Regulation of plasma for fractionation in the United States
Introduction
Plasma for fractionation (PF) is a precious biological resource used as a raw material to manufacture essential (1), life-saving, plasma-derived medicinal products (PDMPs) including clotting factors, albumin, and immunoglobulins. As a biological product derived from human donors, its collection, quality, and availability raise a host of ethical, safety, and scientific issues. The United States (U.S.) is the largest supplier of PF in the world, given its policy of allowing frequent donations from paid donors, while most other countries seek to obtain plasma from volunteer unpaid donors (VUDs). Some have questioned the safety and quality of U.S. plasma, and the health of U.S. donors considering the monetary incentive to donate to be a corrupting influence. Here we will discuss the current world-wide demand for PF, challenges in meeting that demand, the voluntary standards of the commercial fractionation industry, and the regulatory role of the U.S. Food and Drug Administration (FDA) in assuring that PF collected from healthy U.S. donors meets international standards of quality, safety, and potency. We will also compare the U.S. regulatory requirements for components of PF to some of those in Europe, and to the recommendations of the World Health Organization (WHO).
Unmet need for PF
The demand for PF to make PDMPs is great, and growing, especially in low and middle-income countries (LMIC). The World Federation of Hemophilia (WFH) estimates that only about 25% of people with hemophilia receive adequate treatment globally. The International Patient Organization for Primary Immune Deficiencies calculates that at least 70% of individuals with primary immune deficiencies do not have the care they need because they lack access to immunoglobulin products (2). The total demand for plasma in China in 2016 was 12 million liters, but their collection centers only produced about 5.5 million liters (3). Hundreds of thousands of newborns with Rh hemolytic disease of the newborn die or have brain damage each year because anti-D hyper immunoglobulin is unavailable (4-6). Some countries make no attempt to diagnose patients simply because no specific PDMP is available to treat them (6).
Current global collection of PF
In 2015, roughly 60% of PF worldwide came from North America, 20% from Europe, 14% from Asia and Oceania, and the remainder from Latin America, the Middle East, and Africa (2). U.S. commercial blood establishments supply the largest proportion of the plasma used for PF. They obtain plasma by plasmapheresis, mostly from paid donors, and label it exclusively for manufacturing (source plasma). Collection of source plasma in the U.S. has expanded rapidly over the past few years to meet increasing demand, especially for immunoglobulins. The Plasma Protein Therapeutics Association (PPTA), the international trade and voluntary standard setting association representing the largest plasma derivative manufacturers, reported that collections from U.S. donors jumped from 29 million in 2013 to 38 million in 2016 (7). This translates to 30 million liters of plasma collected in 2016, assuming a plasmapheresis volume 800 mL per donation. Members of PPTA, including those in Germany, the Czech Republic, Hungary and Austria, provided more than 80% of the world’s Source Plasma for fractionation in 2017 (8). PF collected from U.S. donors not only fulfills the needs of U.S. licensed fractionators, but supplies much of the rest of the world, including over 60% of PF used in Europe (9).
Recovered plasma, i.e., plasma obtained as a byproduct from a whole blood donation and used for fractionation (6), comprises a smaller proportion of PF than source plasma. The volume of recovered plasma, collected at not-for-profit blood facilities from VUDs, had remained steady for many years but now is declining in the developed world (2,10). Improvements in blood management, and changes in surgical procedures have lessened the need for blood components (2), and in 2015 recovered plasma accounted for only 13% of fractionated plasma worldwide (4). In the U.S. in 2013, recovered plasma was less than 10% of the total plasma used for PF (7,11), and blood collection for transfusion from not-for-profit blood facilities have continued to decline precipitously through 2017 (12).
Although the volume of recovered plasma collected in the developed world has decreased, it has increased in low and moderate income countries (LMICs). A WHO survey (13) reported that, from 2008 to 2013, the number of blood donations from VUDs increased by 10.7 million in 159 countries. The highest increase was in the South-East Asian (75%) and African (37%) regions.
Filling the gap
Relying on a single major source of PF is unwise. The supply of PF can be interrupted by regional emerging infectious diseases, e.g., variant Creutzfeldt-Jakob disease in Great Britain and Europe; industry shutdowns for prolonged periods, e.g., voluntary suspensions by U.S. fractionators in the late 1990’s to meet regulatory requirements (14); and other natural and man-made disasters (2).
The WHO strongly supports increasing the availability of plasma for PF from LMICs as one way to help meet the demand. Plasma is frequently wasted in LMICs because they lack national and local blood programs; quality control systems; screening tests and in vitro diagnostic devices (IVDs); and the technical capacity to meet international quality standards, e.g., inadequate control of plasma temperature or storage conditions (4). WHO, along with the European Commission and local authorities has helped to create national blood systems governed by national blood policies to promote uniform standards of quality and safety, as well as supplying written guidelines and technical support (15-20).
However, counter to expanding donations from all sources, WHO, the Council of Europe (CoE), the International Plasma and Fractionation Association (IPFA), and many national regulatory authorities, other than the FDA, have adopted World Health Assembly resolution WHA28.72 [1975], and reiterated in WHA63.12 [2010] (21), that limits the collection of blood and plasma from paid donors. The resolution urges all WHO Member States “to develop national blood systems based on voluntary unpaid donations and to work towards the goal of self-sufficiency” (21). The objective to increase plasma donations, especially through plasmapheresis, exclusively from VUDs is contentious (22-24), and is based on ethical (25-28) and safety considerations (e.g., paid donors are exploited, and are more at risk for infectious diseases than VUDs), that have their roots in the “wild cat days” of blood collection in the 1960’s and ‘70s (29).
Starting in the mid 1960’s, the demand for PF increased sharply with the discovery that antihemophilic concentrates (FVIII) could be made from cryoprecipitate, and that the development of apheresis equipment permitted frequent, high volume plasma donations. Commercial establishments paid plasmapheresis donors to obtain the large amount of plasma needed to manufacture FVIII. They often collected blood from undesirable sources including from prisoners and placentas (29), and from vulnerable, exploitable donor populations, in slums and in low-income countries, where hepatitis and other chronic infections were prevalent. The number of donations per week and volume of donations were unregulated. Clinicians recognized, however, that patients transfused with plasma from paid donors developed hepatitis more frequently than those treated with VUD plasma. It was against this background of unprincipled commercial blood collection, and threats to donor and patient health, that major improvements in regulations, guidance, and standards for PF were developed in the U.S. and throughout the world, in the early 1970’s.
