
Acquired immune thrombocytopenia: an update on pathophysiology, diagnosis and management
Introduction
Acquired thrombocytopenia occurs when platelet loss and/or consumption in the circulation exceeds their production from megakaryocytes in bone marrow. The following four mechanisms can be distinguished although more than one of them often contributes to thrombocytopenia in the individual patient: (I) hemodilution; (II) platelet consumption; (III) decreased platelet production; (IV) increased sequestration of platelets by immune-mediated response against platelets (1).
In acquired immune thrombocytopenia (AITP) platelet-reactive antibodies, such as autoantibodies (AAbs), alloantibodies, or drug-dependent antibodies (DDAbs) are responsible for the reduced platelet (PLT) lifespan in immune thrombocytopenia (ITP), drug-induced thrombocytopenia (DITP) and heparin-induced thrombocytopenia (HIT), respectively. These antibodies usually target platelet surface glycoproteins (GPs) (Table 1) (7-9). In most of the cases platelet counts decrease significantly and severe thrombocytopenia (<20×109/L) is often present. With the exception of HIT, mucocutaneous bleeding is the predominant symptom in patients with platelet counts below 10–20×109/L.
Table 1
| Glycoprotein | Number of copies per platelet | Function | Ligands |
|---|---|---|---|
| GPIb-IX-V | ~25,000 | Adhesion to extracellular matrix-, endothelial cells-and leukocytes, procoagulant activity, intracellular signaling | vWF, P-selectin |
| GPIIb-IIIa | ~50,000–80,000 | Platelet aggregation | Fibrinogen, vWF, thrombospondin vitronectin, fibronectin |
| GPVI | ~4,000–6,000 | Interaction with collagen of extracellular matrix leads to platelet activation and aggregation | Collagen, laminin, CRP, convulxin |
Summarized from references (
In this review we will provide current insights onto pathophysiology, diagnosis and management of AITP with a focus on ITP, DITP and HIT.
ITP
ITP, previously known as idiopathic thrombocytopenic purpura, is an autoimmune disease characterized by an isolated decrease in PLT count (<100×109/L) with bleeding tendency. The diagnosis of ITP is made based on the exclusion of underlying causes that might be related to thrombocytopenia and in this case is defined as primary ITP. In contrast, in the presence of additional autoimmune disorders and infections, such as HCV and HIV, the term secondary ITP is used (10,11).
Pathophysiology of ITP
It is thought that the low PLT count in ITP is due to accelerated PLT clearance in the circulation and inhibited PLT production in the bone marrow (12). The mechanisms leading to a decrease in PLT count are mainly induced by anti-platelet AAbs including Fc-dependent, Fc-independent phagocytosis, activation of complement or by direct cell lysis through cytotoxic T-lymphocytes (13-15). The impairment of PLT production in the bone marrow through suppression of thrombopoiesis is an additional process mediated by anti-platelet AAbs, which appears to be involved in the pathophysiology of ITP (16).
Clinical course of ITP
The incidence and clinical course of ITP is age dependent (17). The incidence of ITP is estimated to be between 1.6 and 3.9 per 100,000 persons per year in adults and between 1.9 and 6.4 per 100,000 persons per year in children (18-20). Severe bleeding in ITP children was reported rarely and most cases undergo spontaneous remission. Intriguingly, accumulating evidence suggests the risk for chronicity increases with age, with higher rates of chronic disease in adolescents (10,21).
Conversely to the pediatric course, ITP in adults is associated with a higher rate of chronicity and bleeding risk. Factors for prediction of severe bleeding are gender, comorbidities and an age older than 60 years (22).
Diagnosis of ITP
Although different guidelines are currently available, the diagnosis of ITP still represents a challenging task for physicians (10,23). In fact several case reports showed that the lack of sensitive and specific markers can lead to misdiagnosis and consequently to inappropriate treatments of thrombocytopenic patients (24). The diagnostic approach in cases suspected to have ITP is based on a deep and complete clinical patient’s history, physical examination and review of the complete blood count. Furthermore, analysis of the peripheral blood smear is mandatory in order to exclude pseudothrombocytopenia, inherited thrombocytopenia and other pathognomonic alterations like micro-hemolytic anemia (fragmented red cells in TTP) (23).
The mean PLT volume may be increased in patients with ITP. However, ITP patients always show a heterogeneous PLT population with up to ~40% enlarged PLTs. If more than 60% large or even giant PLTs (less than two PLTs fit into one red blood cell) are present, hereditary macrothrombocytopenia is much more likely the underlying cause of thrombocytopenia. Bone marrow examination is not routinely required and is generally not useful for diagnosing ITP but should be performed to exclude other causes of thrombocytopenia when atypical features such as unexplained anemia, lymphadenopathy, or splenomegaly have been observed. Megakaryocyte number is typically normal or even increased in the bone marrow of patients with ITP.
The use of antibody testing is controversial and is recommended in only a few of the available guidelines (23,25). Of note, since the non-responsiveness of particular anti-platelet AAbs to rituximab was described in 2017, there has been a need for reliable anti-platelet AAb testing (26). The detection of anti-platelet AAbs is commonly performed using the monoclonal antibody immobilization of platelet antigens (MAIPA) or flow cytometric assays (Table 2). Of note, current studies are investigating the detection of biomarkers like the expression of CD61 (P-selectin) and the use of mass cytometry which appear to be auspicious procedures for diagnosing and especially tailoring individual ITP therapies (Table 2) (27-32).
