
Diagnosis of von Willebrand disease in Argentina: a single institution experience
Introduction
von Willebrand disease (VWD) is the most common inherited bleeding disorder, affecting 0.1% to 1% of the total population (1,2), although the prevalence of symptomatic patients is closer to 0.01% (1). However, the true prevalence of VWD is unknown, and difficult to determine (3). Since the early eighties, our institution has been studying patients referred to us because of personal and/or family bleeding histories or abnormal pre-surgery hemostasis assays. Since then, we have established a reference center for VWD diagnosis, laboratory testing, and treatment in Argentina, with growing experience over the last four decades. During these years, we have improved our evaluation of patients through the incorporation of additional discriminating assays, including RIPA mixing tests and cryoprecipitate challenge for the differential diagnosis between platelet-type VWD (PT-VWD) and type 2B VWD, and the genotyping study of GP1BA.
In recent years, considerable advances have been made world-wide regarding the diagnosis of VWD, and including:
- the age-related increase of von Willebrand factor (VWF) levels in type 1 VWD patients may result in a normalization of hemostatic parameters and sometimes bleeding symptoms, thereby potentially also changing a patient’s ‘diagnosis’ (4); this finding has only been confirmed in the ‘mild’ VWD subgroup, with less definitive changes seen in the definite type 1 VWD subgroup (5). Increasing bleeding score (BS) in type 1 VWD (6) but no change in VWF and FVIII levels with an increased bleeding risk in type 2 VWD with aging was described (7). However, it has less extensively been explored as to whether the hemorrhagic phenotype improves with aging (8).
- findings related to the involvement of different polymorphisms on VWF:Ag levels among different ethnic and racial groups (9,10). This makes necessary the better characterization of ethnic and racial variations in VWF, otherwise resulting in ambiguity or erroneous conclusions that might have an impact on the diagnosis of VWD;
- the development of new methods to measure VWF activity (11), including: (i) VWF:GPIbR: based on the ristocetin-induced binding of VWF to a recombinant wild-type GPIb fragment (12); (ii) VWF:GPIbM: based on the spontaneous binding of VWF to a gain-of-function variant GPIb fragment (13); (iii) VWF:Ab: based on the binding of a monoclonal antibody to a VWF A1 domain epitope (12); (iv) VWF:2b3aB: to measure VWF binding to αIIb β3 (14); (v) the use of type IV and VI collagen for VWF:CB assay (15); (vi) the development of automated methods for VWF:CB assay (16);
- the development of new methods for molecular genetic testing approaches that include the use of a multi-gene panel, and next-generation sequencing (12);
- the last approach of ISTH-bleeding assessment tool (ISTH-BAT), the semi quantitative system to measure the amount of clinical bleeding, named BS, applied to VWD patients (17,18);
- the description of pathogenic variants, which have been identified in 60–65% of individuals with type 1 VWD (19,20).
Our aim in this paper is to show the phenotypic and genotypic characteristics of the VWD population assessed by our institution. Genetic testing was unfortunately only performed in a minority of our patients, given the high cost of these studies. We have therefore focused genetic testing for type 2 VWD patients.
Criteria and strategy for diagnosis
The rational diagnostic strategy for VWD patients is based on phenotypic analysis and, in specific cases, additionally on genotypic analysis. All patients undergo a minimum of two separate laboratory studies. As usual in clinical practice, the most abnormal value for any given test is considered for the final diagnosis. Possible associations between VWD and other disorders, such as hemophilia A, and B, and different thrombopathies should also be considered (21). Previously, the BS was calculated according to the “Vicenza” BS; subsequently, it has been changed to the ISTH- BAT (17), being defined as normal if 0–3 in adult males, 0–5 in adult females, and 0–2 in both male and female children (18).
Major bleeding (MB) is defined as per Schulman et al. (22), and includes transfusion requirements [recombinant or blood derivatives infusion, DDAVP (1-deamino-8-D-arginine vasopressin) or antifibrinolytic agents], need of hospitalization, surgical re-intervention, or any other urgent procedures or decisions.
Types 1 and 3 variants of VWD
It is relatively easy to distinguish type 1 from type 3 VWD by its milder bleeding tendency and VWF deficiency. Differentiation of type 1 and possible type 1 requires more effort, given the incomplete penetrance of the phenotype, the variable expression of bleeding symptoms, and difficulties in standardizing diagnostic tests (23). Subjects with low VWF (31–50 IU/dL) and mild bleeding symptoms may be considered as “low VWF” (24) or “possible” type 1 VWD (25). In these cases, family history may be negative (12). To be considered as having type 1 VWD, the subject should have VWF:RCo below the blood group specific normal range, bleeding symptoms (26) and a family history or a causative mutation. Notably, variations in the VWF sequence have been described in only 44% of type 1 patients with VWF:Ag levels ≥30 IU/dL (3).
Changes in VWF:Ag and VWF:RCo levels according to aging have been described, with an increase of 0.17 and 0.15 IU/mL per decade (4) in the normal population. It is also known that individual with blood O group have VWF levels around 25% lower than those of non-O group, probably resulting from differences in glycosylation that may alter VWF clearance rates (27). The difference is more pronounced in elderly individuals, where the VWF increases only 1.25-fold in O-group, against 1.71-fold increase in non-O group (27). This consideration may be particularly important in type 1 VWD.
Our criteria for diagnosing quantitative disorders of VWD are: possible type 1: VWF:RCo within 31–49 IU/dL, type 1 VWD with a VWF:RCo within 15–30 IU/dL, and severe type 1 (type 1S) with VWF:RCo within 5–15 IU/dL, and with a poor response to DDAVP. Type 1C comprises those cases in which VWF shows a shortened survival after DDAVP challenge test and high VWFpp ratio (28).In all these cases, VWF:RCo/VWF:Ag (RCo/Ag) are ≥0.6. Type 3 are those patients with VWF <5 IU/dL, FVIII:C usually <10 IU/dL. The use of DDAVP in type 3 VWD patients is known to be ineffective.
Type 2 variants of VWD
Type 2 VWD is characterized by an impaired VWF function, towards platelets or FVIII (29). Type 2A, 2B and 2M VWD is intially identified by RCo/Ag <0.6, with normal or slightly reduced levels of VWF:Ag and FVIII:C. In type 2N, the deficiency of VWF resides in the binding of FVIII:C to VWF; type 2N VWD is suspected when FVIII:C is very low, the FVIII:C/VWF:Ag is <0.7 and furthermore suggested when the FVIII-VWF binding assay (VWF:FVIIIB/VWF:Ag) is <0.8, with normal VWF. Until we find a candidate mutation, however, we refer to these cases as “probable VWD2N”.