The aim of WHO and others to exclude paid donors based on safety concerns, especially for transfusion, was a reasonable precaution in the ‘70’ s, considering the association between paid donations and hepatitis, and the lack of pathogen inactivation for PDMPs or tests for most transfusion, transmitted infections (TTI). However, although the objective of self-sufficiency through VUD donations has been in place for many years, plasma from this source is still insufficient to meet global demand for PDMPs. Out of 180 reporting countries surveyed by WHO, only 51 produced PDMPs through fractionation of PF collected in their own country (13).
FDA approach to PF collection
In contrast to the WHO resolution, FDA regulations are silent about preferring blood donations solely from VUDs. FDA justified paying plasmapheresis donors by considering the need for substantial amounts of plasma to meet the high demand for PDMPs, and that plasmapheresis can be an uncomfortable and lengthy procedure (30).
FDA, however, recognizing the higher risks of paid donations for transfusion, indirectly stopped transfusion services from using paid donations through regulations introduced in the 1970’s that require units of whole blood and components to be labeled “paid” or “volunteer”. While blood collection facilities are theoretically free to trade units of paid or volunteer donations, hospitals and transfusion services only accept “volunteer” units, considering the safety risk. These labeling requirements do not apply to source plasma or to PDMPs (30).
FDA issued additional regulations during the 1970’s that require licensure of all plasmapheresis facilities, establish safeguards to protect the health of the donor, ensure product potency, and create standards for blood collection. FDA published standards for processing source plasma in 1973. A final rule in 1975 required that all facilities that process blood or blood components adhere to current good manufacturing practices (cGMP).
The regulations, promulgated in the 1970’s, were only the beginning of an increase in FDA oversight of the not-for-profit and commercial blood industries. Prior to the AIDS epidemic in the 1980’s, the blood collection and fractionation industry focused on reducing the risk of hepatitis through improvements in donor screening (e.g., hepatitis B in 1970) rather than by eliminating infectious agents from PDMPs. Although hepatitis still occurred [later identified as hepatitis C (HCV)] in transfusion and derivative recipients, the public and industry accepted the risk and did not make the elimination of hepatitis a high priority (30).
The AIDS epidemic which devastated the hemophilia community in the 1980s spurred the FDA and other regulatory bodies, industry, and patient groups, to become much more aggressive in ensuring blood and blood product safety (31). FDA became more vigilant in assessing potential threats to the blood supply from TTIs, enforcing standing regulations and promulgating new ones, changing donor acceptance criteria, and inspecting blood establishments and fractionation facilities. Industry developed procedures to clear pathogens from PDMPs, and voluntary standards to improve the quality of PF. Additionally, consumer groups pressed regulatory agencies and industry to develop purer and safer products. The National Hemophilia Foundation, the Committee of Ten Thousand, the Hemophilia Federation of America, the Immune Deficiency Foundation, the WFH, and the Alpha-1 Foundation are prominent organizations that have contributed to this effort in the U.S.
Regulations and standards for source and recovered plasma in the US: ensuring donor and donation safety, and PF quality
How FDA makes and implements regulations and standards
U.S. blood and plasma are collected, processed and distributed by private industry that is regulated by the FDA under two national laws: the Public Health Service (PHS) Act, and the Federal Food, Drug and Cosmetic (FD & C) Act (Table 1). Title 21 of the Code of Federal Regulations (21 CFR), implement the statutes of the PHS and FDC Acts, and most regulations that apply to blood are in the 21 CFR Part 600’s (32) (Table 2). The CFR annually codifies regulations and standards that are in the Federal Register. Amending regulations is an open, public, and laborious process: FDA places the notice of a proposed regulation in the Federal Register; the proposed rule is available for public notice and comment, e.g., at Blood Products Advisory Committee (BPAC) meetings, workshops, FDA docket, etc.; and if accepted, the FDA places the final rule in the Federal Register along with FDA responses to public comments (33).
Table 1
| PHS Act (42 USC 202 et. seq.) |
| Addresses biologics and communicable disease controls |
| Major provisions of the PHS Act |
| Section 351 (biologics regulation*) |
| Defines and requires licenses for biological products; authorizes suspension and revocation of licenses |
| Allows interstate commerce of approved products |
| Prohibits false labeling |
| Authorizes federal inspections |
| Allows for penalties |
| Section 361 requires control of communicable diseases |
| FD & C Act (21 USC 302 et. seq.) |
| Addresses drugs and medical devices |
| Blood organizations also comply with State laws and voluntary standards (e.g., AABB, PPTA) |
| Major provisions of the FD & C Act |
| Prohibits adulteration or misbranding |
| Requires registration of producers of drugs and devices |
| Authorizes manufacturing facilities inspections |
| Provides penalties for violations, including court injunction |
| Manufacturers must prove a drug is safe and effective before marketing |
*Note that licensed biological products concurrently are drugs or devices under the FD & C Act. FDA, the United States Food and Drug Administration; PHS, the Public Health Service; USC, United States Code; FD & C, the Federal Food, Drug and Cosmetic .
Table 2
| Source plasma |
| 600—general biologic products |
| 601—licensure |
| 606—cGMP’s |
| 607—registration |
| 610—infectious disease testing |
| 630—general donor notification, education, screening, eligibility |
| 640.60–640.76—SP donor and product standards (includes labeling, frequency, holding, and storage) |
| Recovered plasma |
| 600—general biologic products |
| 600.22—short supply |
| 606—cGMP’s (& labeling) |
| 607.65—registration exemption |
| 610—infectious disease testing |
| 630—general donor notification, education, screening, eligibility |
| 640.1–640.5—whole blood standards |
CFR, Code of Federal Regulations; cGMP, current good manufacturing practices; SP, Source Plasma.
The FDA also issues Guidance documents that are recommendations on how to comply with regulations. Guidance documents describe new policies and procedures; they do not bind industry, and industry may offer alternatives (which in practice rarely happens) if they satisfy the requirements of the applicable regulations. PPTA and AABB (an international not-for-profit U.S. based association involved in transfusion medicine, standard setting and accreditation) also provide voluntary standards which further enhance the minimum requirements of the CFR.