Table 2
| Methods | Measured parameter | Targeted antigens |
|---|---|---|
| MAIPA (Kiefel |
Photometric extinction | Antiplatelet antibodies against: GPIIb/IIIa + GPIb/IX |
| Flowcytometric immunobead assay (Zhai |
Flow cytometric fluorescence activity | Antibody binded GPIIb/IIIa + GPIb/IX |
| Functional platelet assay (Frelinger |
Flow cytometric fluorescence activity | CD62p (P-selectin), activated GPIIb/IIIa, GPIbα |
| Mass cytometry (Blair |
Detection of metal-tagged antibody masses | Different surface glycoproteins |
MAIPA, monoclonal antibody immobilization of platelet antigens.
Therapeutic options of ITP
Considering the complexity of ITP in terms of different extent of symptoms and pathophysiologic mechanisms, adequate and individualized treatment is usually required (33). The main goal of the therapy is to minimize the risk of severe bleeding and enhance PLT count (10,34-36).
First line treatment in acute ITP is based on the use of corticosteroids, IVIGs and anti-D-immunoglobulin (Anti-D). Although standard corticosteroids (such as prednisone) have a key role in the initial treatment of ITP, their use is often limited through adverse reactions, lack of patient compliance and delayed response. Hence, the efficacy of high dose dexamethasone has been reported by several studies to be higher compared to standard prednisone (37). However, further investigations about the role of high dose dexamethasone in ITP treatment are needed in order to elucidate toxicity and long-term effects. Whereas the addition of IVIGs in first line treatment is well described, the role of Anti-D does not seem to be consistent. The administration of Anti-D is limited to patients who are expressing the RhD-antigen and are not splenectomized. The exact mechanism of Anti-D remains highly speculative (38).
Second line treatment in ITP includes splenectomy and rituximab. While the role of splenectomy is currently under debate due to the development of alternative therapies such as thrombopoietin receptor agonists (TPO-RAs) (39), emerging evidence suggests that rituximab may be effective in refractory ITP patients. Rituximab, a humanized monoclonal anti-CD20 antibody which leads to peripheral B-cell depletion and reduction of circulating AAbs, was shown to increase PLT count (>50×109/L) and induce sustained response in ITP patients (40). Furthermore, the combination of rituximab with corticosteroid treatment looks to be a promising approach to treat refractory ITP patients (41). Nevertheless, more investigations involving rituximab and its combination with other agents are necessary in order to overcome the high rates of side effects as recently reported (42).
At the time current guidelines were published, TPO-RAs represented a new class of substances in treatment of ITP. At the time, the lack of data regarding safety, long-term effects and toxicity led to the decision that TPO-RAs should be considered as an alternative drug in third line treatment (10). Nowadays, the two FDA approved TPO-RAs, romiplostin and eltrombopag, have transformed the management of ITP and risen to be well-established second line agents with high effectivity and long-term safety (43,44). Furthermore, the use of TPO-RAs in emergency patients with severe ITP, in combination with first line agents like corticosteroids and IVIGs, is becoming more and more common due to the better outcome of patients has been reported (45).
Drug-induced ITP (DITP)
More than 300 drugs have been found to be associated with DITP. A systematic analysis of individual patient data found that the most commonly mentioned drugs with a definite or probable causal relation to thrombocytopenia were: quinine, quinidine, trimethoprim/sulfamethoxazole, vancomycin, penicillin, rifampin, carbamazepine, ceftriaxone, ibuprofen, mirtazapine, oxaliplatin, suramin, and the GPIIb/IIIa inhibitors abciximab, tirofiban and eptifibatide (46).
The pathophysiology of DITP
There are several pathogenic mechanisms that have been associated with DITP (Table 3): (I) quinine-type DDAbs: the classic drug dependent antibodies (DDAbs) attach tightly to PLTs only in the presence of the sensitizing drug and most often target GPIIb/IIIa or GPIb/IX. A recent study showed that a hybrid paratope consisting of quinine and reconfigured antibody, the complementarity-determining regions (CDRs) of the DDAbs, plays a critical role in recognition of its target epitope by an antibody (49). (II) Hapten-dependent DDAbs: small molecules (<5,000 daltons; e.g., penicillin) require a covalent coupling to a larger carrier protein, mostly GPIIb/IIIa, to elicit drug-specific antibodies, which then binds to the small molecule drug rather than to the PLT protein. (III) Fiban-type DDAbs: this mechanism is reported by GPIIb/IIIa inhibitors such as tirofiban and eptifibatide. These drugs can induce conformational changes in the protein structure leading to an immune response against PLTs. (IV) Drug-specific DDAbs: usually reported after administration of drugs with a murine component such as abciximab, a chimeric (mouse-human) monoclonal antibody Fab fragment specific for GPIIIa, used primarily to prevent PLT aggregate formation. (V) Autoantibody mechanism: these antibodies arise after drug exposure (especially gold-therapy); however, the presence of the drug is not required for their binding to PLTs. Although immune response in DITP is thought to be specific to the immunizing drug, a recent study showed that patients treated with oxaliplatin (a conventional cancer drug) produce multiple DDAbs that are specific for other agents to which the patients were exposed (50).
