
Preface to Special Issue: diagnosis and management of von Willebrand disease—diverse approaches to a global and common bleeding disorder
It is a pleasure to present the readership of Annals of Blood (AOB) with this special issue compilation on von Willebrand disease (VWD). It was with some substantial personal gratification for me to put this issue together, in part to reprise a similar issue that I prepared for the journal Seminars in Thrombosis and Hemostasis nearly a decade ago (1). The most intriguing question, of course, is whether or not great strides have been made in the field since that time!
VWD is typically identified as being the most common inherited bleeding disorder, although some forms of VWD can alternatively be considered as rare (2). The exact prevalence of VWD is often debated. In epidemiological studies, VWD has been reported to affect up to 1% of the general population. However, using data related to symptomatic patients, as presenting to clinicians for diagnosis and treatment, or according to bleeding registry data, the prevalence may alternatively be identified as being as low as 0.01% (1 in 10,000) of the general population (1). Moreover, there is great debate also on the (relative) incidence of different forms of VWD (1,2).
VWD is caused by deficiencies and/or defects in von Willebrand factor (VWF), a large and complex multimeric plasma protein, which otherwise would facilitate both primary and secondary hemostasis, by binding to platelets, factor VIII (FVIII), and subendothelial matrix components such as collagen (1-5). Congenital VWD would primarily arise from mutations in VWF. Acquired forms of VWD are also recognized, called acquired von Willebrand syndrome (AVWS), and may perhaps reflect up to 25% of patients presenting to clinicians with a ‘VWD-like’ disorder.
Diagnosis of VWD or AVWS requires evidence of personal history (which may be life-long for VWD, or ‘recent’ for AVWS), and for congenital VWD perhaps also family history, of (mainly) mucocutaneous bleeding, confirmed by laboratory test results that can identify quantitative deficiency and/or qualitative defects in VWF (1-8). Current classification of patients with VWD is into one of 6 types, and based on whether VWF quantitative deficiencies (VWD types 1 and 3), or qualitative defects [type 2 (A, B, M or N) VWD] are present (3).
Type 1 VWD is typically considered the most common form of VWD, particularly in developed countries (1). Type 3 VWD is considered the rarest form of VWD (2). However, this situation can be reversed in developing countries. In part, this is since there is a higher rate of consanguineous relationships in developing countries, leading to a higher occurrence of genetic homozygosity, and thus an increasing risk of rare bleeding disorders such as type 3 VWD (2). Moreover, type 1 VWD may be under-diagnosed in developing countries, because bleeding symptoms are less severe, and patients may not present to clinicians, given the costs of investigation and treatment, the difficulties in finding local experts to provide definitive diagnosis and treatment, as well as the economic burden of not undertaking any paid employment during this time. In contrast, type 1 VWD is no doubt over-diagnosed in developed countries, since inappropriate diagnosis is sometimes made on insufficient findings (9).
Type 2 VWD actually comprises a disparate group of qualitative VWF disorders, and in general represents a group of patients that is under-diagnosed or else misdiagnosed as having a different disorder. This is largely due to the general difficulties in VWD diagnosis, and that some forms of type 2 VWD may look like other forms of VWD, or indeed other clinical conditions entirely, depending on what investigations are performed.
For example, type 2B VWD may be incorrectly diagnosed as ITP (immune thrombocytopenia), because ITP is more common, and because mild thrombocytopenia is often present in 2B VWD, and because VWF levels in type 2B VWD are quantitatively often within the normal reference range. Moreover, definitive diagnosis or exclusion of type 2B VWD requires specific performance of a test called RIPA (ristocetin induced platelet aggregation) (10), and this test is not commonly available, and furthermore requires the use of fresh patient blood and specialized equipment, and so can only be performed at expert sites close to the patient location (i.e., testing cannot be remotely performed).
Similarly, type 2N VWD is commonly misdiagnosed as hemophilia A, because both conditions present with a similar laboratory test phenotype (low FVIII, potentially normal level of VWF protein) when using standard tests, and since hemophilia A is a more common disorder. Moreover, definitive diagnosis or exclusion of type 2N VWD requires specific performance of a test called the VWF:FVIII binding assay (VWF:FVIIIB) (11) (which like RIPA is not generally widely available), or else requires genetic testing of the VWF (vs. F8) gene, which is not commonly applied in VWD diagnostics.