Source plasma regulation and standards
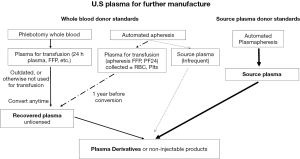
The for-profit commercial blood establishment industry which collects plasma primarily, but not exclusively, from paid donors, follow Source Plasma standards and regulations in the CFR (Figure 1, Table 2).
A blood establishment that collects Source Plasma must show (21CFR 601) in a license application that it can produce safe and potent products. The application avers that the establishment has available appropriate laboratory tests and equipment, donor safety measures, appropriate manufacturing methods, data establishing stability of the product through the dating period, specimens of the labels, and the address of each location involved in the manufacture of the product (Table 3). FDA reviews the license application, inspects the facility to observe manufacturing, and decides whether the facility is ready for licensure. FDA routinely inspects licensed facilities every two years, or “for cause”, to ensure that they meet applicable regulations and standards (Table 4).
Table 3
| Manufacturers name (organization) |
| Establishment name |
| Procedures for determining donor suitability including medical history, examination by a physician |
| Laboratory testing, methods of preparing the venipuncture site and collecting the plasma |
| Methods to prevent circulatory embolism and to assure return of red cells to the proper donor |
| Minimum intervals between donations and maximum frequency of donation |
| Techniques for immunizing donors |
| Laboratory tests of collected plasma |
| Techniques of preparing source plasma and storing it |
| Methods to ensure proper storage conditions and identification of units |
| Label control systems |
| Shipping conditions and procedures |
Table 4
| License application process |
| Manufacturer submits biologics license application (BLA) |
| Desk review of documents: |
| Completeness, consistency with published regulations and recommendations |
| Readiness for inspection |
| Facility inspection to observe manufacturing: |
| Inspect operations and records for compliance with regulations, consistency with applicable regulations and recommendations, and commitments made in the application |
| Observe operations and review records |
| Complete review of submission |
| Administrative processing of application |
| Approval letter sent to manufacturer |
| Post—approval, post—licensure inspections; recalls, withdrawals |
| FDA routinely inspect Source Plasma and recovered plasma facilities |
| Inspections performed by investigators from the FDA’s Office of Regulatory Affairs |
| Trained cadre of investigators and product experts |
| Routine biennial, or “for cause” |
| Follow compliance program guidance manual: |
| Inspection of source plasma establishments—7342.002 |
| Inspection of licensed and unlicensed blood banks, brokers, reference laboratories, and contractors—7342.001 |
| Systems examined: quality assurance, donor eligibility, product testing, product collection and processing, quarantine/storage/disposition |
| Recalls and withdrawals based on post-donation information, obtained from blood product deviation reports. “Lookback” product retrieval and recipient notification based on subsequent testing |
FDA, the United States Food and Drug Administration.
Further requirements and mandatory standards to ensure donor health and product quality are as follows:
General eligibility requirements
Source Plasma donors must meet general donor eligibility requirements 21 CFR 630.10a (Table 5). The Source Plasma establishment must find that the donor is in good health, and that the donor understands the risks of donation. The establishment must check to see if the donor is on a deferred donors list to avoid use of collections from previously unsuitable donors. Other factors for deferral include travel to endemic areas, use of illegal drugs, pregnancy, etc. A donor history questionnaire facilitates collection of this information (34). FDA requires proof of identity and a postal address to be able to notify a donor about reasons for a potential deferral.
Table 5
| Factors that determine donor eligibility |
| Donor must be in good health and free from transfusion-transmitted infections |
| Donation should not affect the health of the donor, or the safety, purity, potency of the blood or blood product |
| Educational material for the donor, given before determining eligibility |
| Provide educational materials understandable by donor |
| Inform about risk factors associated with RTTI; donor with a risk factor should not donate |
| Determine eligibility of donor on day of donation (with few exceptions, see |
| Procedures to determine the eligibility of the donor |
| Inspect records of deferred donors |
| Assure interval since last donation is appropriate |
| Assess the donor’s medical history |
| Determine if the donor is in good health |
| Identify risk factors associated with exposure to clinical evidence of an RTTI infection |
| Determine if there are other conditions that may adversely affect the health of the donor or the safety, purity, or potency of the blood product, e.g., travel to endemic area, pregnancy, etc. |
| Perform a physical assessment of the donor |
| Temperature; Blood pressure; hemoglobin; pulse; weight; skin—free of infection, or indication of drug use |
| Additional requirements |
| Proof of identity and postal address |
Acknowledgment from the donor of: having reviewed the educational material; not donating if posing risk to recipient; being notified if deferred, and reason for deferral; being informed of the risks of donation procedure; and having the opportunity to ask questions and withdraw from the donation procedure.
Recent amendments to the CFR in 2015 (35) demonstrate FDA’s continuous efforts to improve the quality of blood donations and the safety of donation. Requirements include record keeping to prevent deferred or ineligible donors from donating at the collection location or at locations operating under the same license. The rule also requires a person certified in cardiopulmonary resuscitation to be available whenever collections are preformed, and raises the minimum standard of hemoglobin for male donors from 12.5 grams of hemoglobin per deciliter of blood to 13 grams.
Additional requirements for frequent donors (Table 6
Table 6
| Procedure | Source Plasma | Recovered plasma |
|---|---|---|
| Donor selection | In addition to general donor eligibility requirements: (I) physical exam initially/annually 21 CFR 640.65; (II) total protein; (III) serum protein electrophoresis initially/every 4 months; (IV) informed consent | RP obtained from whole blood donors; requirements for whole blood donors in 21 CFR 630.1, etc. |
| Collection frequency | Frequent—2 days apart; no more than 2×/week; infrequent—once every 4 weeks or less frequent | RP is not a collected product; it is made from whole blood or plasma products originally intended for transfusion |
| Collection/preparation method | Automated (or manual) plasmapheresis | Phlebotomy or apheresis |
| Quarantine | 60-day hold | Not specified in regulations |
| Shelf life | 10 years | Not specified in regulations |
| Storage temperature | Injectable—immediately after filling, store at −20 °C or colder; non-injectable—store at temperatures appropriate for intended use | Not specified in regulations; must meet final product manufacturer’s specifications |
| Infectious disease testing | HIV; HCV; HBsAg; Syphilis initially/every 4 months | HIV, HCV, HBsAg, Syphilis, HTLV I/II, west nile virus, chagas disease |
RP, recovered plasma.