Table 3
| Drug | Mechanism | Example of drug |
|---|---|---|
| Quinine-type | Drug binds DDAbs and subsequently to PLT integrin | Quinine, sulfonamide-antibiotics, nonsteroidal anti-inflammatory drugs |
| Hapten-dependent | Drug links covalently to membrane protein and induces drug-specific binding by DDAbs | Penicillin, some cephalosporines |
| Fiban-type | Drug reacts with GP IIb-IIIa and induces neoepitope(s) for DDAbs | Tirofiban, eptifibatide |
| Drug specific | Drug recognizes murine component of chimeric Fab-fragment specific for GP IIIa | Abciximab |
| Autoantibody | Drug induces antibody that reacts with autologous platelet in absence of drug | Gold salts, procainamide |
DDAb, drug dependent antibody; PLT, platelet; GP, glycoprotein (
Clinical course of DITP
DITP presents a life-threatening clinical syndrome that is associated with a high risk of hemorrhage. A review of 247 case reports of DITP found an incidence of major and fatal bleeding of 9% and 0.8%, respectively (47). Symptoms emerge approximately 1–2 weeks after initial drug exposure, with median nadir PLT counts of <20×109/L. An exception is thrombocytopenia induced by the GPIIb/IIIa antagonists, which may present within hours of exposure (early onset) due to naturally occurring antibodies.
Diagnosis of DITP
Pivotal for diagnosing DITP is a high grade of clinical suspicion and careful workup of a patient’s history to reveal the causative drug. There are five clinical criteria, which can help to manifest the diagnosis of DITP (46): (I) exposure to the candidate drug preceded thrombocytopenia; (II) recovery from thrombocytopenia was complete and sustained after discontinuing candidate drug; (III) candidate drug was the only drug used before the onset of thrombocytopenia, or other drugs were continued or reintroduced after discontinuation of the candidate drug with a sustained normal platelet count; (IV) other causes for thrombocytopenia were excluded; and (V) re-exposure to the candidate drug resulted in recurrent thrombocytopenia.
Since DITP often occurs in hospitalized patients who are taking multiple medications and who have comorbidities that can also cause thrombocytopenia, relating thrombocytopenia to a particular drug depending solely on clinical information is commonly difficult. Therefore, most investigators agree that confirmation is required either by a drug-challenge or the in vitro demonstration of DDAbs. Several laboratory methods have been developed to detect antibodies that bind to PLTs in the presence of drug or its metabolite. Test methods must show drug-dependence, immunoglobulin binding, PLT specificity and ideally should be reproducible across laboratories. Recently, recommendations for laboratory testing for DITP have been published by the international society of thrombosis and hemostasis, in which the authors provided helpful methodological guidelines to increase the specificity and sensitivity of the used assays (51).
Management of patients suspected for DITP
Treatment for DITP involves discontinuation of the offending drug. The PLT count usually starts to recover after 4–5 half-lives of the responsible drug or drug metabolite. In cases of severe thrombocytopenia and increased risk of bleeding, highly dosed IVIGs can be given; although this recommendation is based only on case reports (48,52). PLT transfusion is generally ineffective as long as the drug or its metabolite(s) are present in plasma.
Heparin induced thrombocytopenia (HIT type II)
HIT is a prothrombotic disorder that occurs after exposure to unfractionated heparin (UFH) or low molecular weight heparin (LMWH) (53).
Pathophysiology of HIT
The in vivo formation of highly immunizing multimolecular complexes, consisting of negatively charged polyanions and the cationic protein platelet factor 4 (PF4), results in antibody formation in many heparin-exposed patients. Although the immunization rate against PF4/heparin is high, only a subset can induce HIT. In fact, only a minority of IgG anti-PF4/heparin antibodies are able to cross-link the Fc receptors on PLT surface leading to their activation. This results in release of PLT granules, formation of PLT microparticles, thrombin generation and ultimately PLT aggregation (53). Endothelium and monocyte activation with tissue factor expression is also involved in the pathophysiology of HIT (54). These processes together are thought to be responsible for the hypercoagulable state of HIT and the frequent occurrence of thrombotic complications in the absence of appropriate anticoagulation.
Clinical manifestations of HIT
Patients with HIT can present with a wide spectrum of symptoms. The cardinal clinical feature is a fall in PLT count >50% (from the highest value after start of heparin treatment) typically between days 5 to 14 after start of heparin. A PLT count fall within the first hours after the start of heparin (rapid onset HIT) is sometimes observed in preimmunized patients who had received heparin within the past 30 days. While a mean nadir between 50–80×109/L was most often found in larger cohort studies, HIT cases that are complicated by disseminated intravascular coagulation (DIC) may result in a deeper drop in PLT count <20×109/L.
Besides thrombocytopenia, HIT may also be associated with thrombosis which is the most severe complication of HIT and largely contributes to disease morbidity and mortality. About half of untreated patients with acute HIT develop a new thrombotic complication. Deep-vein thrombosis (DVT), with or without pulmonary embolism, is the most common complication. Arterial thrombosis is less frequent than venous thrombosis in HIT patients and typically involves lower-limb, cerebral, coronary, mesenteric and brachial arteries (53). Rarely, severe HIT-associated DIC leads to microthrombosis and critical limb ischemia even in the absence of warfarin therapy. Other (rare) complications observed in HIT patients include skin necrosis at the heparin injection sites and adrenal hemorrhagic necrosis.
Management of patients with suspected HIT
When HIT is strongly suspected, all sources of heparin must be stopped. In addition, anticoagulation should be continued using an alternative non-heparin anticoagulant to prevent new thromboembolic complications. However, alternative anticoagulants are rarely used outside the niche indication HIT and many physicians have limited experience handling these drugs. This may increase the risk for both bleeding and thrombotic events. Therefore, clinicians must distinguish the (relatively) uncommon patient with HIT among the many without.
Identifying the probability of HIT
To assist clinicians in this process, several clinical scoring systems for HIT have been developed. The most extensively studied scoring system, the 4Ts, incorporates four typical clinical features of HIT: (I) thrombocytopenia; (II) timing of onset of thrombocytopenia; (III) thrombosis or other clinical sequelae; and (IV) the likelihood of other causes of thrombocytopenia (55). The negative predictive probability of a 4Ts score below 4 has been shown to be very high (99.8%). However, the positive predictive value of an intermediate or even high 4Ts score is unsatisfactory (14% and 64%, respectively) (56). Other scoring systems such as The HIT expert probability score and the Lillo-Le Louët score require more validation in prospective studies before a firm conclusion can be drawn on their performance in the diagnostic work-up of HIT.
Laboratory investigations for HIT
Two classes of assays are available: functional (platelet activation) assays and (PF4-dependent) immunoassays (57,58). The presence of PLT-activating antibodies can be established only using functional assays. Although recent studies have indicated the feasibility to detect PLT-activating antibodies using the whole blood impedance analyzer (59), assays using washed PLTs such as the Heparin-Induced PLT Activation (HIPA) assay and Serotonin Release Assay (SRA) are the gold standard in the laboratory diagnosis of HIT (Figure 1) (61,62). Functional assays combine both high sensitivity and specificity for clinically relevant HIT antibodies. The performance of the SRA can be further improved by the addition of exogenous PF4 (63). Although both functional assays are considered the “gold standard” for diagnosing HIT, these assays are difficult to perform, require selected healthy PLT donors and are restricted to few reference laboratories. A recent study showed that PLT activating antibodies can be detected by flow cytometer (64). Using the PF4-dependent P-selectin expression assay, the authors demonstrated in a follow up study that the addition of PF4 enabled detection of pathogenic antibodies before the SRA became positive in two patients with HIT (65).
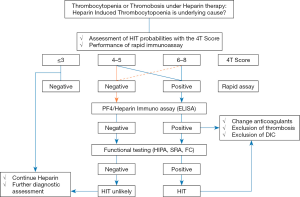
In contrast to functional assays, enzyme-linked immunosorbent assays (ELISA) and particle-based immunoassays can be easily used to detect anti-PF4/heparin antibodies.
ELISAs have an excellent negative predictive value to rule out HIT. However, their specificity is not satisfactory (40–80%) (57). Several approaches may increase the diagnostic specificity of ELISAs including the exclusive detection of anti-PF4/heparin IgG antibodies, consideration of the magnitude of the OD value and implementation of a confirmative inhibition step. Recent meta-analysis, however, did not find a significant advantage of IgG-specific over polyspecific ELISAs to improve the overall performance characteristics of the immunoassays (66).
Particle-based immunoassays are easily performed and reactions can be detected either visually after centrifugation as in the particle gel immunoassay or using lateral flow technology. The major advantage of these assays is its rapid turnaround time. Recent studies showed high negative predictive values of these assays (67). Automated particle-based immunoassays have also been introduced. A systematic meta-analysis investigated recently the diagnostic accuracy of rapid immunoassays for HIT. Data from this study showed that rapid immunoassays for HIT have high negative predictive value and can be used to exclude HIT, particularly in patients with low or intermediate clinical probability (68).
Anticoagulation of patients with suspected HIT
Patients with high clinical suspicion of HIT should be promptly treated with a non-heparin anticoagulant, while awaiting laboratory confirmation or exclusion of the diagnosis. Different anticoagulants are currently used to treat patients with HIT.
Activated factor X inhibitors
Danaparoid was shown to be efficient in preventing new, progressive, or recurrent thromboembolic complications (including thrombotic death) or limb amputation in HIT (69). This seems to be mediated by (I) low cross-reactivity rate with HIT antibodies; (II) the suppression of HIT antibody-induced platelet activation by replacing PF4/heparin complexes from the platelet surface; and (III) disruption of PF4/heparin complexes.
Fondaparinux has been increasingly used off-label for the management of HIT (70) and was found to be safe in patients with acute thrombosis with heparin-dependent platelet-activating antibodies (71).
Direct thrombin inhibitors
Argatroban is a synthetic direct thrombin inhibitor that reversibly binds to the thrombin active site. It is capable of inhibiting both free and clot-associated thrombin. Two multicenter trials showed that argatroban therapy reduces death, amputation and thrombosis when compared to historical controls (72).
Bivalirudin is another synthetic thrombin inhibitor and best investigated in non-HIT patients with coronary disease, including acute coronary syndrome and requiring coronary intervention (73).
Direct oral anticoagulants (DOACs)
Rivaroxaban, apixaban and edoxaban directly inhibit activated factor X, while dabigatran is a direct thrombin inhibitor. DOACs appear to be safe and effective without occurrence of new thrombosis. However, published experience with these drugs in patients with acute HIT is limited and does not allow final conclusion on their advantage. Of a particular importance are the observed low trough levels of the drug, which might cause inadequate protection for HIT patients.
IVIG
High dose IVIG was shown to inhibit HIT antibody-mediated platelet activation. Accumulating evidence from case reports suggests that patients with prolonged thrombocytopenia refractory to standard treatment may benefit from IVIG therapy (74).
Prevention of HIT
As mentioned above, the formation of multimolecular complexes consisting of negatively charged polyanions and the cationic protein PF4 results in antibody formation in many heparin-exposed patients. Interestingly, a 10-fold lower risk of HIT is observed with use of a prophylactic dose of LMWH compared to UFH. One recent study showed a dramatic reduction of the cases with suspected HIT after implementation of a strategy to avoid UFH and replace it with LMWH if required (75).
Protamine/heparin induced thrombocytopenia (PHIT)
Protamine and heparin form multimolecular complexes which result in high rates of immunization in post-cardiac surgery patients. A subset of these anti-protamine/heparin IgGs activates platelets through their FcγIIA receptors.
Diagnosis of PHIT
Anti-protamine/heparin antibodies can be detected using ELISAs. Heparin has been shown to increase binding of anti-protamine antibodies compared to protamine alone (76,77). In fact, protamine undergoes conformational changes after complexing with heparin (78,79), making it very likely that these antibodies bind to neoepitopes expressed on protamine only after complex formation with heparin. The ability of anti-protamine/heparin antibodies to activate platelets can be investigated in vitro using different laboratory methods including SRA and HIPA assay (79-83).
Clinical presentation of anti-protamine/heparin antibodies
While the presence of anti-protamine/heparin antibodies (IgG/A/M) reportedly had no overall impact on the postoperative PLT count evolution (80,82), PLT-activating antibodies against protamine/heparin complexes prior to surgery has been shown to be associated with lower postoperative platelet counts and increased demand on protamine to neutralize heparin after cardiac surgery (79,83). In addition, thromboembolic complications have been reported in patients with PLT-activating antibodies against protamine/heparin complexes (76,84). Currently, no sufficient experience exists regarding the treatment of protamine/HIT. One recent case series reported on the use of argatroban in four patients with PHIT. Platelet count recovered after starting argatroban and adverse events occurred (84).
Conclusions
As outlined in this review platelet count is a sensitive indication for disturbed balance between PLT production and survival. As it may be the first symptom of a severe underlying disease, AITP requires prompt clinical attention and careful diagnostic work-up. Gaining new insights into pathological states of the immune mechanisms is of major relevance to improve the quality patient care. Further understanding of these mechanisms also bears the possibility to identify new strategies for treatment, but also for prevention of thrombocytopenia as a side effect of infection and autoimmune disease.
Acknowledgments
The authors thank Stephen Bosher and Karina Althaus for helpful discussion.
This work was supported by a grant from the German Research Society and the German Red Cross.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Sentot Santoso) for the series “Platelet Immunology” published in Annals of Blood. The article has undergone external peer review.
Conflicts of Interest: The series “Platelet Immunology” was commissioned by the editorial office without any funding or sponsorship. T Bakchoul reports receiving honorarium for a scientific talk from Aspen Germany, CSL Behring, Stago gGmbH German, and research Grants from the German Society of Research, the German Society for Transfusion Medicine and German Red Cross. J Zlamal has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the manuscript and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McMahon CM, Cuker A. Hospital-acquired thrombocytopenia. Hosp Pract (1995) 2014;42:142-52. [PubMed]
- Modderman PW, Admiraal LG, Sonnenberg A, et al. Glycoproteins V and Ib-IX form a noncovalent complex in the platelet membrane. J Biol Chem 1992;267:364-9. [PubMed]
- Kovacsovics TJ, Hartwig JH. Thrombin-induced GPIb-IX centralization on the platelet surface requires actin assembly and myosin II activation. Blood 1996;87:618-29. [PubMed]
- Enayat S, Ravanbod S, Rassoulzadegan M, et al. A novel D235Y mutation in the GP1BA gene enhances platelet interaction with von Willebrand factor in an Iranian family with platelet-type von Willebrand disease. Thromb Haemost 2012;108:946-54. [Crossref] [PubMed]
- Curtis BR, McFarland JG. Human platelet antigens - 2013. Vox Sang 2014;106:93-102. [Crossref] [PubMed]
- Michelson AD. Platelets. 3rd edition. Academic Press, 2012:195-248.
- Cines DB, Cuker A, Semple JW. Pathogenesis of immune thrombocytopenia. Presse Med 2014;43:e49-59. [Crossref] [PubMed]
- Visentin GP, Ford SE, Scott JP, et al. Antibodies from patients with heparin-induced thrombocytopenia/thrombosis are specific for platelet factor 4 complexed with heparin or bound to endothelial cells. J Clin Invest 1994;93:81-8. [Crossref] [PubMed]
- Curtis BR. Drug-induced immune thrombocytopenia: incidence, clinical features, laboratory testing, and pathogenic mechanisms. Immunohematology 2014;30:55-65. [PubMed]
- Neunert C, Lim W, Crowther M, et al. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood 2011;117:4190-207. [Crossref] [PubMed]
- Rodeghiero F, Ruggeri M. ITP and international guidelines: what do we know, what do we need? Presse Med 2014;43:e61-7. [Crossref] [PubMed]
- Cines DB, Liebman HA. The immune thrombocytopenia syndrome: a disorder of diverse pathogenesis and clinical presentation. Hematol Oncol Clin North Am 2009;23:1155-61. [Crossref] [PubMed]
- Bakchoul T, Walek K, Krautwurst A, et al. Glycosylation of autoantibodies: insights into the mechanisms of immune thrombocytopenia. Thromb Haemost 2013;110:1259-66. [Crossref] [PubMed]
- McMillan R. The pathogenesis of chronic immune thrombocytopenic purpura. Semin Hematol 2007;44:S3-11. [Crossref] [PubMed]
- McMillan R, Nugent D. The effect of antiplatelet autoantibodies on megakaryocytopoiesis. Int J Hematol 2005;81:94-9. [Crossref] [PubMed]
- Ballem PJ, Segal GM, Stratton JR, et al. Mechanisms of thrombocytopenia in chronic autoimmune thrombocytopenic purpura. Evidence of both impaired platelet production and increased platelet clearance. J Clin Invest 1987;80:33-40. [Crossref] [PubMed]
- Kühne T, Buchanan GR, Zimmerman S, et al. A prospective comparative study of 2540 infants and children with newly diagnosed idiopathic thrombocytopenic purpura (ITP) from the Intercontinental Childhood ITP Study Group. J Pediatr 2003;143:605-8. [Crossref] [PubMed]
- Kurata Y, Fujimura K, Kuwana M, et al. Epidemiology of primary immune thrombocytopenia in children and adults in Japan: a population-based study and literature review. Int J Hematol 2011;93:329-35. [Crossref] [PubMed]
- Terrell DR, Beebe LA, Vesely SK, et al. The incidence of immune thrombocytopenic purpura in children and adults: A critical review of published reports. Am J Hematol 2010;85:174-80. [PubMed]
- Moulis G, Palmaro A, Montastruc JL, et al. Epidemiology of incident immune thrombocytopenia: a nationwide population-based study in France. Blood 2014;124:3308-15. [Crossref] [PubMed]
- Bennett CM, Neunert C, Grace RF, et al. Predictors of remission in children with newly diagnosed immune thrombocytopenia: Data from the Intercontinental Cooperative ITP Study Group Registry II participants. Pediatr Blood Cancer 2018;65: [Crossref] [PubMed]
- Fogarty PF. Chronic immune thrombocytopenia in adults: epidemiology and clinical presentation. Hematol Oncol Clin North Am 2009;23:1213-21. [Crossref] [PubMed]
- Matzdorff A, Meyer O, Ostermann H, et al. Immunthrombozytopenie - aktuelle Diagnostik und Therapie: Empfehlungen einer gemeinsamen Arbeitsgruppe der DGHO, OGHO, SGH, GPOH und DGTI. Oncol Res Treat 2018;41:5-36. [Crossref] [PubMed]
- Arnold DM, Nazy I, Clare R, et al. Misdiagnosis of primary immune thrombocytopenia and frequency of bleeding: lessons from the McMaster ITP Registry. Blood Adv 2017;1:2414-20. [PubMed]
- McMillan R, Wang L, Tani P. Prospective evaluation of the immunobead assay for the diagnosis of adult chronic immune thrombocytopenic purpura (ITP). J Thromb Haemost 2003;1:485-91. [Crossref] [PubMed]
- Arnold DM, Vrbensky JR, Karim N, et al. The effect of rituximab on anti-platelet autoantibody levels in patients with immune thrombocytopenia. Br J Haematol 2017;178:302-7. [Crossref] [PubMed]
- Basu SS, Deutsch EC, Schmaier AA, et al. Human platelets as a platform to monitor metabolic biomarkers using stable isotopes and LC-MS. Bioanalysis 2013;5:3009-21. [Crossref] [PubMed]
- Frelinger AL 3rd, Grace RF, Gerrits AJ, et al. Platelet Function in ITP, Independent of Platelet Count, Is Consistent Over Time and Is Associated with Both Current and Subsequent Bleeding Severity. Thromb Haemost 2018;118:143-51. [Crossref] [PubMed]
- Zhai J, Ding M, Yang T, et al. Flow cytometric immunobead assay for quantitative detection of platelet autoantibodies in immune thrombocytopenia patients. J Transl Med 2017;15:214. [Crossref] [PubMed]
- Porcelijn L, Huiskes E, Oldert G, et al. Detection of platelet autoantibodies to identify immune thrombocytopenia: state of the art. Br J Haematol 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Kiefel V, Santoso S, Weisheit M, et al. Monoclonal antibody--specific immobilization of platelet antigens (MAIPA): a new tool for the identification of platelet-reactive antibodies. Blood 1987;70:1722-6. [PubMed]
- Blair TA, Michelson AD, Frelinger AL 3rd. Mass Cytometry Reveals Distinct Platelet Subtypes in Healthy Subjects and Novel Alterations in Surface Glycoproteins in Glanzmann Thrombasthenia. Sci Rep 2018;8:10300. [Crossref] [PubMed]
- Lozano ML, Revilla N, Gonzalez-Lopez TJ, et al. Real-life management of primary immune thrombocytopenia (ITP) in adult patients and adherence to practice guidelines. Ann Hematol 2016;95:1089-98. [Crossref] [PubMed]
- Provan D, Newland AC. Current Management of Primary Immune Thrombocytopenia. Adv Ther 2015;32:875-87. [Crossref] [PubMed]
- Neunert CE. Management of newly diagnosed immune thrombocytopenia: can we change outcomes? Blood Adv 2017;1:2295-301. [Crossref] [PubMed]
- Matzdorff A, Giagounidis A, Greinacher A, et al. Diagnosis and therapy of autoimmune thrombocytopenia. Recommendations of a joint Expert Group of DGHO, DGTI, DTH. Onkologie 2010;33:2-20. [Crossref] [PubMed]
- Din B, Wang X, Shi Y, et al. Long-term effect of high-dose dexamethasone with or without low-dose dexamethasone maintenance in untreated immune thrombocytopenia. Acta Haematol 2015;133:124-8. [Crossref] [PubMed]
- Depré F, Aboud N, Mayer B, et al. Efficacy and tolerability of old and new drugs used in the treatment of immune thrombocytopenia: Results from a long-term observation in clinical practice. PLoS One 2018;13:e0198184 [Crossref] [PubMed]
- Cuker A, Cines DB, Neunert CE. Controversies in the treatment of immune thrombocytopenia. Curr Opin Hematol 2016;23:479-85. [Crossref] [PubMed]
- Patel VL, Mahevas M, Lee SY, et al. Outcomes 5 years after response to rituximab therapy in children and adults with immune thrombocytopenia. Blood 2012;119:5989-95. [Crossref] [PubMed]
- Gudbrandsdottir S, Birgens HS, Frederiksen H, et al. Rituximab and dexamethasone vs dexamethasone monotherapy in newly diagnosed patients with primary immune thrombocytopenia. Blood 2013;121:1976-81. [Crossref] [PubMed]
- Shoukat BA, Ali O, Kumar D, et al. Hypogammaglobulinemia Observed One Year after Rituximab Treatment for Idiopathic Thrombocytopenic Purpura. Case Rep Med 2018;2018:2096186 [Crossref] [PubMed]
- Kuter DJ, Macahilig C, Grotzinger KM, et al. Treatment patterns and clinical outcomes in patients with chronic immune thrombocytopenia (ITP) switched to eltrombopag or romiplostim. Int J Hematol 2015;101:255-63. [Crossref] [PubMed]
- Wong RSM, Saleh MN, Khelif A, et al. Safety and efficacy of long-term treatment of chronic/persistent ITP with eltrombopag: final results of the EXTEND study. Blood 2017;130:2527-36. [Crossref] [PubMed]
- Elgebaly AS, Ashal GE, Elfil M, et al. Tolerability and Efficacy of Eltrombopag in Chronic Immune Thrombocytopenia: Meta-Analysis of Randomized Controlled Trials. Clin Appl Thromb Hemost 2017;23:928-37. [Crossref] [PubMed]
- Arnold DM, Kukaswadia S, Nazi I, et al. A systematic evaluation of laboratory testing for drug-induced immune thrombocytopenia. J Thromb Haemost 2013;11:169-76. [Crossref] [PubMed]
- George JN, Raskob GE, Shah SR, et al. Drug-induced thrombocytopenia: a systematic review of published case reports. Ann Intern Med 1998;129:886-90. [Crossref] [PubMed]
- Arnold DM, Nazi I, Warkentin TE, et al. Approach to the diagnosis and management of drug-induced immune thrombocytopenia. Transfus Med Rev 2013;27:137-45. [Crossref] [PubMed]
- Zhu J, Zhu J, Bougie DW, et al. Structural basis for quinine-dependent antibody binding to platelet integrin alphaIIbbeta3. Blood 2015;126:2138-45. [Crossref] [PubMed]
- Curtis BR, Hsu YS, Podoltsev N, et al. Patients treated with oxaliplatin are at risk for thrombocytopenia caused by multiple drug-dependent antibodies. Blood 2018;131:1486-9. [Crossref] [PubMed]
- Arnold DM, Curtis BR, Bakchoul T, et al. Recommendations for standardization of laboratory testing for drug-induced immune thrombocytopenia: communication from the SSC of the ISTH. J Thromb Haemost 2015;13:676-8. [Crossref] [PubMed]
- Tvito A, Bakchoul T, Rowe JM, et al. Severe and persistent heparin-induced thrombocytopenia despite fondaparinux treatment. Am J Hematol 2015;90:675-8. [Crossref] [PubMed]
- Greinacher A. CLINICAL PRACTICE. Heparin-Induced Thrombocytopenia. N Engl J Med 2015;373:252-61. [Crossref] [PubMed]
- Kasthuri RS, Glover SL, Jonas W, et al. PF4/heparin-antibody complex induces monocyte tissue factor expression and release of tissue factor positive microparticles by activation of FcgammaRI. Blood 2012;119:5285-93. [Crossref] [PubMed]
- Lo GK, Juhl D, Warkentin TE, et al. Evaluation of pretest clinical score (4 T's) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J Thromb Haemost 2006;4:759-65. [Crossref] [PubMed]
- Cuker A, Gimotty PA, Crowther MA, et al. Predictive value of the 4Ts scoring system for heparin-induced thrombocytopenia: a systematic review and meta-analysis. Blood 2012;120:4160-7. [Crossref] [PubMed]
- Bakchoul T, Zollner H, Greinacher A. Current insights into the laboratory diagnosis of HIT. Int J Lab Hematol 2014;36:296-305. [Crossref] [PubMed]
- Nagler M, Bakchoul T. Clinical and laboratory tests for the diagnosis of heparin-induced thrombocytopenia. Thromb Haemost 2016;116:823-34. [Crossref] [PubMed]
- Morel-Kopp MC, Mullier F, Gkalea V, et al. Heparin-induced multi-electrode aggregometry method for heparin-induced thrombocytopenia testing: communication from the SSC of the ISTH. J Thromb Haemost 2016;14:2548-52. [Crossref] [PubMed]
- Bakchoul T, Hinz A. Diagnostik von angeborenen und erworbenen Thrombozyten-Erkrankungen. Haemotherapie 2017;28:4-12.