It is also my belief that 2M VWD is generally under-diagnosed or else may be misdiagnosed as type 2A or type 1 VWD (3,12). This is because the differential diagnosis of 2A vs. 2M VWD would require the performance of VWF multimer assessment (13) or some differential pattern of test results using VWF activity assays (12,14). Unfortunately, VWF multimer assessment is rarely performed in VWD investigations, being time-consuming, specialized, complex and costly; alternatively, if performed, it may be poorly performed and subject to higher error rates than standard VWF assays (15,16). In terms of assessment of differential patterns of test results using VWF activity assays, most laboratories do not perform sufficient discriminatory VWF activity assays, or may not be able to properly interpret the resulting test patterns (12).
This special issue of AOB aims to highlight some of the difficulties in diagnosis of VWD, and also explore some differences in ways that laboratories approach the diagnosis, as well as differences in treatment of VWD based on geographical location. Thus, VWD is actually the same disease in every country world-wide, but VWD is not diagnosed the same way or as effectively all across the world, and may also be subject to different types of treatment, based on where the patient lives.
This issue of AOB is not meant to be comprehensive. Indeed, VWD is potentially diagnosed and treated in every country of the world, and within each country there may be scores or even hundreds of VWD diagnosis and treatment centers. Instead, this issue of AOB is meant to provide a snapshot of VWD diagnosis and management in the year 2018, across ‘the four corners’ of the world. Invited contributions were received from the countries identified in Figure 1, and as representing both developed and developing countries. Recognized workers in the field were simply asked to prepare a paper on the diagnosis and treatment of VWD in their country/locality. Only two items were mandated for these contributions, these being information or statistics on VWD type/subtype distribution and an algorithm that described the diagnostic (and optional – management) process in their country/locality. Authors were otherwise free to decide the remaining content of their contributions, and it is always of interest to see the different ways that different authors approach such a task. This issue of AOB has not been officially ‘closed’, and contributions are still invited if they add significantly to the discussion.
My own contribution to this issue of AOB, written with some clinical colleagues, is provided as an example of the diagnosis and treatment of VWD in Australia, representing a developed country in the South Pacific region (6). Like most developed countries, type 1 VWD is recognized as being the most common form of VWD in Australia (>60% of all VWD cases), and type 3 VWD alternatively identified as the rarest form of VWD (<5% of all VWD cases) (Figure 2). Within type 2 VWD, types 2B and 2N are the rarest forms identified in Australia, and types 2A and 2M are the most common. Nevertheless, type 2 VWD in total represents <30% of all cases of VWD identified in Australia. We believe that Australians have access to the best diagnostic laboratory tools, and we are fortunate to be in this position. Treatment of VWD in Australia comprises many options, but primarily desmopressin (1-deamino-8-d-arginine vasopressin, DDAVP) for short term therapy in type 1 and some type 2 VWD, with plasma derived VWF concentrates (typically also containing factor VIII; FVIII) employed for long term therapy in all forms of VWD, or for short term therapy in cases of VWD where DDAVP is ineffective or considered contraindicated. Although recombinant VWF concentrate is available as a treatment in some geographies (17), it is not yet available in Australia. Where recombinant VWF concentrate is unavailable, the therapeutic material employed is typically plasma derived (18). In Australia, we also use the DDAVP response pattern to help characterize the VWD type in patients with unclear type based on standard testing (6,19).
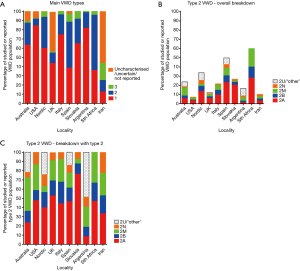
There are several additional contributions from other developed countries. For example, the contribution from Flood and colleagues provides one perspective from the United States of America (USA) (20). In terms of broad VWD type frequencies, their data (centered on the Milwaukee area of the USA), provides a similar breakdown to that of Australia in terms of the main VWD types (Figure 2), with type 1 VWD most highly represented. Further specific breakdown of type 2 VWD was not provided by these workers, but previous data from this group and the USA suggests type 2A as being the most common ‘subtype’, with 2M being less common, and again types 2B and 2N VWD being least common (21) (Figure 2). Although also a developed country, the laboratory testing process is somewhat different in the USA compared to Australia, being sometimes driven by what tests the FDA (Food and Drug Administration) has ‘approved’ (or ‘cleared’). In the USA, many fewer VWF tests are regulatory approved than in Europe or even Australia, leading to potentially compromising VWD diagnosis, unless laboratories are willing to take on the additional ‘risks’ of using ‘research only tests’ in diagnostics (22,23). From a treatment perspective, VWD management would be broadly similar to that in Australia, except that recombinant VWF concentrate is now available for treatment of VWD in the USA (17).