Source Plasma donor standards permit collection of plasma twice within a seven-day period. The volume of collected plasma is based on the donor’s weight, and one must weigh at least 110 lbs. to donate. Given the frequency of collection, additional safeguards include a physical exam initially, and then annually; total protein determination; and serum protein electrophoresis initially and then every 4 months. U.S. licensed screening assays and cleared IVDs (36) test for TTIs including HIV, HCV, HBsAg and Syphilis.
Do frequent donations affect donor health?
U.S. regulations permit more frequent donations and greater total volume withdrawn per year than other countries (Table 7). Does the frequency/volume of collection affect the health of the donor or the quality of plasma? There are no definitive, well controlled clinical trials extending over several years that have assessed the health exclusively of donors who donate 102 times per year versus those who donate less frequently. However, a recent extensive review of available data (39), concluded that although total serum protein and immunoglobulin levels initially decrease and then level off in frequent plasmapheresis donors (40), “there are no reports of clinical consequences of these proteins falling below normal range, other than the need for deferral once limits have been reached.” The review also concluded that blood facilities should monitor candidate plasmapheresis donors to exclude those who have low protein and immunoglobulin levels, and to closely follow those who enter the plasmapheresis program with therapeutic protein levels close to the minimum ranges.
Table 7
| Collection parameters | United States* | Council of Europe* | Germany* | U.K.+ |
|---|---|---|---|---|
| Maximum Donation Frequency | 104/yr | 33/yr | 45/yr | 24/yr |
| Maximum times/week | Twice/week | Once/week | Once/week | Twice/week |
| Maximum volume/donation (weight dependent) | 800 mL | 750 mL | 850 mL | 1,050 mL |
| Volume/year | 83 L | 25 L | 38 L | 15 L |
*, Williams AE. FDA Considerations Regarding Frequent Plasma Collection Procedures (
Note: FDA requires blood collection establishments to immediately notify the Agency if a donor has a fatal reaction that is in any way associated with plasmapheresis. Loss of plasma can be medically significant, leading to an elevated heart rate; drop in blood pressure; hemoconcentration with resultant hyperviscosity and a hypercoagulable state; and if sever enough, hypovolemic shock like that caused by hemorrhage (37). FDA analysis of data from 2005–2011 showed no change in fatality rate when corrected for changes in collection rates, with a 3-year moving average of 2–7 reported fatalities per ten million donations.
Source Plasma processing standards
Source Plasma must meet standards of collection, freezing, storage, and shipping, (21 CFR 640.69). The standard “immediately after filling, plasma intended for manufacturing into injectable products shall be stored at a temperature not warmer than −20 °C” (Table 8) is especially problematic. FDA accepted these requirements for freezing, in the 1970’s, to accommodate the freezing equipment of the time, and they were not based on optimizing the quality of plasma. Later studies showed that a rapid rate of freezing, e.g., plasma frozen to a core temperature of ≤−25 °C in ≤12 hr, or ≤−30 °C in ≤1 hr, better preserves the activity of labile proteins such as Factor VIII.
Table 8
| Processing elements | Source plasma | Recovered plasma |
|---|---|---|
| Collection method | Plasmapheresis | Whole blood or apheresis |
| Time from collection to freezing | immediately | Undefined |
| Freezing conditions, temperature | ≤−20 °C | Undefined |
| Storage expiration | ≤−20 °C, 10 yr | Undefined |
| Shipping temperature | ≤−5 °C | Undefined |
| Allowable deviation | Can exceed <−20 °C for <72 hr total; never >−5 °C, always frozen | Undefined |
European requirements and recommendations (Table 9) specify the latter conditions (41). Fortunately, the FDA regulation sets a minimum requirement for freezing temperature, and therefore U.S. blood establishments can follow the more stringent European parameters that are science-based. The European standards for temperature of freezing have become industry standards for both Source Plasma and recovered plasma, which is one factor that makes PF derived from these plasmas suitable for marketing in Europe as well as the U.S.
Table 9
| Processing elements | European Pharmacopoeia | Council of Europe | |||
|---|---|---|---|---|---|
| Purpose | Labile proteins for fractionation | Non labile protein for fractionation | For transfusion | ||
| Collection method | Plasmapheresis or plasma from whole blood | Plasmapheresis | Plasma from whole blood | Whole blood plasma | Apheresis |
| Time from collection to freezing | ≤24 hrs | ≤24 hrs | ≤72 hrs | ≤18 hrs (≤6 hrs optimal); if 20–24 °C, then ≤24 hrs | ≤6 hrs; if 20–24 °C, then ≤24 hrs |
| Freezing conditions, temperature | Frozen to a core temp of ≤−25 °C in ≤12 hr | Chamber at ≤−20 °C | To <30 °C within 1 hr | ||
| Storage | ≤−20 °C | If −18 to −25 °C, 3 months; if <−25 °C, 36 months | |||
| Shipping temperature | ≤−20 °C | −18 to −25 °C for 3 months storage; <−25 °C, for 36 months storage | |||
| Allowable deviation | Exceeds −20 °C not more than 72 h total; one time >−15 °C; never >−5 °C | None | |||
Placing Source Plasma in the freezer “immediately after filling” (in practice, within 2 hours of filling) is a more problematic requirement. Separating plasma from cells by plasmapheresis and freezing it rapidly is the optimal way to preserve protein activity and recovery. This requirement, however, limits the ability of non-commercial blood establishments to collect apheresis plasma for manufacturing on mobile units because mobile units often cannot return to a central facility in time to freeze plasma (see discussion below, recovered plasma).
Additional regulatory standards for Source Plasma
FDA added requirements 640.69 (e) and (f), to Source Plasma regulations in 2015 (35).