Type 2A and 2M show low affinity of VWF for GPIb, reduced or absent RIPA at 1.2 mg/mL ristocetin, with normal multimers in type 2M. Type 2B shows a gain of function of VWF for GPIb and subsequently enhanced RIPA at low doses (<0.8 mg/mL), with absence of large multimers of VWF. The platelet count may range from low to normal (30). In situations of stress, such as pregnancy or response to DDAVP, thrombocytopenia is accentuated. An atypical form of type 2B has also been described, characterized by normal platelet count, normal multimers, and normal RCo/Ag (31). A proposal has been made to distinguish these as type 2B-I, being those patients lacking in large multimers, versus type 2B-II, being those with normal multimeric profile, with or without a normal VWF synthesis, in order to better classify type 2B VWD patients (32). PT-VWD is a very rare bleeding disorder, caused by the presence of mutations in GP1BA, which result in a platelet function defect, evidenced by an increased affinity of platelets for VWF. Seven mutations in the GP1BA gene related to PT-VWD have so far been described, and about 50 patients are currently reported world-wide (http://pt-vwd.org/. Accessed: November 2017). One of these mutations has been newly described by us (33). Herein, VWF is not involved per se. However, because PT-VWD patients show similar bleeding symptoms and laboratory abnormalities as type 2B VWD, their study is typically included in VWD reviews. The differential diagnosis between the two entities is especially challenging, as evidenced by high levels of misdiagnosis of both conditions, but particularly PT-VWD, which has been reported as type 2B VWD in ~15% of cases (34). Both PT-VWD and type 2BVWD may also be misdiagnosed as idiopathic thrombocytopenia (34). For this reason, the real PT-VWD incidence is unknown (34). The therapeutic management of the two disorders is different, so that discrimination is clinically essential.
Methods
Sample preparation
Peripheral venous blood is drawn in 3.13% sodium citrate for clotting tests, in 2% EDTA for DNA analysis, and with anti-proteases (2.8% sodium citrate; 60 mM N-ethylmaleimide, 50 mM EDTA (disodium ethylenediaminetetraacetate dihydrate), and 2,000 KIU/mL aprotinin) for multimeric profile. Laboratory investigation is offered to all available members of affected families.
Phenotypic analysis
The following tests are performed: complete blood count that includes platelet count (Plt); bleeding time (BT) [Ivy method, normal value (nv) ≤4.5 min]; factor VIII (FVIII:C) (35) [one-stage method, normal range (nr): 50-150 IU/dL]; VWF antigen (VWF:Ag) (36) (ELISA, nr: 50–150 IU/dL); VWF ristocetin cofactor (VWF:RCo) (37) (aggregometry, nr: 50–150 IU/dL); VWF collagen binding (VWF:CB) (ELISA, collagen type 1, nr: 60–130 IU/dL); VWF FVIII binding (VWF:FVIIIB), VWF propeptide (VWFpp) (38) (ELISA); VWF multimeric analysis (39), using SDS 1% and 1.7% agarose gel electrophoresis. FVIII/VWF:Ag (nv ≥0.7), VWF:RCo/VWF:Ag (RCo/Ag) (nv ≥0.6), VWF:CB/VWF:Ag (CB/Ag) (nv ≥0.6), and VWFpp/VWF:Ag (VWFpp ratio) (nr: 0.92–2.14) are calculated. The presence of anti-VWF antibodies in patients with either prolonged activated partial thromboplastin time (APTT) or VWF:RCo <10 IU/dL or with clinical suspicion of acquired von Willebrand syndrome (AVWS) is performed as previously described (40); however, antibodies to nonfunctional domain will be missed on such functional testing. We therefore developed an ELISA technique to detect anti-VWF antibodies as previously described (41), but with some modifications. Ristocetin induced platelet aggregation (RIPA) at 1.2 mg ristocetin/mL is the first RIPA test performed. Where RIPA 1.2 mg/mL is normal, testing is repeated using ristocetin 0.5–0.7 mg/mL, looking for the threshold ristocetin concentration; where RIPA 1.2 mg/mL is absent, testing is repeated using ristocetin at 1.5 and if required 2 mg/mL (http://www.isth.org/?page=ssc_minutes/; 56th SSC meeting of ISTH 2010. Accessed: November 2017).
When PT-VWD is suspected, discriminating methods (RIPA mixing assay and cryoprecipitate challenge assay) are performed as previously described (42,43).
Not all these tests are routinely performed on all patients. VWF:CB, multimeric analysis, and VWFpp are performed in selected patients, according to the results of previous phenotypic studies. A local plasma-pool obtained from 20 healthy donors is used as a secondary standard calibrated against the standard 07/316 of the National Institute of Biological Standards and Control (NIBSC).
As described before (3), given our lower limit of detection for VWF:RCo is 10 IU/dL, levels <10 IU/dL were taken as 5 IU/dL for calculation of means and ratios.
Genotypic analysis
There are several situations in which differential diagnosis in VWD is mandatory: between hereditary congenital and AVWS (44), between type 2N VWD and mild hemophilia A (45) and between type 2BVWD and PT-VWD (42). In all these situations, the definitive diagnosis remains the identification of mutations.
Genetic testing for VWD is not performed routinely. Since 1998, we have performed genotypic studies in patients with suspected type 2N, and subsequently in patients with suspected 2A, 2M, 2B, and PT-VWD. Genomic DNA is extracted from peripheral blood using standard methods. Exons of interest are amplified using polymerase chain reaction (PCR) technique. In probable type 2N VWD patients, exons 17 to 27 of VWF gene are analyzed. In type 2A, 2M and 2B patients, given the high frequency of mutations located in the exon 28, we start genotypic studies through the analysis of this exon (46). Subsequently, where no mutations are found, exons 26, 27, 29, 30, 31 and 52 are also analyzed. When PT-VWD is suspected, GPIBA is analyzed. Primers for PCR were designed in our laboratory. Both forward and reverse strands are directly sequenced by automated sequencing technology (ABI Prism 310 Genetic Analyzer; Applied Biosystems, Foster City, CA, USA). When mutations are found in the index cases, genotypic studies are offered to all available relatives to confirm familial transmission.
Figure 1 shows an algorithm that describes the VWD diagnostic strategy.
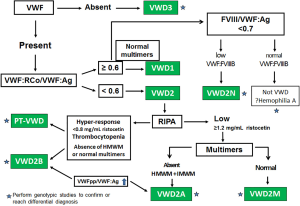
Biologic response to desmopressin
DDAVP is a synthetic analog of the vasopressin, which increases two to five times the basal level of circulating FVIII/VWF within 30–60 min and last by 6–8 hours post infusion, returning to basal levels after 24 hours (47). This effect is achieved by the FVIII/VWF releases from storage. In cases of increased clearance, levels return to base-line after 1–4 hours. DDAVP does not normally show significant side effects and it is safe from the risk of transmitting blood viruses. It is generally contraindicated in type 2B VWD because of the transient appearance or aggravation of thrombocytopenia (30). However, DDAVP was described as appropriate in type 2B VWD patients with normal multimeric pattern and platelet count (32). Because of its antidiuretic properties, such as hyponatremia, and volume overload, it should be used with caution in elderly subjects with cardiovascular or atherosclerosis disease (25), and in children below the age of 2 years (25).