Several other reports from developed countries are presented in this issue of AOB, and generally they present similar findings. One report that represents the ‘Nordic perspective’ is of interest as it represents a consortium of individually small European countries (24). Here, VWD management/treatment is similar to that in Australia, albeit using different commercial forms of VWF/FVIII concentrates (18,24). Diagnostic practice would also be similar to that in Australia and the USA, although differences in the reported proportions of VWD types (24) (Figure 2) would suggest it is not entirely identical. In particular, for example, type 2M VWD appears to be under-diagnosed, potentially instead being identified as type 2A VWD, as otherwise previously reported in comparative studies (12,14). Sometimes, however, it is not misdiagnosis per se, but just a preference to classify a patient differently. For example, the contribution from Spain (25) even discusses the concept of 2A/2M, representing patients with particular VWF mutations that sometimes leads to a diagnosis of type 2A and other times as 2M. Sometimes, the differential diagnosis between 2A and 2M hinges on the concept of ‘significant reduction’ in high molecular weight (HMW) VWF multimers – this being generally clear in type 2A VWD. However, sometimes researchers may apply this concept also to some cases of 2M in which others would only assign as ‘minor loss of HMW VWF multimers’, or where there may be other evidence VWF multimer changes but no loss of HMW VWF. Overall, the report from Spain provides the most up to date assessment around genetics in VWD in this issue of AOB, given their exploration of next generation sequencing (25). It is important to note also that the frequencies shown in Figure 2 for VWD do not always reflect a national database, but sometimes only represent local experience. For example, in the report from Spain (25), the authors have focused their data on the results of a local genetics-focused study, and so this would reflect selection bias associated to VWD patients with more severe disease (e.g., type 3 VWD) or with qualitative defects (i.e., type 2 VWD). Thus, the frequency of type 1 VWD would be under-represented in their report (25), and not because type 1 VWD is less frequent in Spain. In addition, these authors provide data on composite VWD disorders as well as ‘carriers’ of VWD (e.g., heterozygous type 3 and 2N, who may or may not be clinically symptomatic).
Additional perspectives from developed countries and information of VWD type distributions is provided from the United Kingdom (UK) (26), Italy (27), and Slovakia (28) (Figure 2). Some interesting similarities and differences can be highlighted in these reports. For example, there is a greater variety of commercial VWF concentrate products available in some locations, such as the UK (26). Again, the differences in reported VWD type frequencies can be highlighted (Figure 2). The contention in regards to the perennial question of type 2A vs. 2M also continues upon review of the differential data presented from these locations. Like the report from Spain (25), the report from Italy (27) also presents much genetic data, including a report of the most frequent genetic mutations presenting in Italy. The report from the UK (26) has an interesting and in-depth discussion of inhibitors. The report from Slovakia (28) also has several interesting insights, and reflects an unusually high proportion of type 3 VWD cases compared to other developed countries. I was fortunate to recently visit the Slovakian laboratory, and was impressed by the level of hemostasis related activity undertaken in the small township of Martin.
Additional insights into VWD diagnosis and management, from the perspective of developing or economically challenged countries are also available in this special issue of AOB, as represented by reports from Argentina (29), South Africa (30) and Iran (31). In developing countries, financial considerations can severely limit clinical investigation and laboratory testing, and also potentially compromise treatment/management of patients. Each of these contributions therefore gives unique insights into the difficulties faced is such localities in order to effect an accurate diagnosis of VWD, and thus facilitate the best treatments/management. Additional strategies may therefore be employed to improve local practice. For example, in Argentina (29), the DDAVP challenge test, like Australia (6,7,19) is used not only to assess clinical efficacy of treatment, but also as a VWD diagnostic tool for difficult cases. Genetics testing is also increasingly being employed in Argentina. I have been fortunate to also visit the Argentinean laboratory, and was saddened to hear that Dr. Lazzari has recently passed away.
In South Africa (30), the sustainability of the diagnostic service is enabled by judicious use of laboratory testing and mostly by performing in-house developed assays. One big challenge in South Africa is the (poor) quality of the samples received, and which jeopardizes effective diagnosis of VWD (30). That samples often need to be transported over large distances to a central laboratory can lead to a variety of pre-analytical issues and thus causes false diagnosis of (especially type 2) VWD (30,32). Transport costs, and additional costs involved in patient attendance to clinics, also helps explain the predominance of type 2 VWD samples identified in this report (30) (Figure 2).
Finally, the report from Iran (31) also identifies a relatively low proportion of type 1 VWD for reasons that have already been mentioned (Figure 2). In Iran, however, what is perhaps most striking is the comparatively very high proportion of type 3 VWD cases, as assessed against other localities. As mentioned earlier, this is likely to be due to a combination of factors, including that consanguinity is more common in Iran than many other countries, and also since type 3 VWD represents the most severe form of VWD, and thus those patients most likely to be investigated in a financially constrained environment.
Also provided within each of the issue contributions is an algorithm representing the diagnostic strategy used by each authorship group or geographical region. Again, this reflects somewhat on the variable approaches taken in different geographies, perhaps reflecting the dominant technologies (or else reflective of the lack of commercial tests) as available or regulatory approved, as well as the respective biases of the groups involved in diagnosis. As a reiterated example, my own group has continuously touted the value of the VWF collagen binding assay (6,7,33,34), not only to firm up a diagnosis or otherwise of type 2 VWD, but to also help better subtype VWD patients into (for example) the 2A vs. 2M VWD ‘camp’. Some other workers in this field also utilize this assay in their diagnostic workup, as identified in this special issue of the journal. In contrast, in the USA, given lack of any FDA-cleared VWF:CB assay (22,23), this method would be much more selectively utilized.
It is hoped that this special issue compilation on VWD is found to be of interest to the readership of AOB. It is also hoped that we can learn from one another, and adopt the best of the best in terms of methods and strategies for VWD diagnosis, as well as improve the treatment of VWD—a globally widely distributed and common bleeding disorder, but with diverse approaches to its diagnosis and management.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Blood for the series “Diagnosis and Management of von Willebrand Disease: Diverse Approaches to a Global and Common Bleeding Disorder”. The article did not undergo external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/aob.2018.10.03). The series “Diagnosis and Management of von Willebrand Disease: Diverse Approaches to a Global and Common Bleeding Disorder” was commissioned by the editorial office without any funding or sponsorship. EJF served as an unpaid Guest Editor of the series. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclaimer: The opinions expressed in this article are those of the author, and not necessarily those of New South Wales Health Pathology.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Favaloro EJ. Von Willebrand disease: local diagnosis and management of a globally distributed bleeding disorder. Semin Thromb Hemost 2011;37:440-55. [Crossref] [PubMed]
- Favaloro EJ. Rare forms of von Willebrand disease. Ann Transl Med 2018;6:345. [Crossref] [PubMed]
- Sadler JE, Budde U, Eikenboom JC, et al. Working Party on von Willebrand Disease Classification. Update on the pathophysiology and classification of von Willebrand disease: a report of the Subcommittee on von Willebrand Factor. J Thromb Haemost 2006;4:2103-14. [Crossref] [PubMed]
- Nichols WL, Hultin MB, James AH, et al. von Willebrand disease (VWD): evidence-based diagnosis and management guidelines, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA). Haemophilia 2008;14:171-232. [Crossref] [PubMed]
- Laffan MA, Lester W, O'Donnell JS, et al. The diagnosis and management of von Willebrand disease: a United Kingdom Haemophilia Centre Doctors Organization guideline approved by the British Committee for Standards in Haematology. Br J Haematol 2014;167:453-65. [Crossref] [PubMed]
- Favaloro EJ, Pasalic L, Curnow J. Diagnosis and management of von Willebrand disease in Australia. Ann Blood 2018;3:31. [Crossref]
- Favaloro EJ, Pasalic L, Curnow J. Laboratory tests used to help diagnose von Willebrand disease: an update. Pathology 2016;48:303-18. [Crossref] [PubMed]
- Just S. Laboratory Testing for von Willebrand Disease: The Past, Present, and Future State of Play for von Willebrand Factor Assays that Measure Platelet Binding Activity, with or without Ristocetin. Semin Thromb Hemost 2017;43:75-91. [PubMed]