Under 640.69 (e), a Source Plasma donation from a paid donor must not be used for manufacturing until the donor has been found to meet general donor requirements and to have a record of negative test results for applicable relevant TTIs (RTTIs) (e.g., HIV, HBV, HCV, etc.) on at least two occasions in the past 6 months. Thus, this restriction on using a donation from a one-time-only donor for further manufacturing “results in committed donors and eliminates the risk that so-called ‘test-seekers’ would be accepted.” (35). This requirement supports the observation that repeat, committed donors are safer donors (30).
Regulation 640.69 (e) requires establishments to hold plasma obtained from paid Source Plasma donors for a minimum of 60 days before using it to manufacture an injectable product. Establishments cannot release plasma from quarantine if the donor is subsequently deferred because of having a reactive screening test or failing to meet general donor eligibility requirements in a subsequent donation. The 60-day hold significantly reduces the residual risk of RTTIs from paid donors to closely match the residual risk of VUDs (42).
These new requirements are based on PPTA’s voluntary Qualified Donor Standard and Inventory Hold (QSEAL) standards. Industry’s voluntary standards have advantages over government regulations in that a trade organization can write and implement them quickly to adjust to emerging scientific, epidemiological, or sociological findings. As PPTA represents a multinational industry, their voluntary standards help to harmonize practices globally. However, voluntary standards do not have legal standing or enforceability; a given manufacturer could choose to withdraw from PPTA at any time despite public opprobrium. Although a downside of placing a voluntary standard in the CFR is that it is difficult to remove or modify as technology advances, FDA determined that these particular voluntary standards improved the safety of Source Plasma sufficiently to codify them.
Industry’s voluntary standards
In addition to the minimal FDA regulatory requirements in the CFR, PPTA has instituted multiple voluntary standards to further reduce potential RTTI contamination of Source Plasma, and to promote donor health.
In 1991, PPTA initiated the International Quality Plasma Program (IQPP) that focused on improving the quality of a facility’s donors, and its plasma collection operations. Within the IQPP are the Center Management Standards which includes: Viral Marker Standard, Personal Education and Training Standard, Professional Plasma Collection Facility Standard, and Quality Assurance. Importantly an “independent third-party evaluation and recognition of a centers adherence to industry standards for source plasma” is in place to monitor implementation of these standards (43).
The QSEAL program, started in 1997, has, in addition to what are now FDA required standards cited above, in-process testing for Parvovirus B19, the use of Nucleic Acid Amplification Technology (NAT) screening at the donation or pool level, and a Viral Marker Standard whereby a Source Plasma center must not exceed a national standard for positive RTTI test results, or lose their industry IQPP certification.
Among PPTA’s standards are two that PPTA designed specifically to promote donor health. The IQPP Donor Fluid Administration standard requires plasma centers to administer fluids as part of the donation process to assist donors in maintaining hydration. The Cross Donation Management Standard prevents donors from donating in the same or a different plasma center, owned by the same or a different company, more than twice a week. Limiting the volume of donation helps to ensure that the donor will remain healthy.
PPTA’s Community Based Donor Standard requires donors to submit evidence of a local residence, and prohibits those living outside a defined area from donating. Also, commercial establishments do not accept those living in half-way houses, homeless shelters or missions. Note that this standard extends the existing FDA regulation which requires proof of residence to permit notification in case of deferral.
In October 2017 PPTA submitted a draft of a proposal for public comment to improve their IQPP standard to “monitor, manage, and document donor adverse events… This IQPP Standard serves as the foundation for establishing industry-wide requirements for adverse event definitions and classification.” (44). This is an example of industry’s on-going efforts to ensure donor safety.
The safety of Source Plasma from paid donors
Does payment for donations promote a class of “professional donors” who support themselves through selling their blood? Some (45) have argued that this is not the case in the U.S. Blood establishments compensate donors an average of $35 per donation, and even for donors who donate 104 times per year, this amounts to $3,600 per year, hardly a living wage (45). While $3,600 is not enough money to support an individual, it would be a substantial supplement for a person earning $12,228 per year or less, the poverty threshold in the U.S. in 2016. Forty million people were below the official poverty threshold in 2016 in the U.S. (46). Whether one wishes to consider payment as altruistic, e.g., “Interventions to remove barriers and disincentives to donation experienced by those disposed to donate” or non-altruistic, e.g., “Financial incentives that leave the donor in a better financial position as a result of donating.” (47) is an open question. The practical effect is that payment does incentivize many donors who donate Source Plasma in the U.S. (48).
At the same time, regulations and standards of the FDA and PPTA reduce donations from donors with potential RTTIs, and those whom the donation process could harm, so that only “Qualified Donors” can contribute. A recent study (49) compared estimates of a manufacturer releasing for fractionation a potentially infectious unit (virus below NAT detection level, i.e., window period residual risk) of Source Plasma from paid donors, to units of recovered plasma from VUDs. The residual risk of Source Plasma over recovered plasma was 1.4 for HIV, 2 for HCV, and 9.8 for HBV. These differences are insignificant relative to the pathogen clearance procedures of PDMP manufacture that reduce residual risk by many millions fold (50)
FDA and industry regulations and standards protect the health of the donor, ensure the quality of plasma, and permit collection of sufficient plasma to meet the needs of patients. There has been no confirmed case of transmission of viral infection in more than two decades from U.S. licensed PDMPs. Importantly, adoption of voluntary standards by all fractionators who are part of PPTA, including those outside the U.S., should reduce the concern about the health and welfare of paid Source Plasma donors and the quality of PF collected by international fractionators, if regulators and manufacturers strictly enforce these voluntary standards.
Recovered plasma
Recovered plasma is plasma derived from single units of Whole Blood as a byproduct in the preparation of blood components from Whole Blood collection, and intended for further manufacturing [Compliance Policy Guides (CPG 7134.12), section 230.100]. Not-for-profit facilities collect recovered plasma from the donations of donors who meet Whole Blood donor requirements (21 CFR 630.1) (Table 4, Figure 1) which apply to blood and blood components for transfusion collected by phlebotomy or automated apheresis. In the U.S, the American Red Cross (ARC) collects about 40% of VUD blood (51); community based independent collectors, represented by Americas Blood Centers, account for about 50% (52); and hospitals and military facilities most of the remainder. VUDs can receive benefits or rewards such as time off from work or lotteries, if they are not readily convertible to cash (53).