In our institute, DDAVP is infused intravenously (0.3 µg/kg body weight during 20 min in 50 mL saline solution). Blood samples are obtained before, 60 and 120 min after the end of the infusion. In case of children below 20kg of body weight, samples are obtained before and 90 min after (48). In accordance with the National Heart, Lung and Blood Institute guidelines on VWD (25) we do not use DDAVP in children <2 years of age. The time courses of Plt, BT, FVIII:C, VWF:Ag, VWF:RCo and euglobulin clot lysis time are analyzed. A good response is considered when all abnormal parameters reach normal levels. A poor response is when FVIII, VWF:Ag or VWF:RCo do not reach normal levels or when these levels fall rapidly (i.e., within 2 h) after the infusion. No response means that all the parameters remain abnormal, or in type 2N VWD patients, where FVIII:C does not reach plasma levels required for hemostasis (≥50 IU/dL) (49). In these cases of accelerated fibrinolytic response, the dose of DDAVP is reduced to 0.2 µg/kg body weight, for further treatments. The success of the test depends on the infusion time, which must be strictly observed and the time between the end of the infusion and the start of the bleeding event to be prevented.
Minor local bleeding (nasal or oral bleeding) may be managed with topical hemostatic agents or local pressure for long time. The most common topical agents are: fibrin sealants, topical thrombin, micronized collagen strips (25).
The most frequent pharmacologic agents are: fibrinolysis inhibitors such as aminocaproic acid, and tranexamic acid, which are very frequently used in dental procedures and menorrhagia (50), and hormonal treatments with estrogen-progesterone combined oral contraceptives that are useful in treatment of menorrhagia (25).
In silico evaluation
In silico evaluation is being used more and more frequently by several groups to predict effects of nucleotidic changes on the integrity of the resulting protein. In our laboratory, the in silico analysis was performed using PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/. Accessed: November 2017), Panther (http://pantherdb.org/ Accessed: November 2017), and Mutation Taster (http://www.mutationtaster.org/. Accessed: November 2017). The sequence alignment of the proteins is performed using the informatics application UniProt KB (www.uniprot.org. Accessed: November 2017).
Results
Characteristics of our VWD population
Our country has nearly 44 million inhabitants. From 1982 to December 2016, a total of 5,439 patients were studied at our institution and the final VWD diagnosis was reached in 2,482 patients. We do not have an estimation of prevalence of VWD in our country, because this is a report from a single institution. A total of 83.2% of patients were diagnosed as having a quantitative VWD, whereas 16.8% were type 2 VWD. Two patients were diagnosed as PT-VWD and six patients as AVWS. The true global prevalence of the different VWD variants is unknown, but type 1 VWD is believed to represent 65–80% of all the VWD population (51). The prevalence of type 1 VWD variants in our population is quite similar to those of other reports (Table 1). Seventy-five percentage of patients were under 13 years of age at enrollment; the more severe VWD, the younger the patients. Blood O group was shown in 74% of all patients. The highest frequency of this blood group was observed in type 1 (83.1%), possible type 1 (76.1%), and type 2B (70.5%), whereas in our normal population is near 50% (52). Only 64.7% of the patients have family history of bleeding. The prevalence of females was 60.7%, especially in type 1 VWD. In patients with type probable 2N and combined type 1+probable 2N, the frequency of males is higher than females (P<0.0001).
Table 1
| Type of VWD | Overall, n (%) | Females (%) | Blood O group (%) | ≤13 years-old (%) | With family history (%) | With MB (%) |
|---|---|---|---|---|---|---|
| Quantitative variants, n=2,066 (83.2%) | ||||||
| Possible type 1 | 1,626 (78.7) | 70 | 76.1 | 24 | 61.6 | 12.8 |
| Type 1 | 308 (14.9) | 66.4 | 83.3 | 24 | 67.8 | 15.2 |
| Type 1S | 30 (1.5) | 54.8 | 72,2 | 41.9 | 66.6 | 33.3 |
| Type 1C | 8 (0.4) | 62.5 | 50 | 20 | 100 | 40 |
| 1+probable 2N | 60 (2.9) | 13.9 | 74.2 | 46.8 | 60.8 | 60.2 |
| Type 3 | 34 (1.6) | 55.8 | 73.6 | 41.2 | 82.1 | 62 |
| Qualitative variants, n=416 (16.8%) | ||||||
| Probable 2N | 31 (7.5) | 37.6 | 53.5 | 17 | 80.5 | 18 |
| 2N | 14 (3.4) | 2.4 | 50 | 28.5 | 90 | 30 |
| 2A | 35 (8.4) | 55.5 | 37.5 | 25 | 93.1 | 60 |
| 2B | 43 (10.3) | 53.4 | 70.5 | 25.6 | 84.6 | 60.4 |
| 2M | 91 (21.9) | 56.1 | 62.3 | 39.3 | 88.4 | 38.4 |
| 2 unclassified | 202 (48.6) | 54.5 | 64.4 | 30.5 | 54.8 | 20.6 |
| AVWS | 6 | 40 | 50 | 0 | 0 | 100 |
| PT-VWD | 2 | 0 | 50 | 0 | 50 | 100 |
Number and percentage of patients within their variant group. VWD, von Willebrand disease; MB, major bleeding; AVWS, acquired von Willebrand
Although 0.29% of parental consanguinity rate in normal births were reported in our country, we have one case of recognized parental consanguinity in one 2-year girl with type 3 VWD whose parents (brother and sister) were diagnosed as type 1 VWD.
Quantitative subtypes
Phenotypic analysis
Within our patients belonging to quantitative variants, possible type 1 is the most frequent. Type 3 accounts for only 1.6%, and type 1C that includes type Vicenza, for 0.4%. The laboratory data of the quantitative variants are summarized in Table 2.
Table 2
| VWD | BS | VWF:Ag, IU/dL | FVIII:C, U/dL | FVIII/Ag | VWF:RCo, IU/dL | RCo/Ag | BT, min | VWFpp ratio |
|---|---|---|---|---|---|---|---|---|
| Possible type 1 | 3.5±2.4 | 43.5±11.7 | 51±19 | 1.18±0.3 | 41.6±5.3 | 1.2±0.6 | 4.8±1.8 | ND |
| Type 1 | 4.2±2.8 | 35±11.6 | 50.6±30.3 | 1.4±0.5 | 25.8±4.2 | 0.9±0.3 | 5.2±2.6 | ND |
| Type 1S | 5.7±3.2 | 14.8±8.6 | 38±11.8 | 2.7±0.4 | 7.9±6.0 | NA | 6.2±2.7 | 1.5±0.2 |
| Type 1C | 4.7±2 | 14.1±5.8 | 35.6±13.1 | 2.5±0.9 | 10.9±5.3 | 0.9±0.2 | 5.8±2.4 | 5.3±3.5 |
| Type 1 probable 2N | 6.4±3.1 | 43±9.6 | 9.7±8.5 | 0.22±0.1 | 40.2±7 | 1±0.25 | 4.6±1.7 | ND |
| Type 3 | 9±4.8 | 1.7±1.9 | 12.4±8.9 | 7.3±1.5 | 1±0 | NA | 11.8±2.9 | ND |
VWD, von Willebrand disease; BS, bleeding score; FVIII:C, factor VIII; VWF:Ag, von Willebrand antigen; VWF:RCo, ristocetin cofactor activity; RCo/Ag, ristocetin cofactor activity/antigen ratio; BT, bleeding time; VWFpp ratio, propeptide/antigen ratio. NA, not applicant; ND, not done.
The most frequent bleeding symptoms seen in quantitative variants were bleeding after tooth extraction, bleeding related to surgery, epistaxis, bleeding related to parturition/delivery, and in females of fertile age, menorrhagia. Joint bleeding was reported in 0.77% of patients (14.7% of type 3 VWD patients). Overall, lower levels of VWF:RCo, a higher percentage of patients with bleeding symptoms, especially epistaxis and bleeding, related to surgeries.
The median of age at the enrollment in possible type 1 was 22 years (range: 1–73 years), and in type 1 patients 23 years (range: 1–67 years), both variants with 24% of patients below 13 years old. In these cases, it is important to consider that at that age of diagnosis, patients have had less time to accumulate bleeding symptoms, as highlighted previously (3). This is the case for possible type 1 VWD patients, who show a wide heterogeneity not only in their BS, but also in their MB incidence. Unexpectedly for their VWF levels, BS ≥10 was observed in 2% of patients, quite similar to that observed in type 1 (5% of patients). MB incidence was 12.8% in possible type 1 VWD, versus 15.2%in type 1 VWD (P=0.01; RR, 1.19; 95% CI: 0.89–1.60). On the other hand, 10.2% of possible type 1 VWD patients do not have personal bleeding symptoms, quite similar to type 1 patients (9.5%) (P=ns), with no difference in the frequency of blood O group (P=ns). In addition, normal levels of FVIII are found in 44% of type 1 and possible type 1 variants. Then, considering all these findings and the variability in FVIII and VWF over time, the differences between the possible types 1 and type 1 are subtle. Asymptomatic patients with low values of VWF should be regarded with caution, as many of them might not have had a hemostatic challenge to manifest a bleeding tendency. This implies a risk for bleeding that acquires an important value in challenging situations, such as surgeries.
Recently a non-significant difference in BS between blood O group and non-O blood group was described in type 1 VWD patients, with no correlation with VWF:Ag (3). In agreement with the authors, in our cohort, when possible type 1 and type 1 VWD patients were grouped according to their BS and blood group, no correlation was observed between FVIII:C, VWF:Ag, VWF:RCo and BT between blood O group and non-O group (Table 3). In addition, no difference was observed in the percentage of patients with similar BS between blood O group and non-O group.
Table 3
| VWD | Laboratory tests | Blood group (O group/non-O group) | ||
|---|---|---|---|---|
| BS ≥10 | BS from normal to 9 | Normal BS | ||
| Type 1 | % of patients | 7.3/4* | 40.2/24* | 52.5/72* |
| FVIII:C, IU/dL | 60±28/20 | 48±17/62±36* | 49±18/54±21* | |
| VWF:Ag, IU/dL | 31±6.9/20 | 32±11/41±14* | 34±11/35±17* | |
| VWF:RCo, IU/dL | 25±4.5/25 | 26±4/28±3* | 27±4/24±4* | |
| BT, min | 6±2.5/– | 5±2/3±1* | 5±2/5±2* | |
| Possible type 1 | % of patients | 2.6/2.3* | 27.3/21* | 70/76.7* |
| FVIII:C, IU/dL | 44.2±18/63±27* | 51±18/52±25* | 50±17/53±22* | |
| VWF:Ag, IU/dL | 42.6±8/47±4.7* | 42±9/43±10* | 42±11/45±12* | |
| VWF:RCo, IU/dL | 41.2±8/43±6.2* | 41±5/43±6* | 42±5/42±5* | |
| BT, min | 4.9±2.2/6±1.7* | 5±2/5±2* | 5±2/5±2* | |
VWD, von Willebrand disease; BS, bleeding score; FVIII:C, factor VIII; VWF:Ag, von Willebrand antigen; VWF:RCo, ristocetin cofactor activity; BT, bleeding time; *, not significant.
Individuals with both low VWF and FVIII/VWF:Ag <0.7 resulted in a more severe bleeding phenotype: patients with type 1+ probable 2N showed higher frequency of MB (P<0.0001; RR, 3.37, 95% CI, 2.42–4.68) than probable 2N. The same finding was observed when we compared type 1+ probable 2N versus possible type 1 (P=0.0001; RR, 4.71; 95% CI, 3.78–5.87).
Regarding type 3 VWD, the median age of enrollment was 16 years (range: 1–61years), with 41.2% of patients below 13 years and 29.4% ≥40 years. Joint bleeding was reported in 14.7% of patients. Four patients (11.7%) within seven months to 6 years of age are currently under long-term prophylactic treatment because of the severity of their bleeding episodes.
Genotypic analysis
Eight cases of type 1C VWD have been identified: two patients with type Vicenza: one of them, with heterozygous p.R1205H, and the other patient, with combined heterozygous p.R1205H and p.R924Q (53). In the remaining six patients, p.R1315C was identified in heterozygous state.
Qualitative subtypes
Phenotypic analysis
It has been described that type 2M VWD is more often misidentified than correctly identified as 2M VWD (47), giving an under-reported incidence of this variant in the literature. In our patients belonging to qualitative variants, type 2M was the most frequent, followed by type 2B, and type 2A, and type 2N. In 56% of patients overall, the subtype could not be identified because the necessary additional phenotypic and genotypic studies could not be performed.
The most frequent symptoms were bleeding after tooth extraction, bleeding related to surgery, menorrhagia, hematomas, and epistaxis. Joint bleeding was reported in 8.6% of patients (5.7% of type 2A VWD patients). The qualitative group showed higher bleeding tendency than the quantitative group (P=0.000; RR, 1.57; 95% CI, 1.33–1.87). Type 2B VWD was the group with the highest MB frequency (60.4%), followed by type 2A (60%), 2M (38.4%), type 2N (30%), and probably 2N (18%) (Table 1). Type 2A patients showed a significant higher bleeding tendency than type 2M patients (P=0.025; RR, 1.67; 95% CI 1.13–2.46), but not significantly higher than those of type 2B patients. However, a 3-year-old girl with type 2M VWD is currently under long-term prophylactic treatment.
The laboratory data are summarized in Table 4.
Table 4
| VWD | BS | VWF:Ag, IU/dL | FVIII:C, IU/dL | FVIII/Ag | VWF:RCo, IU/dL | RCo/Ag | VWF:CB, IU/dL | CB/Ag | BT, min | VWFpp ratio |
|---|---|---|---|---|---|---|---|---|---|---|
| Probable 2N | 5.4±3.2 | 88.4±30.7 | 24.4±15.3 | 0.27±0.15 | 81.4±24.4 | 0.95±0.2 | ND | ND | 4.5±1.7 | ND |
| 2A | 7.5±4.1 | 43.5±23.6 | 42.2±18.7 | 0.97±1.2 | 2.9±4.5 | 0.1±0.1 | 11.3±5.6 | 0.3±0.1 | 9±3.4 | 2.7±1.1 |
| 2B | 5.8±2.5 | 53.3±30.8 | 52.2±24.9 | 0.96±0.1 | 28.7±22.4 | 0.6±0.4 | 22±18.2 | 0.41±0.6 | 8.3±2.1 | 2.3±0.5 |
| 2M | 5.1±3.7 | 48.3±26.8 | 51.8±27.2 | 1.07±0.2 | 8.9±11.5 | 0.2±0.2 | 23.6±29 | 0.7±0.6 | 7±2.9 | 2.07±0.8 |
| 2 unclassified | 4.1±3 | 78.1±41.9 | 51±28.8 | 0.70±0.1 | 26.6±15.6 | 0.3±0.2 | 71±48 | 1±0.4 | 6.6±3.3 | ND |
| PT-VWD | 10±1 | 95±68 | 73±38 | 0.76±0.1 | 5±4.5 | 0.08±0.02 | 50.5±27.5 | 0.58±0.03 | >9 | 1.5±0.2 |
VWD, von Willebrand disease; BS, bleeding score; FVIII:C, factor VIII; VWF:Ag, von Willebrand antigen; VWF:RCo, ristocetin cofactor activity; RCo/Ag, ristocetin cofactor activity/antigen ratio; CB/Ag, collagen binding/antigen ratio; BT, bleeding time; VWFpp ratio, propeptide/antigen ratio; ND, not done.
Genotypic analysis
From the 416 patients with qualitative phenotype, responsible mutations were identified in 156 patients (37.5%).
Patients with type 2A VWD
From our total of 35 type 2A patients, the exon 28 was sequenced in 33 individuals. Six mutations were identified in heterozygosis in 30 patients: p.C1272F (n=2), p.G1505R (n=3), p.Y1542D (n=1), p.R1597W (n=16), p.I1628T (n=6) and p.F1654L (n=2). In three patients, no mutations were observed in this exon. The p.R1597W, located in the A2 domain, was the most frequent mutation in this variant.
Patients with type 2B VWD
From our total of 43 type 2B patients, seven mutations in heterozygosis were identified in 38 patients: p.Y1258C (n=1), p.P1266L (n=1), p.M1304V (n=3), p.R1306W (n=8), p.R1308C (n=9), p.S1310F (n=4), and p.V1316M (n=12).Two mutations, p.P1266L and p.M1304V were associated with atypical type 2B VWD, given the presence of normal HMWM and platelet count (54), according to Casonato’s description (31). The p.V1316M was the most frequent mutation in this variant.
Patients with type 2M VWD
Eighty out of 89 type 2M VWD patients were available for sequencing of exon 28; 14 mutations were identified in 74 patients: in heterozygosis: p.F1293C (n=2), p.R1315C (n=2), p.G1324S (n=1), p.S1325F (n=1), p.R1334Q (n=1), p.R1374C (n=18), p.R1374L (n=1), p.A1437T (n=5), p.T1468I (n=1), p.L1503P (n=2), p.E1549K (n=32), p.R1564W (n=1), p.R1583W (n=1), and p.I1628T (n=4); and in homozygosis: p.R1374C (n=2; two sisters). In the remaining 6 patients, no mutations were observed in this exon. The p.E1549K was the most frequent mutation, observed in a big family (four generations) and in one unrelated individual.
Patients with type probable 2N and type 1+probable 2N VWD
We could genotypically study 215 patients with FVIII/VWF:Ag <0.7. In this group, we found three mutations in heterozygosis in 14 patients: p.R854Q (n=6); p.R924Q (n=6), p.R816W (n=1), and a novel mutation p.D1195Y (n=1). Although type 2N VWD has been described as a recessive trait, in our case, all the patients had mutations in heterozygous state. No mutations were found in the exons 17–27 of VWF gene in the remaining 201 patients. Given that 20% of patients with responsible mutations showed normal VWF:FVIIIB/VWF:Ag, this test was not performed anymore. These patients were referred to look for mutations related to hemophilia A.
Patients with PT-VWD
Two patients with phenotypic tests suggesting type 2B VWD were studied. Discriminating methods suggested PT-VWD diagnosis. Two mutations were found in GP1BA: p.W246L (33) in one patient, and p. M255V in the other patient. In these patients, no mutations were found in exon 28 of VWF gene.
Patients with AVWS
Six patients with bleeding symptoms of late appearance showed phenotypic studies suggesting type 2 VWD. The absence of mutations to explain the phenotype helped the medical staff to redirect the studies over the search of AVWS. Elevated VWFpp ratio, the detection of antiphospholipid antibodies, platelet-associated antibodies or anti-VWD immunoglobulins (IgG, IgA or IgM) were common features among these patients. AVWS related to monoclonal gammopathy of undetermined significance was diagnosed in four patients, and related to antiphospholipid syndrome was diagnosed in the other two patients.
Discrepancies in genotype-phenotype relationships
Diagnosis for VWD can also be complicated by incomplete penetrance and variable expressivity of the conditions described above. For example, ethnicity can influence the diversity seen in VWF. Some genomic variants of VWF are seemingly pathogenic in Caucasian populations, but benign in African individuals, which may contribute to an increase in the rate of false-positive diagnoses (10,55).
Discrepancies between the VWD variants and the candidate mutations related to different phenotypes have been reported in the ISTH-SSC VWF online database (http://www.ragtimedesign.com/vwf/. Accessed: November 2017). This is the case of p.R1315C, which has been reported in association with type 2M (56), type unclassified, type 1 (57), type 2A (58), type 1C (59) and type 3. In our population, this mutation was found in nine patients with normal multimers, in whom the initial phenotype was not clear. These cases could be solved through VWFpp ratio and the DDAVP challenge test. In six patients, with high basal VWFpp ratio, very low VWF and RCo/Ag <0.6, the normalization of RCo/Ag but a rapid clearance of VWF after DDAVP infusion, suggested a type 1C phenotype; another two patients, with normal VWFpp ratio and discordant RCo/Ag before and after DDAVP infusion showed a type 2M phenotype, and other patient could not be differentiated as type 1C or 2M.
We also found discrepancy in ten patients with p.I1628T; six patients showed a 2AVWD phenotype, whereas another four patients showed a 2M VWD phenotype. This mutation was reported in the ISTH-VWF database as type 2AVWD and polymorphism (SNP). Those patients with a 2A VWD phenotype showed significant longer BS than those with type 2M phenotype (P=0.03). These data show some diagnostic problems to be taken into account during patient investigations (46).
Another real challenge was the identification of two mutations with heterozygous and trans presentation in one of our patients with a type 2M VWD phenotype. The first mutation, p.R1426C, not previously described, responsible for probable type 1 in his mother, and the second mutation, the p.Pro1648fs*45, previously described associated with type 1VWD in the VWF SSC VWF database, which is absent in parents, suggesting a de novo presentation in the patient. The combined effect of both mutations resulted in a type 2M VWD, despite being individually associated to type 1 VWD (60).
De novo mutations
Occurrence of de novo mutations is a rare event in VWD families, although there are several cases described (61-64). Its frequency within families was reported as 14.3% (62), and 26.7% (61). In our cohort, five de novo mutations were identified among 52 families, with a frequency of 9.61%: two mutations associated with type 2B VWD (p.V1316M and p.S1310F) (54), one mutation associated with type 1C (Vicenza) (p.R1205H), which has been described in combination with p.R924Q (53), one deletion associated to type 1 VWD according to the ISTH VWF database (p.Pro1648fs*45) and another mutation related to type 2A VWD (p.Y1542D).
DDAVP challenge test
Results of DDAVP challenge tests in our VWD population are summarized in Table 5. The median age of patients at the moment of testing was 18.4 (range: 3–55) years. From a total of 932 tests performed, 138 (14.8%) were <13 years.
Table 5
| VWD type | Response % of patients | ||
|---|---|---|---|
| Good | Partial | Absent | |
| Quantitative subtypes (n=843) | |||
| Possible 1 (n=677) | 99 | 1 | 0 |
| Type 1 (n=119) | 91.6 | 7.6 | 0.8 |
| Type 1S (n=14) | 43 | 57 | 0 |
| Type 1C (n=5) | 40 | 60 | 0 |
| Type 1+ probable 2N (n=18) | 38.8 | 50 | 11.2 |
| Type 3 (n=10) | 0 | 0 | 100 |
| Qualitative subtypes (n=89) | |||
| Probable 2N (n=18) | 46.5 | 32.5 | 20.9 |
| Type 2A (n=6) | 16.6 | 83.4 | 0 |
| Type 2B (n=9) | 33.3 | 55.5 | 11.1 |
| Type 2M (n=27) | 44.4 | 48.1 | 7.4 |
| Type 2 unclassified (n=29) | 62.1 | 31 | 6.9 |
VWD, von Willebrand disease; DDAVP, 1-deamino-8-D-arginine vasopressin.
Quantitative subtypes: over a total of 843 tests done, 93% of patients showed a good response whereas 4.2% showed a partial response and 1.5% did not respond. A 12.3% of patients were <13 years. Only one type 1 VWD patient was 3 years old.
Qualitative subtypes: DDAVP response test was assessed in 89 patients. A total of 47.4% of these patients showed a good response, whereas 40.4% had a partial response, and 12.2% did not respond. A 38.2% of patients were <13 years, especially in probable 2N VWD. Only five patients were between 2–4 years old.
DDAVP challenge test was very useful in identifying some patients with difficult interpretation of tests results, and the subsequent classification such as discriminating type 3 from severe type 1, and type 2M from type 1C when the VWF levels were inconclusive or too low. This was the case of two patients, who developed thrombocytopenia, and by this way, the diagnostic approach towards type 2B VWD was addressed. Another similar case was our group of patients with the mutation p.R1315C, which was commented on previously.
Conclusions
The wide heterogeneity of clinical symptoms and laboratory data from possible type 1 implies a risk for bleeding that becomes crucial in challenging situations.
In our population, type 2M VWD was found more frequent than type 2A variant.
Because of the absence of significant side effects in our patients, its effectiveness and low cost, DDAVP should be considered as the first-choice therapy in VWD, especially in developing countries.
DDAVP challenge test, VWF propeptide and genotypic studies were very useful tools for discriminating those cases with difficult diagnoses, such as type 2M versus type 1C VWD. Elevated VWFpp ratio, the detection of antiphospholipid antibodies, platelet-associated antibodies or anti-VWF immunoglobulins (IgG, IgA or IgM) and the negative genotypic screening were useful to discriminate inherited VWD from AVWS. Genotypic testing also helped us to discriminate between type 2B versus PT-VWD and type 2N VWD versus mild hemophilia A.
Acknowledgments
We are grateful to Maria Marta Casinelli for her excellent technical assistance. This work was supported by CONICET, Fundación René Barón, and Academia Nacional de Medicina, Buenos Aires city, Argentina.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Emmanuel J. Favaloro) for the series “Diagnosis and Management of von Willebrand Disease: Diverse Approaches to a Global and Common Bleeding Disorder” published in Annals of Blood. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob.2017.12.04). The series “Diagnosis and Management of von Willebrand Disease: Diverse Approaches to a Global and Common Bleeding Disorder” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional ethical approval was waived. This study is included within the framework of the project whose number of minutes is 92/November 10/2016. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sadler JE, Mannucci PM, Berntorp E, et al. Impact, diagnosis and treatment of von Willebrand disease. Thromb Haemost 2000;84:160-74. [PubMed]
- Bowman M, Hopman WM, Rapson D, et al. The prevalence of symptomatic von Willebrand disease in primary care practice. J Thromb Haemost 2010;8:213-6. [Crossref] [PubMed]
- Flood VH, Christopherson PA, Gill JC, et al. Clinical and laboratory variability in a cohort of patients diagnosed with type 1 VWD in the United States. Blood 2016;127:2481-8. [Crossref] [PubMed]
- Rydz N, Grabell J, Lillicrap D, et al. Changes in von Willebrand factor level and von Willebrand activity with age in type 1 von Willebrand disease. Haemophilia 2015;21:636-41. [Crossref] [PubMed]
- Borghi M, Guglielmini G, Mezzasoma AM, et al. Increase of von Willebrand factor with aging in type 1 von Willebrand disease: fact or fiction? Haematologica 2017;102:e431-3. [Crossref] [PubMed]
- Tosetto A, Rodeghiero F, Castaman G, et al. A quantitative analysis of bleeding symptoms in type 1 von Willebrand disease: results from a multicenter European study (MCMDM-1 VWD). J Thromb Haemost 2006;4:766-73. [Crossref] [PubMed]
- Sanders YV, Giezenaar MA, Laros-van Gorkom BA, et al. von Willebrand disease and aging: an evolving phenotype. J Thromb Haemost 2014;12:1066-75. [Crossref] [PubMed]
- Abou-Ismail MY, Ogunbayo GO, Secic M, et al. Outgrowing the laboratory diagnosis of type 1 von Willebrand disease: A two decade study. Am J Hematol 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Flood VH, Gill JC, Morateck PA, et al. Common VWF exon 28 polymorphisms in African Americans affecting the VWF activity assay by ristocetin cofactor. Blood 2010;116:280-6. [Crossref] [PubMed]
- Bellissimo DB, Christopherson PA, Flood VH, et al. VWF mutations and new sequence variations identified in healthy controls are more frequent in the African-American population. Blood 2012;119:2135-40. [Crossref] [PubMed]
- Bodó I, Eikenboom J, Montgomery R, et al. Platelet-dependent von Willebrand factor activity. Nomenclature and methodology: communication from the SSC of the ISTH. J Thromb Haemost 2015;13:1345-50. [Crossref] [PubMed]
- Goodeve A, James P. von Willebrand Disease. In: Adam MP, Ardinger HH, Pagon RA, et al. editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle, 1993-2017.
- Flood VH, Gill JC, Morateck PA, et al. Gain-of-function GPIb ELISA assay for VWF activity in the Zimmerman Program for the Molecular and Clinical Biology of VWD. Blood 2011;117:e67-74. [Crossref] [PubMed]
- Montgomery RR, Flood VH. What have we learned from large population studies of von Willebrand disease? Hematology Am Soc Hematol Educ Program 2016;2016:670-7.
- Flood VH, Schlauderaff AC, Haberichter SL. Crucial role for the VWF A1 domain in binding to type IV collagen. Blood 2015;125:2297-304. [Crossref] [PubMed]
- Favaloro EJ, Mohammed S. Evaluation of a von Willebrand factor three test panel and chemiluminescent-based assay system for identification of, and therapy monitoring in, von Willebrand disease. Thromb Res 2016;141:202-11. [Crossref] [PubMed]
- Rodeghiero F, Tosetto A, Abshire T, et al. ISTH/SSC bleeding assessment tool: a standardized J Thromb Haemost 2010;8:2063-5. [Crossref] [PubMed]
- Elbatarny M, Mollah S, Grabell J, et al. Normal range of bleeding scores for the ISTH-BAT: adult and pediatric data from the merging project. Haemophilia 2014;20:831-5. [Crossref] [PubMed]
- Batlle J, Pérez-Rodríguez A, Corrales I, et al. Molecular and clinical profile of von Willebrand disease in Spain (PCM-EVW-ES): Proposal for a new diagnostic paradigm. Thromb Haemost 2016;115:40-50. [Crossref] [PubMed]
- Yadegari H, Driesen J, Pavlova A, et al. Mutation distribution in the von Willebrand factor gene related to the different von Willebrand disease (VWD) types in a cohort of VWD patients. Thromb Haemost 2012;108:662-71. [Crossref] [PubMed]
- Woods AI, Sánchez-Luceros A, Meschengieser SS, et al. Diagnosis and management of von Willebrand disease in a single institution of Argentina. Semin Thromb Hemost 2011;37:568-75. [Crossref] [PubMed]
- Schulman S, Kearon C. Definition of major bleeding in clinical investigation of antihemostatic medicinal products in non-surgical patients. Scientific and Standardization Committee Communication. J Thromb Haemost 2005;3:692-4. [Crossref] [PubMed]
- Castaman G, Linari S. Diagnosis and Treatment of von Willebrand Disease and Rare Bleeding Disorders. J Clin Med 2017;6:E45 [Crossref] [PubMed]
- Sadler JE. Low von Willebrand factor: sometimes a risk factor and sometimes a disease. Hematology Am Soc Hematol Educ Program 2009;106-12. [Crossref] [PubMed]
- Nichols WL, Hultin MB, James AH, et al. von Willebrand disease (VWD): evidence-based diagnosis and management guidelines, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA). Haemophilia 2008;14:171-232. [Crossref] [PubMed]
- Favaloro EJ, Lillicrap D, Lazzari MA, et al. von Willebrand disease: laboratory aspects of diagnosis and treatment. Haemophilia 2004;10:164-8. [Crossref] [PubMed]
- Albánez S, Ogiwara K, Michels A, et al. Aging and ABO blood type influence von Willebrand factor and factor VIII levels through interrelated mechanisms. J Thromb Haemost 2016;14:953-63. [Crossref] [PubMed]
- Haberichter SL, Balistreri M, Christopherson P, et al. Assay of the von Willebrand factor (VWF) propeptide to identify patients with type 1 von Willebrand disease with decreased VWF survival. Blood 2006;108:3344-51. [Crossref] [PubMed]
- Sadler JE, Budde U, Eikenboom JC, et al. Update on the pathophysiology and classification of von Willebrand disease: a report of the Subcommittee on von Willebrand Factor. J Thromb Haemost 2006;4:2103-14. [Crossref] [PubMed]
- Federici AB, Mannucci PM, Castaman G, et al. Clinical and molecular predictors of thrombocytopenia and risk of bleeding in patients with von Willebrand disease type 2B: a cohort study of 67 patients. Blood 2009;113:526-34. [Crossref] [PubMed]
- Casonato A, Gallinaro L, Cattini MG, et al. Reduced survival of type 2B von Willebrand factor, irrespective of large multimer representation or thrombocytopenia. Haematologica 2010;95:1366-72. [Crossref] [PubMed]
- Casonato A, Daidone V, Galletta E, et al. Type 2B von Willebrand disease with or without large multimers: A distinction of the two sides of the disorder is long overdue. PLoS One 2017;12:e0179566 [Crossref] [PubMed]
- Woods AI, Sánchez-Luceros A, Bermejo E, et al. Identification of p.W246L as a novel mutation in the GP1BA gene responsible for platelet-type von Willebrand disease. Semin Thromb Hemost 2014;40:151-60. [Crossref] [PubMed]
- Hamilton A, Ozelo M, Leggo J, et al. Frequency of platelet type versus type 2B von Willebrand disease. An international registry based study. Thromb Haemost 2011;105:501-8. [Crossref] [PubMed]
- Proctor RR, Rapaport SI. The partial thromboplastin time with kaolin. A simple screening test for first stage plasma clotting factor deficiencies. Am J Clin Pathol 1961;36:212-9. [Crossref] [PubMed]
- Taylor LD. The application of the biotin-avidin system to the von Willebrand factor antigen immunoassay. Thromb Haemost 1988;59:251-4. [PubMed]
- Macfarlane DE, Stibbe J, Kirby EP, et al. Letter: A method for assaying von Willebrand factor (ristocetin cofactor). Thromb Diath Haemorrh 1975;34:306-8. [PubMed]
- Borchiellini A, Fijnvandraat K, ten Cate JW, et al. Quantitative analysis of von Willebrand factor propeptide release in vivo: effect of experimental endotoxemia and administration of 1-deamino-8-d-arginine vasopressin in humans. Blood 1996;88:2951-8. [PubMed]
- Farias C, Kempfer AC, Blanco A, et al. Visualization of the multimeric structure of von Willebrand factor by immunoenzymatic stain using avidin-peroxidase complex instead of avidin-biotin peroxidase complex. Thromb Res 1989;53:513-8. [Crossref] [PubMed]
- Kasper CK. Laboratory tests for factor VIII inhibitors, their variation, significance and interpretation. Blood Coagul Fibrinolysis 1991;51:7.
- Siaka C, Rugeri L, Caron C, et al. A new ELISA assay for diagnosis of acquired von Willebrand Syndrome. Haemophilia 2003;9:303-8. [Crossref] [PubMed]
- Favaloro EJ. Phenotypic identification of platelet-type von Willebrand disease and its discrimination from type 2B von Willebrand disease: a question of 2B or not 2B? A story of nonidentical twins? Or two sides of a multidenominational or multifaceted primary-hemostasis coin? Semin Thromb Hemost 2008;34:113-27. [Crossref] [PubMed]
- Enayat MS, Guilliatt AM, Lester W, et al. Distinguishing between type 2B and pseudo-von Willebrand disease and its clinical importance. Br J Haematol 2006;133:664-6. [Crossref] [PubMed]
- Federici AB. Acquired von Willebrand syndrome: an underdiagnosed and misdiagnosed bleeding complication in patients with lymphoproliferative and myeloproliferative disorders. Semin Hematol 2006;43:S48-58. [Crossref] [PubMed]
- Mazurier C, Hilbert L. Type 2N von Willebrand disease. Curr Hematol Rep 2005;4:350-8. [PubMed]
- Woods AI, Kempfer A C, Keller L, et al. Genotypic diagnosis of VWD: preliminary results in the analysis of exon 28. J Thromb Haemost 2009;7. Abstract 610.
- Favaloro EJ, Pasalic L, Curnow J. Type 2M and Type 2A von Willebrand Disease: Similar but Different. Semin Thromb Hemost 2016;42:483-97. [Crossref] [PubMed]
- Sánchez-Luceros A, Meschengieser SS, Woods AI, et al. Biological and clinical response to desmopressin (DDAVP) in a retrospective cohort study of children with low von Willebrand factor levels and bleeding history. Thromb Haemost 2010;104:984-9. [Crossref] [PubMed]
- Woods AI, Meschengieser SS, Blanco AN, et al. Clinical features and laboratory patterns in a cohort of consecutive Argentinian patients with von Willebrand`s disease. Haematologica 2001;86:420-7. [PubMed]
- Kouides PA, Byams VR, Philipp CS, et al. Multisite management study of menorrhagia with abnormal laboratory haemostasis: a prospective crossover study of intranasal desmopressin and oral tranexamic acid. Br J Haematol 2009;145:212-20. [Crossref] [PubMed]
- Swystun LL, James PD. Genetic diagnosis in hemophilia and von Willebrand disease. Blood Rev 2017;31:47-56. [Crossref] [PubMed]
- Dean L. Blood Groups and Red Cell Antigens [Internet]. Bethesda, MD: National Center for Biotechnology Information (US), 2005: Chap 2.
- Woods AI, Kempfer AC, Sánchez-Luceros A, et al. Clinical profile of the association of p.R1205H and p.R924Q in a patient with von Willebrand disease Haemophilia 2013;19:e180-1. (letter). [Crossref] [PubMed]
- Woods AI, Kempfer AC, Paiva J, et al. Phenotypic parameters in genotypically selected type 2B von Willebrand Disease patients: a large single center experience including a new novel mutation. Semin Thromb Hemost 2017;43:92-100. [PubMed]
- Johnsen JM, Nickerson DA, Reiner AP. Massively parallel sequencing: the new frontier of hematologic genomics. Blood 2013;122:3268-75. [Crossref] [PubMed]
- Casaña P, Martínez F, Espinós C, et al. Search for mutations in a segment of the exon 28 of the human von Willebrand factor gene: new mutations, R1315C and R1341W, associated with type 2M and 2B variants. Am J Hematol 1998;59:57-63. [Crossref] [PubMed]
- James PD, Notley C, Hegadorn C, et al. The mutational spectrum of type 1 von Willebrand disease: Results from a Canadian cohort study. Blood 2007;109:145-54. [Crossref] [PubMed]
- Ribba AN, Hilbert L, Lavergne JM, et al. The arginine-552-cysteine (R1315C) mutation within the A1 loop of von Willebrand factor induces an abnormal folding with a loss of function resulting in. type 2A-like phenotype of von Willebrand disease: study of 10 patients and mutated recombinant von Willebrand factor. Blood 2001;97:952-9. [Crossref] [PubMed]
- Robertson JD, Yenson PR, Rand ML, et al. Expanded phenotype-genotype correlations in a pediatric population with type 1 von Willebrand disease. J Thromb Haemost 2011;9:1752-60. [Crossref] [PubMed]
- Woods AI, Paiva J, Kempfer AC, et al. Combined effects of two mutations in von Willebrand disease 2M phenotype. Res Pract Thromb Haemost 2017; In Press. [Crossref]
- Shen MC, Chen M, Ma GC, et al. De novo mutation and somatic mosaicism of gene mutation in type 2A, 2B and 2M VWD. Thromb J 2016;14:36. [Crossref] [PubMed]
- Ahmad F, Oyen F, Jan R, et al. Germline de novo mutations and linkage markers vs. DNA sequencing for carrier detection in von Willebrand disease. Haemophilia 2014;20:e311-7. [Crossref] [PubMed]
- Lester WA, Guilliatt AM, Surdhar GK, et al. Inherited and de novo von Willebrand disease 'Vicenza' in UK families with the R1205H mutation: diagnostic pitfalls and new insights. Br J Haematol 2006;135:91-6. [Crossref] [PubMed]
- Murray EW, Giles AR, Lillicrap D. Germ-line mosaicism for a valine-to-methionine substitution at residue 553 in the glycoprotein Ib-binding domain of von illebrand factor, causing type IIB von Willebrand disease. Am J Hum Genet 1992;50:199-207. [PubMed]
Cite this article as: Woods AI, Kempfer AC, Paiva J, Blanco AN, Sánchez-Luceros A, Lazzari MA. Diagnosis of von Willebrand disease in Argentina: a single institution experience. Ann Blood 2017;2:22.