- Sadler JE. Von Willebrand disease type 1: a diagnosis in search of a disease. Blood. 2003;101:2089-93. [Crossref] [PubMed]
- Frontroth JP, Favaloro EJ. Ristocetin-Induced Platelet Aggregation (RIPA) and RIPA Mixing Studies. Methods Mol Biol 2017;1646:473-94. [Crossref] [PubMed]
- Mohammed S, Favaloro EJ. Laboratory Testing for von Willebrand Factor: Factor VIII Binding (for 2N VWD). Methods Mol Biol 2017;1646:461-72. [Crossref] [PubMed]
- Favaloro EJ, Bonar RA, Meiring M, Duncan E, Mohammed S, Sioufi J, Marsden K. Evaluating errors in the laboratory identification of von Willebrand disease in the real world. Thromb Res 2014;134:393-403. [Crossref] [PubMed]
- Favaloro EJ, Oliver S. Evaluation of a new commercial von Willebrand factor multimer assay. Haemophilia 2017;23:e373-7. [Crossref] [PubMed]
- Favaloro EJ, Bonar RA, Mohammed S, et al. Type 2M von Willebrand disease – more often misidentified than correctly identified. Haemophilia 2016;22:e145-55. [Crossref] [PubMed]
- Chandler WL, Peerschke EI, Castellone DD, et al. Von Willebrand factor assay proficiency testing. The North American Specialized Coagulation Laboratory Association experience. Am J Clin Pathol 2011;135:862-9. [Crossref] [PubMed]
- Meijer P, Haverkate F. An external quality assessment program for von Willebrand factor laboratory analysis: an overview from the European concerted action on thrombosis and disabilities foundation. Semin Thromb Hemost 2006;32:485-91. [Crossref] [PubMed]
- Favaloro EJ. Towards personalised therapy for von Willebrand disease: a future role for recombinant products. Blood Transfus 2016;14:262-76. [PubMed]
- Favaloro EJ, Franchini M, Lippi G. Biological therapies for von Willebrand Disease. Expert Opin Biol Ther 2012;12:551-64. [Crossref] [PubMed]
- Favaloro EJ, Pasalic L, Curnow J. Monitoring Therapy during Treatment of von Willebrand Disease. Semin Thromb Hemost 2017;43:338-54. [PubMed]
- Flood VH, Abshire TC, Christopherson PA, et al. Von Willebrand disease in the United States: perspective from the Zimmerman program. Ann Blood 2018;3:7. [Crossref] [PubMed]
- Flood VH, Gill JC, Friedman KD, et al. Von Willebrand disease in the United States: a perspective from Wisconsin. Semin Thromb Hemost 2011;37:528-34. [Crossref] [PubMed]
- Favaloro EJ, Plebani M, Lippi G. Regulation in hemostasis and thrombosis: part I-in vitro diagnostics. Semin Thromb Hemost 2013;39:235-49. [Crossref] [PubMed]
- Favaloro EJ, Lippi G. Recent advances in mainstream hemostasis diagnostics and coagulation testing. Semin Thromb Hemost 2018; In press.
- Szanto T, Lassila R, Funding E, et al. Von Willebrand disease—the Nordic perspective. Ann Blood 2018;3:2. [Crossref]
- Batlle J, Pérez-Rodríguez A, Corrales I, et al. Diagnosis and management of von willebrand disease in Spain. Ann Blood 2018;3:5. [Crossref]
- Keeney S, Goodeve A. Diagnosis and management of von Willebrand disease in the United Kingdom. Ann Blood 2018;3:29. [Crossref]
- Castaman G, Oliovecchio E, Federici AB. Diagnosis and management of von Willebrand disease in Italy. Ann Blood 2018;3:28. [Crossref]
- Kubisz P, Sokol J, Simurda T, et al. Diagnosis and management of von Willebrand disease in Slovakia. Ann Blood 2018;3:9. [Crossref]
- Woods AI, Kempfer AC, Paiva J, et al. Diagnosis of von Willebrand disease in Argentina: a single institution experience. Ann Blood 2017;2:22. [Crossref]
- Meiring M, Haupt L, Conradie C, et al. Challenges in the laboratory diagnosis and management of von Willebrand disease in South Africa. Ann Blood 2017;2:19. [Crossref]
- Dorgalaleh A, Tabibian S, Shams M, et al. Von Willebrand disease in Iran: diagnosis and management. Ann Blood 2018;3:4. [Crossref]
- Favaloro EJ, Lippi G. Pre-analytical issues that may cause misdiagnosis in haemophilia and von Willebrand disease. Haemophilia 2018;24:198-210. [Crossref] [PubMed]
- Favaloro EJ, Mohammed S. Laboratory Testing for von Willebrand Factor Collagen Binding (VWF:CB). Methods Mol Biol 2017;1646:417-33. [Crossref] [PubMed]
- Favaloro EJ. An update on the von Willebrand factor collagen binding assay: 21 years of age and beyond adolescence but not yet a mature adult. Semin Thromb Hemost 2007;33:727-44. [Crossref] [PubMed]
Cite this article as: Favaloro EJ. Preface to Special Issue: diagnosis and management of von Willebrand disease—diverse approaches to a global and common bleeding disorder. Ann Blood 2018;3:43.