Donations must be from healthy donors; regulations limit donation frequency to no more than once in 8 weeks for Whole Blood or Red Blood Cells, and once in 16 weeks for apheresis collection of two units of Red Blood Cells. Like Source Plasma donors, Whole Blood donors are tested for HIV, HCV, HBsAg and Syphilis, but in addition also HTLV-I/II, West Nile Virus, and Chagas disease. Source Plasma is not tested for these additional infectious agents because the manufacturing of PDMPs clears them, but these TTIs have the potential of remaining in blood or blood components for transfusion that have not undergone pathogen reduction.
Plasma for transfusion can take many forms, as described in the CFR and in voluntary standards as defined in the AABB Circular of Information in conjunction with the FDA (41,54). Included among these are:
(I) Fresh Frozen Plasma (FFP) is prepared from whole blood or apheresis collection and frozen at −18 °C or colder within the time frame as specified in the directions for use for the blood collection, processing, and storage system;
(II) Plasma frozen within 24 hours after phlebotomy (PF24): must be separated and placed at ≤−18 °C within 24 hours of whole blood collection;
(III) Liquid Plasma is separated no later than 5 days after the expiration date of the Whole Blood (expiration can be 21 or 35 days depending on anticoagulant). Plasma may be stored at ≤−18 °C. Liquid plasma is stored at refrigerator temperature 1–6 °C [21 CFR 640.34(c)].
Regardless of whether plasma is first made into FFP or from other plasma types, such as PF24, or Liquid Plasma, any plasma collected manually originally for transfusion, but not used for that purpose may be labeled (or relabeled) at any time as recovered plasma, and used for PF to manufacture PDMPs and for non-transfusable products, e.g., test kit reagents (Figure 1).
Recovered plasma is an unlicensed product, and ordinarily U.S. law does not permit shipping an unlicensed product across State lines. To be placed in interstate commerce, recovered plasma must comply with “short supply” regulations 21 CFR 601.22. The term “short supply” embodies the concept that PDMPs, e.g., hyperimmune globulins, are in short supply due to the scarcity of the donor required to manufacture the product. Under these regulations, the licensed manufacturer must have a written, signed “Short Supply Agreement” which establishes “procedures, inspections, tests or other arrangements as will assure full compliance with the applicable regulations... related to continued safety, purity, and potency.”
Although recovered plasma does not have any required standards that apply to the type of plasma comprising it, freezing conditions etc. (Table 8), fractionators specify these parameters in the short supply contract, e.g., PF24 to make labile products such as Factor VIII. In practice, most blood facilities in the U.S. follow the European Pharmacopoeia standards for recovered plasma (Table 9), e.g., rapid freezing of plasma within 24 hours of collection for labile products. Even though the time from collection to freezing is longer than the “immediate” requirement for Source Plasma, fractionators have routinely accepted recovered plasma collected under these less stringent conditions for many years without a reported effect on the quality of the PDMPs made from it.
Source Plasma requirements limit the collection of apheresis plasma from VUDs in the U.S.
Plasma for PF from VUDs is much in demand globally. The non-commercial U.S blood banking community would like to meet this demand to avoid plasma wastage, to improve inventory management, and to add to revenue through sales of plasma for manufacturing.
Blood facilities can currently obtain plasmapheresis plasma for manufacturing from VUDs if they desire by collecting a product called Source Plasma (infrequent) (Figure 1) every 4 weeks or less frequently, as part of an infrequent plasmapheresis program, approved under 21 CFR 640.120. The donors in this program do not undergo the annual physical and total protein determination required for donors of commercial Source Plasma. However, not-for-profit blood facilities must make Source Plasma (infrequent) under the same freezing and storage conditions as Source Plasma. This brings up the challenge alluded to previously, in that Source Plasma must be frozen “immediately after filling” which is problematic, considering that in 2011, blood centers obtained 68% of their collections on mobile units (55), and in 2017, ARC collected 80% of their plasma on mobile units (51).
To increase the amount of plasma from VUDs that could become recovered plasma, AABB, in 2002, requested that FDA change its stance regarding automated apheresis plasma for transfusion collected under whole blood standards (56).
Currently, blood establishments can relabel plasma collected by automated apheresis, with or without cellular components, and intended for transfusion, as recovered plasma after a 1-year expiration period (Figure 1). The year-long delay is meant to assure VUDs that their altruistic apheresis donation to the local not-for-profit blood facility is for transfusion rather than for commercial manufacturing. Holding plasma for a year before conversion into recovered plasma, however, is burdensome for a blood establishment, considering the cost of storage and inventory management.
To resolve this problem, FDA discussed, in Blood Product Advisory Committee meetings (41), permitting various licensed apheresis plasma products intended for transfusion that are co-collected with one or more cellular blood components to be relabeled as licensed Concurrent Plasma (CCP) shortly after collection. This would improve flexibility for plasma inventory management.
Unfortunately, the regulatory pathway to establish CCP has proved more challenging than expected and FDA cannot create CCP simply through Guidance. If FDA removed the requirement for waiting a year before whole blood apheresis plasma became recovered plasma, CCP would assume the definition of Source Plasma in the CFR, i.e., plasmapheresis plasma for further manufacturing, with its attendant rapid freezing requirements. FDA has signaled (57) that creating CCP as a new product will require changes to the CFR, which is especially difficult under our current Administration which has restricted modification of regulations. Nevertheless, FDA will continue to work on this issue to meet the blood banking community’s request and to improve the global availability of VUD apheresis plasma for PF.
Summary
Source plasma from paid U.S. donors will continue to be the major source of PF for many years to come. FDA and the commercial fractionation industry have been effective in ensuring the safety of the donor and donation, and the quality of PF. Underlying principles involve maintaining a strong regulatory system that establishes and enforces standards, regulations and policies; and a resilient industry that is intent on maintaining product quality and availability.
The regulations, standards and policies of the FDA ensure the safety and quality of PF, and the health and safety of the donor related to the donation process. These activities include donor selection criteria, use of registries to avoid use of collections from previously unsuitable donors, laboratory testing for infectious disease markers, quarantining collections pending determination of donor suitability, and monitoring and investigating adverse events to ensure appropriate responses and corrective actions.
The voluntary standards of the fractionation industry significantly enhance the minimal requirements of the FDA. These voluntary standards are measures to recruit and keep healthy donors, and to exclude those who are intent on gaming the system. Although paid Qualified Donors of Source Plasma still have a modestly higher prevalence of TTIs than VUDs, their donations will not compromise the safety of PDMPs because of the overwhelming effectiveness of viral clearance in the manufacturing of PDMPs.
The success of the Agency and industry depends, in part, upon an open and transparent process of developing regulations, policy and standards (e.g., BPAC) that accepts the input and responds to the needs of stakeholders. The actions and opinions of the WHO, other regulatory bodies, trade associations, and consumer organizations, benefit consumers by pressing the commercial fractionation industry and the FDA to continuously seek to improve the quality, safety and availability of plasmapheresis plasma for manufacturing, while protecting the health and safety of the donor.
The efforts that the FDA and industry have taken, as described here, to ensure the quality and safety of PF, and the health of the donor, should allay concerns that countries that import PF might have about these issues. Countries that are in the process of developing a national blood program could benefit from taking some aspects of the U.S. regulatory approach with industry input to expand their donor base of plasmapheresis plasma for manufacturing, beyond aiming to accept plasma only from VUDs.
Acknowledgments
Mikhail Ovanesov, PhD, Office of Tissues and Advanced Therapeutics, Center for Biologics Evaluation and Research, U.S. Food and Drug Administration, Silver Spring, Maryland, United States of America kindly provided some reference articles for this review.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Thierry Burnouf) for the series “Plasma Fractionation” published in Annals of Blood. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob.2017.12.02). The series “Plasma Fractionation” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the manuscript and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Essential medicines and health products 2017. Available online: http://www.who.int/medicines/publications/en/
- Strengers PF, Klein H. Plasma is a strategic resource. Transfusion 2016;56:3133-7. [Crossref] [PubMed]
- Penrod J. The Need for Plasma in Asia. The Source. Available online: http://www.pptaglobal.org/images/source/2017/SUMMER/TheSource_Summer2017.pdf
- Bult JM. A Global Footprint. Presentation Beijing, China. Available online: http://www.pptaglobal.org/images/presentations/2017/PDI_Congress/JMB_-_A_Global_Footprint_R1.pdf
- Farrugia A. Clinical Need for Plasma Protein Therapies, The source. Available online: http://www.pptaglobal.org/images/source/2014/FALL/2._Clinical_Need.pdf
- Improving access to safe blood products through local production and technology transfer in blood establishments World Health Organization. Available online: http://www.who.int/phi/publications/blood-prods_technology_transfer.pdf
- Bult JM. The World Needs Plasma: From The USA And Other Countries. Available online: http://pptaglobal.org/images/presentations/2017/2017-_The_World_Needs_Plasma.pdf
- Gustafson M. Plasma Vigilance: Source Plasma Donor Hemovigilance Activities and Results. Available online:http://www.pptaglobal.org/images/presentations/2017/Gustafson_PlasmaVigilance100817.pdf
- An EU-wide overview of the market of blood, blood components and plasma derivatives focusing on their availability for patients Creative Ceutical Report, revised by the Commission to include stakeholders’ comments. Available online: https://ec.europa.eu/health//sites/health/files/blood_tissues_organs/docs/20150408_cc_report_en.pdf
- Aleccia J. As Loyal Donors Age, Industry Is Out for Young Blood. Kaiser Health News. Available online: http://khn.org/news/as-loyal-blood-donors-age-industry-is-out-for-young-blood/
- Sapiano MRP, Savinkina AA, Ellingson KD, et al. Supplemental findings from the National Blood Collection and Utilization Surveys, 2013 and 2015. Transfusion 2017;57:1599-624. [Crossref] [PubMed]
- Klein HG, Hrouda JC, Epstein JS. Crisis in the Sustainability of the U.S. Blood System. N Engl J Med 2017;377:1485-8. [Crossref] [PubMed]
- WHO Blood safety and availability fact sheet. Available online: http://www.who.int/mediacentre/factsheets/fs279/en/#.WY5_kYgXm6k.google
- Availability of Immune Globulin Intravenous for Treatment of Immune Deficient Patients -- United States, 1997-19. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/00056604.htm
- Annex 4 Recommendations for the production, control and regulation of human plasma for fractionation. World Health Organization WHO Technical Report Series No 941. Available online: http://www.who.int/bloodproducts/publications/TRS941Annex4blood.pdf?ua=1
- Improving access to safe blood products through local production and technology transfer in blood establishments. Available online: http://www.who.int/phi/publications/improving_access_safe_blood_products/en/
- Annex 4 WHO guidelines on good manufacturing practices for blood establishments, World Health Organization WHO Technical Report Series, No. 961. Available online: http://www.who.int/bloodproducts/publications/GMP_Bloodestablishments.pdf?ua=1
- Assessment criteria for national blood regulatory systems. Available online: http://www.who.int/bloodproducts/NationalBloodRegSystems.pdf
- Shoos KL. Setting Up a Regulatory Framework for Blood in Africa. Available online: https://www.wbmt.org/fileadmin/pdf/10_CapeTown_WS1-2014/3late-afternoon/00a-SHOOS_WBMT_Regulatory_Presentation_11_6_14.pdf
- Rossi F. Benefits of Plasma Quality Programs in Blood Establishments, IPFA Asia pacific workshop on plasma quality and supply. Available online: https://www.ipfa.nl/UserFiles/File/WS%202016/Taipei%202016/Proceedings%20publicly%20published/3_4_amended_IPFA_WS_Taipei_2016_Rossi_Dr_IPFA%20%20%20.pdf
- Availability, safety and quality of blood products, sixty-third world health assembly. Available online: http://apps.who.int/gb/ebwha/pdf_files/WHA63/A63_R12-en.pdf
- Penrod J, Farrugia A. Errors and Omissions: Donor Compensation Policies and Richard Titmuss. Available online: https://link.springer.com/article/10.1007/s10730-015-9267-7
- Farrugia A, Penrod J, Bult JM. The Ethics of Paid Plasma Donation: A Plea for Patient Centeredness. HEC Forum 2015;27:417-29. [Crossref] [PubMed]
- Taylor JS. WTH WHO? HEC Forum 2015;27:287-300. [Crossref] [PubMed]
- Buyx AM. Blood Donation, Payment, and Non-Cash Incentives: Classical Questions Drawing Renewed Interest. Transfus Med Hemother 2009;36:329-39. [Crossref] [PubMed]
- Titmuss RM. The Gift Relationship. From Human Blood to Social Policy. 2nd edition. New York: New Press, 1997.
- WHO Expert Group. Expert Consensus Statement on achieving self-sufficiency in safe blood and blood products, based on voluntary non-remunerated blood donation (VNRBD). Vox Sanguinis 2012;103:337-42. [Crossref] [PubMed]
- Farrell AM. The Politics of Blood Ethics, Innovation and the Regulation of Risk. Cambridge: University Press, 2012.
- Starr D. Blood: An Epic History of Medicine and Commerce. HarperCollins, 2002.
- Leveton LB, Sox HC, Stoto MA, editors. HIV and the Blood Supply: An Analysis of Crisis Decision Making. Washington (DC): National Academies Press (US), 1995.
- Epstein JS, Jaffe HW, Alter HJ, et al. Blood system changes since recognition of transfusion-associated AIDS. Transfusion 2013;53:2365-74. [Crossref] [PubMed]
-
Electronic Code of Federal Regulations -
About the Code of Federal Regulations -
Full-length Donor History Questionnaire – Source Plasma Industry - Food and Drug Administration. HHS. Requirements for blood and blood components intended for transfusion or for further manufacturing use. Final rule. Fed Regist 2015;80:29841-906. [PubMed]
-
Complete List of Donor Screening Assays for Infectious Agents and HIV Diagnostic Assays - Williams AE. FDA Considerations Regarding Frequent Plasma Collection Procedures. 15th International Haemovigilance Seminar, Brussels, Belgium. Available online: http://www.ihn-org.com/wp-content/uploads/2012/08/4-WilliamsFreqPlasma-2-21-131.pdf
- Guidelines for the Blood Transfusion Services in the United Kingdom, 7th edition. Available online: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/228828/0117033715.pdf
- Pink J. TS093 Plasma Supply Management Sustainable and Safe Plasmapheresis, European Committee (Partial agreement) on blood transfusion (CD-P-TS) PA/PH/TS(16) 37 R, Strasbourg. Available online: https://sbsc-bsd.ch/dokuman2/Portals/0/BSD/Arbeitsgruppen/AG%20BSH/Sitzungen/2016/5.%20Sitzung/Unterlagen/11.1.1_05-16_BL_EDQM_Plasmapheresis.pdf
- Laub R, Baurin S, Timmerman D, et al. Specific protein content of pools of plasma for fractionation from different sources: impact of frequency of donations. Vox Sang 2010;99:220-31. [Crossref] [PubMed]
- Topic II: Current Considerations on Plasma for Further Manufacturing Obtained from Whole Blood Donors. Blood Products Advisory Committee 100th Meeting, April 28-29, 2011. Available online: https://www.fda.gov/ucm/groups/fdagov-public/@fdagov-afda-adcom/documents/document/ucm272595.pdf
- Blood Plasma Safety: Plasma Product Risks Are Low If Good Manufacturing Practices Are Followed. GAO/HEHS-98-205 Blood Plasma Safety. Available online: https://www.gpo.gov/fdsys/pkg/GAOREPORTS-HEHS-98-205/pdf/GAOREPORTS-HEHS-98-205.pdf
-
International Quality Plasma Program (IQPP) - Public Review Draft 1, Proposed Revision to Version 1.0 of the IQPP Standard for Recording Donor Adverse Events. Available online: http://www.pptaglobal.org/images/IQPP/Public_Comment/10-24-17/STKH17007a_DAERS_Revision_Public_Review_Draft_1.pdf
- Skinner MW, Hoppe PA, Grabowski HG, et al. Risk-based decision making and ethical considerations in donor compensation for plasma-derived medicinal products. Transfusion 2016. Available online: http://www.pptaglobal.org/images/publications/2016/Skinner_et_al-2016-Transfusion_1.pdf
- US Census Bureau, Income and Poverty in the United States: 2016. Available online: https://www.census.gov/programs-surveys/cps.html
- Nuffield Council on Bioethics. Human bodies: Donations for medicine and research. Available online: http://nuffieldbioethics.org/wp-content/uploads/2014/07/Donation_full_report.pdf
- Edin KJ, Shaefer HL. $2.00 A DAY: Living on Almost Nothing in America. Houghton Mifflin Harcourt, 2015.
- Schreiber G. Analysis of US Source Plasma Infectious Disease Residual Risk Compared to Recovered Plasma Residual Risk. IPFA/BCA Global Symposium on the Future for Blood and Plasma Donations. Available online: http://www.pptaglobal.org/images/presentations/2017/SCHREIBER_George_Presentation_September_11_12.pdf
- Kreil TR. The Evolution of Pathogen Safety: A 25-year perspective and thoughts for the future. Available online: http://www.pptaglobal.org/images/source/2017/FALL/4._The_Evolution_of_Pathogen_Safety.pdf
- Facts about American Red Cross Blood Services. Available online: http://www.redcrossblood.org/learn-about-blood/blood-facts-and-statistics#arc-facts
-
Blood Donation FAQs, America’s Blood Centers - CPG Sec. 230.150 Blood Donor Classification Statement, Paid or Volunteer Donor. Available online: https://www.fda.gov/ICECI/ComplianceManuals/CompliancePolicyGuidanceManual/ucm122798.htm
- AABB Circular of Information for the use of human blood and blood components. Available online: https://www.aabb.org/tm/coi/Documents/coi1113.pdf
- Whitaker BI, Hinkins S. The 2011 National Blood Collection and Utilization Survey Report Available online: https://www.hhs.gov/sites/default/files/ash/bloodsafety/2011-nbcus.pdf
- Gregory K. Statement of the American Association of Blood Banks Before the Blood Products Advisory Committee, June 13, 2002. Available online: https://www.fda.gov/OHRMS/DOCKETS/ac/02/briefing/3867b1.htm
- AABB FDA Liaison Meeting – 01/31/17. Available online: http://www.aabb.org/advocacy/government/fdaliaison/bloodcomponents/Pages/flm170131.aspx
Cite this article as: Weinstein M. Regulation of plasma for fractionation in the United States. Ann Blood 2018;3:3.