- Greinacher A, Michels I, Kiefel V, et al. A rapid and sensitive test for diagnosing heparin-associated thrombocytopenia. Thromb Haemost 1991;66:734-6. [Crossref] [PubMed]
- Sheridan D, Carter C, Kelton JG. A diagnostic test for heparin-induced thrombocytopenia. Blood 1986;67:27-30. [PubMed]
- Vayne C, Guery EA, Kizlik-Masson C, et al. Beneficial effect of exogenous platelet factor 4 for detecting pathogenic heparin-induced thrombocytopenia antibodies. Br J Haematol 2017;179:811-9. [Crossref] [PubMed]
- Padmanabhan A, Jones CG, Curtis BR, et al. A Novel PF4-Dependent Platelet Activation Assay Identifies Patients Likely to Have Heparin-Induced Thrombocytopenia/Thrombosis. Chest 2016;150:506-15. [Crossref] [PubMed]
- Jones CG, Pechauer SM, Curtis BR, et al. A Platelet Factor 4-Dependent Platelet Activation Assay Facilitates Early Detection of Pathogenic Heparin-Induced Thrombocytopenia Antibodies. Chest 2017;152:e77-80. [Crossref] [PubMed]
- Nagler M, Bachmann LM, Ten Cate H, et al. Diagnostic value of immunoassays for heparin-induced thrombocytopenia: a systematic review and meta-analysis. Blood 2016;127:546-57. [Crossref] [PubMed]
- Linkins LA, Bates SM, Lee AY, et al. Combination of 4Ts score and PF4/H-PaGIA for diagnosis and management of heparin-induced thrombocytopenia: prospective cohort study. Blood 2015;126:597-603. [Crossref] [PubMed]
- Sun L, Gimotty PA, Lakshmanan S, et al. Diagnostic accuracy of rapid immunoassays for heparin-induced thrombocytopenia. A systematic review and meta-analysis. Thromb Haemost 2016;115:1044-55. [Crossref] [PubMed]
- Lubenow N, Warkentin TE, Greinacher A, et al. Results of a systematic evaluation of treatment outcomes for heparin-induced thrombocytopenia in patients receiving danaparoid, ancrod, and/or coumarin explain the rapid shift in clinical practice during the 1990s. Thromb Res 2006;117:507-15. [Crossref] [PubMed]
- Schindewolf M, Steindl J, Beyer-Westendorf J, et al. Frequent off-label use of fondaparinux in patients with suspected acute heparin-induced thrombocytopenia (HIT)--findings from the GerHIT multi-centre registry study. Thromb Res 2014;134:29-35. [Crossref] [PubMed]
- Warkentin TE, Davidson BL, Buller HR, et al. Prevalence and risk of preexisting heparin-induced thrombocytopenia antibodies in patients with acute VTE. Chest 2011;140:366-73. [Crossref] [PubMed]
- Lewis BE, Wallis DE, Leya F, et al. Argatroban anticoagulation in patients with heparin-induced thrombocytopenia. Arch Intern Med 2003;163:1849-56. [Crossref] [PubMed]
- Warkentin TE, Greinacher A, Koster A. Bivalirudin. Thromb Haemost 2008;99:830-9. [Crossref] [PubMed]
- Padmanabhan A, Jones CG, Pechauer SM, et al. IVIg for Treatment of Severe Refractory Heparin-Induced Thrombocytopenia. Chest 2017;152:478-85. [Crossref] [PubMed]
- McGowan KE, Makari J, Diamantouros A, et al. Reducing the hospital burden of heparin-induced thrombocytopenia: impact of an avoid-heparin program. Blood 2016;127:1954-9. [Crossref] [PubMed]
- Panzer S, Schiferer A, Steinlechner B, et al. Serological features of antibodies to protamine inducing thrombocytopenia and thrombosis. Clin Chem Lab Med 2015;53:249-55. [Crossref] [PubMed]
- Lee GM, Joglekar M, Kuchibhatla M, et al. Serologic characterization of anti-protamine/heparin and anti-PF4/heparin antibodies. Blood Adv 2017;1:644-51. [Crossref] [PubMed]
- Chudasama SL, Espinasse B, Hwang F, et al. Heparin modifies the immunogenicity of positively charged proteins. Blood 2010;116:6046-53. [Crossref] [PubMed]
- Bakchoul T, Zollner H, Amiral J, et al. Anti-protamine-heparin antibodies: incidence, clinical relevance, and pathogenesis. Blood 2013;121:2821-7. [Crossref] [PubMed]
- Pouplard C, Leroux D, Rollin J, et al. Incidence of antibodies to protamine sulfate/heparin complexes incardiac surgery patients and impact on platelet activation and clinical outcome. Thromb Haemost 2013;109:1141-7. [Crossref] [PubMed]
- Singla A, Sullivan MJ, Lee G, et al. Protamine-induced immune thrombocytopenia. Transfusion 2013;53:2158-63. [PubMed]
- Lee GM, Welsby IJ, Phillips-Bute B, et al. High incidence of antibodies to protamine and protamine/heparin complexes in patients undergoing cardiopulmonary bypass. Blood 2013;121:2828-35. [Crossref] [PubMed]
- Grieshaber P, Bakchoul T, