and then a large number of HBV antigens and DNA accumulate in the peripheral blood and liver tissue, which leads to T cell dysfunction and exhaustion, which is characterized as low proliferative ability, decreased antiviral cytokine production and suppressed cytotoxicity. All of the above are important reasons for chronic and persistent HBV infection (5-7).
T cell immunoglobulin and mucin-domain-containing molecule-3 (Tim-3) is a type I transmembrane protein, and involved in a variety of metabolic and immunomodulatory processes. As a kind of T cell surface inhibitory molecule, Tim-3 can combine with Galectin-9 and then drive death of Th1 T cells, promote peripheral tolerance and contribute to the functional inactivation of CD8+ T cells during persistent viral infections (8,9). Tim-3 has been found play an extremely important role in innate and adaptive immunity of chronic HBV infection. Tim-3 can be expressed on the surface of many types of immune cells, such as cytotoxic T lymphocytes, T helper cells, natural killer cells, dendritic cells and macrophages. Tim-3 expression increased and accompanied by impaired function of the above mentioned immunocytes during chronic HBV infection. Moreover, CD8+ Tim-3+ T cells are found to be the major exhausted phenotype of T cells during the active state of HBV infection (10,11). And Tim-3 blockade can at least partially rescue impaired immune function and thus promote viral clearance (12-14).
To gain a comprehensive vision of the T cell exhaustion in CHB patients, several lines of investigations have been initiated to evaluate the phenotype and molecular biology of exhausted T cells in CHB cohorts. However, few studies have been reported to depict the expression of Tim-3 on T cells and its correlation with clinical virological indicators under different immune states of CHB patients. Hence, in this study, we intended to disclose the characteristics of Tim-3 on T cells in different phases of CHB and analyzed the correlation between Tim-3 expression and clinical virological characteristics.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/aob-20-38).
Methods
Ethical statement
The study was approved by the Institute Review Board the Third Affiliated Hospital of the Sun Yat-sen University, and conducted in full compliance with the principles of the Helsinki Declaration and national regulations. Written informed consent was obtained from all patients.
Study design and setting
This cross-sectional descriptive and analytic study about CHB patients was performed at infectious diseases clinic of the Third Affiliated Hospital of the Sun Yat-sen University (Guangzhou, China) between July 2015 and January 2017. Information of patients on the demographics and clinical were collected after enrolled. The classification and denomination of patients were based exclusively on biochemical and serologic parameters in accordance with international treatment guidelines (1). The CHB phases (immune active-IA, immune tolerant phase-IT, inactive CHB-IC, and grey zone-GZ) were classified. Classification criteria was showed in Table 1.
Table 1
| Disease phases classification | ALT | HBV-DNA | HBeAg |
|---|---|---|---|
| Immune active (IA) | Elevated | >20,000 IU/mL | Positive |
| >2,000 IU/mL | Negative | ||
| Inactive CHB (IC) | Normal | Low HBV DNA level | Negative |
| Immune tolerance (IT) | Normal | >1 million IU/mL | Positive |
| Grey zone (GZ) | Not classified as IC, IT or IA |
Upper limit of normal (ULN) of ALT: 35 U/L.
Participants
The locations, times and group-setting of participants are mentioned above. Patients with HBV infection for at least 6 months were enrolled for this study. But patients who were receiving antiviral treatment (interferon or nucleoside analogs) within the previous 6 months; patients with human immunodeficiency virus (HIV), hepatitis C virus, or hepatitis D virus co-infection; and patients with end-stage liver insufficiency, fatty liver disease, autoimmune disorders, immunosuppressive treatment, cirrhosis, and malignancies were excluded. Only qualified patients with informed consent will be included in our study. Seventeen non-HBV infected [HBV markers including HBV surface antigen (HBsAg), hepatitis Be antigen (HBeAg), hepatitis Be antibody (HBeAb), and antibody to HBV core antigen (HBcAb) negative] healthy controls (HC) without history of liver or other systematic diseases were also included in this study, who had normal concentrations of liver function enzymes.
Variables
The participants were invited to come to the hospital in the morning after fasting for at least 8 hours. We collected demographic (age and sex), personal history (alcohol use, tobacco use, medicine use), family history of liver disease (viral hepatitis, cirrhosis, liver cancer), and other symptoms data through a face-to-face interview and fill them in our designed data sheets. We measured their height and weight in order to calculate the body mass index (BMI) and drew blood for HBV markers detection [HBsAg, HBeAg, HBcAb, HBV deoxyribonucleic acid (HBV-DNA)], hepatic panel [alanine transaminase (ALT), aspartate transaminase (AST), albumin (ALB), total bilirubin (TBIL)], Blood routine test [white blood cell count (WBC), red blood cell count (RBC), platelet count (PLT)]. Besides, EDTA-anticoagulant peripheral blood was collected for Tim-3 expression detection [the frequency and mean fluorescence intensity (MFI) of Tim-3 on total T cells, CD4+ T cells, CD8+ T cells]. AST-to-platelet ratio index (APRI) was calculated to evaluation fibrosis.
Data sources and measurements
Their weight was measured (kg) with an electronic scale and their height was measured (m) with a stadiometer close to the scalp. BMI was calculated as weight/height2. Serum HBV DNA level was measured by Roche COBAS Ampliprep/COBAS Taqman HBV test v2.0 (reported unit: IU/mL, dynamic range from 20 to 1.7E+08 IU/mL, Roche Molecular Diagnostics, Branchbug, NJ). Quantified HBsAg level was measured by the Elecsys HBsAg II Quant reagent kits (reported unit: IU/mL, Roche Diagnostics, Indianapolis, IN) according to the manufacture’s instruction. HBeAg and HBcAb were tested on Roche Cobas E601 Electrochemiluminescence immunoassay analyzer (reported unit: COI, Roche Diagnostics, Indianapolis, IN). Hepatic panel (ALT, AST, ALB, TBIL) were tested on AU640 automatic biochemical analyzer (reported unit: U/L, U/L, g/L, umol/L, respectively, OLYMPUS, Shizuoka, Japan). Blood routine (WBC, RBC, PLT) was tested on Sysmex XN2000 automatic hematology analyzer (reported unit: ×109/L, ×1012/L, ×109/L, respectively, Sysmex, Kobe, Japan). Tim-3 expression frequency and MFI were determined by flow cytometry (more details will be mentioned below). A formula for calculating the APRI is given: APRI = [(AST/upper limit of normal range of AST ×100]/platelet count (109/L). The upper limit of normal (ULN) of AST is 40 U/L and 35 U/L for male and female respectively. For HBeAg titer, <1 COI was considered as negative and ≥1 COI was considered as positive. We divided HBsAg levels into three groups: <1,000 IU/mL, 1,000 to 5,000 IU/mL and >5,000 IU/mL. Similarly, we divided HBcAb titer two groups: <0.009 COI and ≥0.009 COI.
Control of the bias
Bias may occur from research design and implementation to data processing and analysis. After finishing all the surveys, data was checked by trained personnel, two people both processing data to ensure data accuracy. In order to improve the reliability of the experiments, we used isotype control in flow cytometry. The proper statistical methods were used for analysis.
Study size
PASS 15.0 software was used to calculate the sample size, at least 139 participants were needed for this study [two-sided test, confidence level (1− α) =0.95, admissible errors (δ) =5].
Statistical analysis
Descriptive analysis, qualitative data using frequency and percentage; quantitative data consistent with the normal distribution with mean and standard deviation, does not meet the normal distribution using the median. We compared the significant difference between patient groups using the Mann-Whitney test or Student t-test for numeric data, chi square analysis for qualitative data. Data are expressed as medians and interquartile range. Spearman correlation analyses and linear regression analyses were performed for examined the association between two continuous variables. Besides, the correlation between Tim-3 expression and clinical virological indicators were examined using single factor linear regression analysis and multivariate linear regression analysis where applicable. There are few missing data in this study, so we just deleted the missing data. Statistical tests were performed using SPSS software 24.0 and GraphPad Prism 8.0. P<0.05 was considered statistically significant.
Flow cytometry analysis
Peripheral blood mononuclear cells (PBMCs) were isolated from fresh blood of patients using Ficoll density gradients (Invitrogen, Grand Island, NY, USA). PBMCs were stained with FITC-CD8, PE-CD8, PE-CF594-CD3, PE-CY7-CD4, APC-Tim-3 (eBioscience, San Diego, CA). Corresponding isotype matched controls were purchased from BD Biosciences (Franklin Lakes, NJ) and eBioscience (San Diego, CA). Data were acquired on a Gallios instrument (Beckman Coulter, Brea, CA) and analysed with FlowJo software (Ashland, OR).
Results
Overview of patients’ characteristics
According to inclusion and exclusion criteria, a total of 320 patients [chronic HBV infection at different stages: immune tolerance stage (IT group) =31 cases, inactive stage (IC) =48 cases, active stage (IA) =184 cases, grey area (GZ group) =57 cases] were included in this study, and 17 normal people were used as control group (HC =17 cases). All these participants were included in our study. Some participants refused to undergo routine blood tests because the program was self-financed, which can lead to missing APRI score data. Demographic data (age, gender), body mass index, virology data [HBsAg quantification, HBeAg (+/−), HBcAb, HBV-DNA], AST, ALT, TBIL, and aspartate aminotransferase-to-platelet ratio index (APRI) score of the four groups were shown in Table 2.
Table 2
| Characteristics | IT (n=31) | IA (n=184) | IC (n=48) | GZ (n=57) | P value |
|---|---|---|---|---|---|
| Age, years, median [quartile] | 26 [24, 31] | 29.5 [25, 34.75] | 32 [28, 39] | 32 [27.5, 38] | <0.001 |
| Sex, n (%) | 0.428 | ||||
| Male | 19 (61.3) | 139 (75.5) | 35 (72.9) | 42 (73.7) | |
| Female | 12 (38.7) | 45 (24.5) | 13 (27.1) | 15 (26.3) | |
| BMI, median [quartile] | 20.55 [18.38, 22.58] | 21.01 [19.26, 22.76] | 22.18 [20.3, 23.39] | 21.19 [19.5, 23.28] | 0.058 |
| AST, U/L, median [quartile] | 25 [21, 28] | 61 [36, 107.75] | 24.5 [21.25, 29] | 26 [22, 30.5] | <0.001 |
| ALT, U/L, median [quartile] | 24 [19, 29] | 101 [55, 172.25] | 22.5 [17, 29.75] | 25 [20, 32] | <0.001 |
| ALB, g/L, median [quartile] | 44.8 [44, 47.1] | 44.8 [42.4, 46.95] | 46.8 [45.1, 48.5] | 46.65 [45.5, 48.03] | <0.001 |
| GLB, g/L, median [quartile] | 29.33 [26.35, 31.5] | 29 [26.42, 32.21] | 28.82 [27.06, 31.2] | 29.25 [26.73, 31.39] | 0.933 |
| TBIL, μmol/L, median [quartile] | 13.05 [9.45, 18.6] | 15.7 [11.75, 20.25] | 12.2 [9.375, 16.65] | 12.05 [9.3, 15.65] | <0.001 |
| HBV-DNA, Log IU/mL, median [quartile] | 8.23 [8.23, 8.23] | 7.88 [6.18, 8.23] | 1.96 [1.36, 3] | 3.78 [3.24, 4.48] | <0.001 |
| qHBsAg, IU/mL, median [quartile] | 42,428 [28,177, 52,000] | 10,433.5 [2,550.75, 33,712.75] | 1,031 [106, 3,701] | 1,278 [176.4, 2,615] | <0.001 |
| HBcAb, n (%) | 0.002 | ||||
| ≥0.009 | 24 (77.4) | 101 (54.9) | 38 (79.2) | 34 (59.6) | |
| <0.009 | 6 (19.4) | 82 (44.6) | 9 (18.8) | 23 (40.4) | |
| Missing | 1 (3.2) | 1 (0.5) | 1 (2.1) | 0 | |
| HBeAg status, n (%) | <0.001 | ||||
| Negative | 0 (0) | 42 (22.8) | 45 (93.8) | 48 (84.2) | |
| Positive | 31 (100.0) | 142 (77.2) | 3 (6.3) | 9 (15.8) | |
| APRI score, median [quartile] | 0.2897 [0.2274, 0.3732] | 0.8824 [0.5696, 1.684] | 0.3034 [0.2395, 0.3548] | 0.2995 [0.2533, 0.4448] | <0.001 |
BMI, body mass index; AST, aspartate transaminase; ALT, alanine transaminase; ALB, albumin; GLB, globulin; TBIL, total bilirubin; HBV-DNA, HBV deoxyribonucleic acid.
Distribution of Tim-3 expression in T cells and its subsets in CHB patients
To display an overall picture of Tim-3 on T cells in treatment naïve CHB, we investigated the expression of Tim-3 on CD3+ T cells, and T cell subset CD4+ and CD8+ T cells. The results were shown in Figure 1.
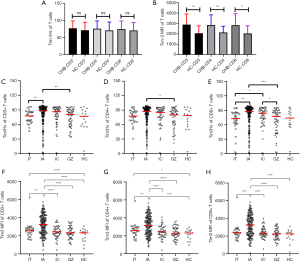
There was no significant difference in the frequency of Tim-3 on CD3+, CD4+ and CD8+ T cells between CHB patients and HC (Figure 1A). But significantly higher Tim-3 MFI expressions on CD3+, CD4+ and CD8+ T cells were observed in CHB patients compared to HC (2,750 vs. 2,326, P=0.0029; 2,733 vs. 2,306, P=0.0067; 2,569 vs. 2,246, P=0.0115, for Tim-3 MFI on CD3+, CD4+ and CD8+ T cells, respectively) (Figure 1B).
The frequency of Tim-3 in each group were 77.4%, 88.4%, 86.1%, 80.6%, and 76.8% on CD3+ T cells, 77.5%, 87.3%, 85.2%, 80.3% and 78.9% on CD4+ T cells, and 72.9%, 87.3%, 83.9%, 77.35% and 73.8% on CD8+ T cells, in the IT, IA, IC, GZ and HC group respectively. The MFI of Tim-3 was 2,598, 3,247, 2,419, 2,318 and 2,326 on CD3+ T cells, 2,593, 3,129, 2,429, 2,239 and 2,306 on CD4+ T cells, and 2,372, 3,251, 2,322, 2,237 and 2,246 on CD8+ T cells, in the IT, IA, IC, GZ and HC group respectively. Both of frequency and MFI of Tim-3 expression level was highest in the IA group in which patients had high ALT levels, among all the groups.
Correlation between Tim-3 on T Cells and HBV DNA
In order to explore the relationship between Tim-3 on T cells and HBV virology, the correlation analysis between the expression of Tim-3 on CD3+ T cells and their subsets (CD4+, CD8+) and HBV DNA was carried out, and the result was displayed in Figure 2. The results showed that: the frequency of Tim-3 expression on both CD3+ T cells and the subsets CD4+ and CD8+ T cells had no correlation with HBV DNA, the correlation coefficients were 0.0008, 0.0583 and 0.0663, and P values were 0.9937, 0.3064, and 0.2442, for CD3+, CD4+ and CD8+ T cells respectively. However, the MFI of Tim-3 on T cells and the subset T cells showed a significant positive correlation with HBV DNA, and the correlation coefficients were 0.2768, 0.2540 and 0.2478, and P values were all less than 0.0001, for CD3+, CD4+ and CD8+ T cells respectively.

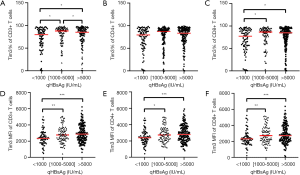
Correlation between Tim-3 on T cells and HBsAg
The correlation between Tim-3 on T cells and HBsAg levels was analyzed (Figure 3). Patients were divided into three groups referred to different HBsAg levels: <1,000 IU/mL (group A), >5,000 IU/ML (group B), and 1,000–5,000 IU/mL (group C). The expressions of Tim-3 on CD3+ T cells and CD8+ T cells were higher in group B and group C than that in group A (P<0.05). The frequency of Tim-3 on CD4+ T cells were not obviously different among the three groups. Moreover, the MFI levels of Tim-3 on CD3+ T cells, CD4+ and CD8+ T cells were all higher in group B and group C than that in group A (P<0.05).
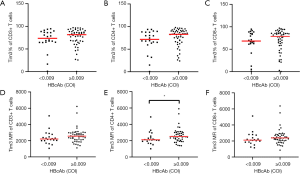
Correlation between Tim-3 on T cells and HBcAb
The correlation between Tim-3 on T cells and HBcAb was also explored (Figure 4). Patients were divided into two groups according to serum HBcAb levels: ≥0.009 COI (group A) and <0.009 COI (group B). The frequency of Tim-3 on CD3+ T cells was 81.6% and 73.6% in group A and B, and that on CD4+ T cells was 82.6% and 71.7%, that on CD8+ T cells was 78.5% and 68.1%, in group A and group B, respectively. The MFI of Tim-3 on CD3+ T cells was 2,557 and 2,209, that on CD4+ T cells was 2,512 and 2,116, and that on CD8+ T cells was 2,382 and 2,090, in group A and group B, respectively. When comparing the expression of Tim-3 between group A and group B, it was found that neither the frequency nor MFI of Tim-3 on T and subset T cells was significantly different between the two groups (P>0.05), except for Tim-3 MFI on CD4+ T cells (P<0.05).
Correlation between Tim-3 on T cells and liver inflammation
Next, we analyzed the correlation between Tim-3 on T cells and liver inflammation (ALT and AST) (Figure 5). We found that the expression levels of Tim-3 on CD3+ T cells and the subset CD4+ and CD8+ T cells were positively correlated with ALT, and the correlation coefficients were 0.213, 0.177 and 0.262, all the P value were less than 0.05, for CD3+, CD4+ and CD8+ T cells respectively. Meanwhile, the MFI of Tim-3 on T cells were also positively correlated with ALT, and the correlation coefficients were 0.473, 0.442 and 0.467, respectively, with all the P values less than 0.0001.
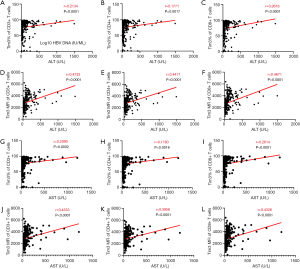
Similar to ALT, the correlation between Tim-3 on T cells and AST was also significant. It was found that both the frequency and MFI of Tim-3 on CD3+, CD4+ and CD8+ T cells were all positively correlated with AST. And correlation coefficients were 0.209, 0.178 and 0.261 (P<0.005), for frequency of Tim-3 on CD3+, CD4+ and CD8+ T cells respectively, and R=0.433, 0.396 and 0.433, for MFI of Tim-3 on CD3+, CD4+ and CD8+ T cells respectively, with all the P values less than 0.0001.
Correlation between Tim-3 on T cells and TBIL
To further explore the relationship between Tim-3 on T Cells and liver function, the correlation between Tim-3 on T cells and TBIL was analyzed (Figure 6). And the results showed that the frequency on CD8+ T cells, MFI on both T cells and the two subsets were significantly positively correlated with TBIL. The correlation coefficient was 0.134 and the P value was 0.021, for frequency of Tim-3 on CD8+ T cells. And the correlation coefficients were 0.274, 0.253 and 0.264, for MFI of Tim-3 on CD3+, CD4+ and CD8+ T cells respectively with all the P values less than 0.0001.
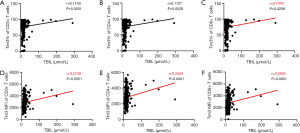
Correlation between Tim-3 on T cells and APRI score
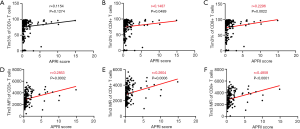
APRI score is a method of evaluating liver fibrosis and cirrhosis using AST and PLT ratios, which is an accurate method in clinic practice recommended by the world health organization. We calculated the APRI score of every patient and analyzed its correlation with Tim-3 frequency and MFI on T cells (Figure 7). The results showed that the MFI of Tim-3 on CD3+, CD4+ and CD8+ T cells were all positively correlated with APRI score, the correlation coefficients were 0.285, 0.260 and 0.486, all the P<0.001, respectively. Meanwhile, the frequency of Tim-3 on CD4+ and CD8+ T cells were positively correlated with the APRI score (correlation coefficients R=0.149, P=0.0489; R=0.229, P=0.0022, respectively).
Discussion
In this study, we focused on the relationship of Tim-3 expression on T cells with liver function, fibrosis and virology in patients with CHB virus infection, and further explored the influence of Tim-3 expression in CHB. Our results showed that the expression of Tim-3 on T and subset T cells were associated with virology (HBV-DNA, HBsAg and HBcAb), liver function (ALT, AST and TBIL), and liver fibrosis APRI score.
In the natural course of chronic HBV infection, viral load plays an important role in liver fibrosis and inflammation. And T lymphocytes continuously exposing to high levels of virus antigen is the main cause of different degree of dysfunction of T cell and HBV specific T cells (15). In HIV and HCV infection, Tim-3 was found highly expressed on CD8+ T lymphocyte, and the expression levels were positively correlated with T cell exhaustion (12,16). Tim-3 blocking could enhance the anti-tumor immunity mediated by T cells in HCC patients (14). Peng et al. found that the expression of Tim-3 on HBV-specific CD8+ T lymphocytes in patients with chronic HBV infection was significantly higher than that in the acute HBV infection patients and the healthy control, and was positively correlated with HBV-DNA virus load (17). This is consistent with the results in our current study, that Tim-3 expression was positively correlated with HBV DNA, HBsAg and HBcAb levels. High HBV antigen levels are believed to be important causes of T cell exhaustion, which eventually leads to persistent viral infection and liver damage, suggesting that Tim-3 expression or its dynamic changes could be used to reflect the exhaustion of T cell immunity.
Transaminase (ALT, AST) and bilirubin (TBIL) are important clinical indicators reflecting liver inflammatory and lesions. T cells play a critical role in HBV clearing, and associate with HBV induced liver inflammation. Liver cell apoptosis and necrosis caused by immune response are associated with the emergence of clinical hepatitis and serum transaminase elevations. Therefore, higher ALT, AST and TBIL mean heavier intrahepatic T cell immune inflammatory response. A large number of researches showed that binding of Tim-3 with its ligand galectin-9 played an important negative role in the regulation of adaptive immune response (18,19). Tim-3, which belongs to the T cell immune globulin mucin gene families, is an immunomodulatory molecular of Th1 cells. Tim-3 can adjust the Th1 cells immune function and lead to Th1 cells apoptosis (20-22). In our study, the results showed that the expression levels of Tim-3 on T cells were positively correlated with ALT, AST and TBIL. The higher the transaminase and bilirubin were, the higher the expression levels of Tim-3 were, indicating that the host may down-regulated the immune inflammation by overexpressing Tim-3 to control the activated T cell response negatively, and reduce the secretion of cytokines, which would alleviate liver inflammation.
During chronic HBV infection, long-term immune inflammatory activities in the liver tissue can cause liver fibrosis, cirrhosis, or even cancer. Therefore, we hope to find indicators that could reflect this and closely monitor liver fibrosis, in order to identify early occurrence of liver cirrhosis. In clinical studies, some CHB HBeAg+ patients have normal transaminase and liver function, but their FibroScan values and APRI score are high. And patients who have high liver fibrosis level without antiviral treatment would progress into liver cirrhosis and liver cancer (23-25). Therefore, clinical items alone are not an accurate item in reflecting immune inflammatory response in liver tissue. Up to now, the relationship between fibrosis APRI score and Tim-3 has been rarely studied, while our current research demonstrated a positive association between APRI score and the expression of Tim-3 on T cells. APRI score is an important index of liver fibrosis, Wu et al. found that, compared with healthy subjects, Tim-3 expression on peripheral blood T lymphocyte was significantly increased with in patients with chronic and acute HBV infection, and was positively correlated with the severity of disease (11). Liao et al. found that the Tim-3 gene polymorphism is associated with HBV infection in chronic HBV infection (26). Blocking Tim-3 during chronic viral infection can enhance the function of T cells. Our study found that the patients with higher the expression of Tim-3 on T cells, had higher the value of APRI score. In chronic HBV infection, the increased expression level of Tim-3 on T cells can lead to the energy and metabolic damage of T cells, and long-term defects in the number and function of T cells are the main reason for persistence of the virus, leading to the progression of liver fibrosis, which could explain the relationship of Tim-3 and APRI score in our study.
During chronic HBV infection, T cells express a variety of inhibitory molecules, and Tim-3 is just one of the inhibitory molecules on T cells. However, there are many factors that could inhibit and regulate T cell immunity besides Tim-3, while other inhibitory molecules, such as PD-1, CTLA-4 and LAG-3 have not been detected in this study, which is the limitation of our research. In future studies, the study of other inhibitory molecules and cytokines and their relationships can be explored.
In summary, this study found that Tim-3 expression on T cells in patients with CHB was associated with virology, liver inflammation and liver fibrosis, suggesting that Tim-3 expression on T cells may play important roles in the disease process and outcome in CHB patients and Tim-3 would be helpful in determination of antiviral treatment in future study.
Acknowledgments
Funding: This study was supported in part by Research and Development Planned Project in Key Areas of Guangdong Province (grant number 2019B110233002) and National Natural Science Foundation of China (grant number 81872006).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/aob-20-38
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob-20-38). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by ethical committee of the Third Affiliated Hospital of Sun Yat-sen University {No. [2014]2-66}, and conducted in full compliance with the principles of the Helsinki Declaration and national regulations. Written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358-80. [Crossref] [PubMed]
- Liaw YF, Chu CM. Hepatitis B virus infection. Lancet 2009;373:582-92. [Crossref] [PubMed]
- Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet 2014;384:2053-63. [Crossref] [PubMed]
- Maini MK, Boni C, Lee CK, et al. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J Exp Med 2000;191:1269-80. [Crossref] [PubMed]
- Webster GJ, Reignat S, Brown D, et al. Longitudinal analysis of CD8+ T cells specific for structural and nonstructural hepatitis B virus proteins in patients with chronic hepatitis B: implications for immunotherapy. J Virol 2004;78:5707-19. [Crossref] [PubMed]
- Walsh R, Locarnini S. Hepatitis B precore protein: pathogenic potential and therapeutic promise. Yonsei Med J 2012;53:875-85. [Crossref] [PubMed]
- Lunemann S, Malone DF, Hengst J, et al. Compromised function of natural killer cells in acute and chronic viral hepatitis. J Infect Dis 2014;209:1362-73. [Crossref] [PubMed]
- McIntire JJ, Umetsu SE, Akbari O, et al. Identification of Tapr (an airway hyperreactivity regulatory locus) and the linked Tim gene family. Nat Immunol 2001;2:1109-16. [Crossref] [PubMed]
- Meyers JH, Sabatos CA, Chakravarti S, et al. The TIM gene family regulates autoimmune and allergic diseases. Trends Mol Med 2005;11:362-9. [Crossref] [PubMed]
- Rong YH, Wan ZH, Song H, et al. Tim-3 expression on peripheral monocytes and CD3+CD16/CD56+natural killer-like T cells in patients with chronic hepatitis B. Tissue Antigens 2014;83:76-81. [Crossref] [PubMed]
- Wu W, Shi Y, Li J, et al. Tim-3 expression on peripheral T cell subsets correlates with disease progression in hepatitis B infection. Virol J 2011;8:113. [Crossref] [PubMed]
- Golden-Mason L, Palmer BE, Kassam N, et al. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J Virol 2009;83:9122-30. [Crossref] [PubMed]
- Wu W, Shi Y, Li S, et al. Blockade of Tim-3 signaling restores the virus-specific CD8(+) T-cell response in patients with chronic hepatitis B. Eur J Immunol 2012;42:1180-91. [Crossref] [PubMed]
- Liu F, Zeng G, Zhou S, et al. Blocking Tim-3 or/and PD-1 reverses dysfunction of tumor-infiltrating lymphocytes in HBV-related hepatocellular carcinoma. Bull Cancer 2018;105:493-501. [Crossref] [PubMed]
- Mueller SN, Ahmed R. High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A 2009;106:8623-8. [Crossref] [PubMed]
- Jones RB, Ndhlovu LC, Barbour JD, et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med 2008;205:2763-79. [Crossref] [PubMed]
- Peng G, Li S, Wu W, et al. PD-1 upregulation is associated with HBV-specific T cell dysfunction in chronic hepatitis B patients. Mol Immunol 2008;45:963-70. [Crossref] [PubMed]
- Nebbia G, Peppa D, Schurich A, et al. Upregulation of the Tim-3/galectin-9 pathway of T cell exhaustion in chronic hepatitis B virus infection. PLoS One 2012;7:e47648 [Crossref] [PubMed]
- Dookie RS, Villegas-Mendez A, Kroeze H, et al. Combinatorial Tim-3 and PD-1 activity sustains antigen-specific Th1 cell numbers during blood-stage malaria. Parasite Immunol 2020; [Crossref] [PubMed]
- Han G, Chen G, Shen B, et al. Tim-3: an activation marker and activation limiter of innate immune cells. Front Immunol 2013;4:449. [Crossref] [PubMed]
- Gorman JV, Colgan JD. Acute stimulation generates Tim-3-expressing T helper type 1 CD4 T cells that persist in vivo and show enhanced effector function. Immunology 2018;154:418-33. [Crossref] [PubMed]
- Shan NN, Hu Y, Hou M, et al. Decreased Tim-3 and its correlation with Th1 cells in patients with immune thrombocytopenia. Thromb Res 2014;133:52-6. [Crossref] [PubMed]
- Tseng TC, Liu CJ, Su TH, et al. Fibrosis-4 Index Helps Identify HBV Carriers With the Lowest Risk of Hepatocellular Carcinoma. Am J Gastroenterol 2017;112:1564-74. [Crossref] [PubMed]
- Poynard T, Vergniol J, Ngo Y, et al. Staging chronic hepatitis B into seven categories, defining inactive carriers and assessing treatment impact using a fibrosis biomarker (FibroTest®) and elastography (FibroScan®). J Hepatol 2014;61:994-1003. [Crossref] [PubMed]
- Graf C, Mondorf A, Knop V, et al. Evaluation of Point Shear Wave Elastography Using Acoustic Radiation Force Impulse Imaging for Longitudinal Fibrosis Assessment in Patients with HBeAg-Negative HBV Infection. J Clin Med 2019;8:2101. [Crossref] [PubMed]
- Liao J, Zhang Q, Liao Y, et al. Association of T-cell immunoglobulin and mucin domain-containing molecule 3 (Tim-3) polymorphisms with susceptibility and disease progression of HBV infection. PLoS One 2014;9:e98280 [Crossref] [PubMed]
Cite this article as: Gu Y, Li J, Gu L, Bi Y, Li X, Liao C, Liao X, Huang Z, Chen L, Huang Y. Tim-3 expression on t cells is correlated with liver inflammation, fibrosis and virological characteristics in treatment-naïve chronic hepatitis B patients: a cross-sectional study. Ann Blood 2020;5:12.


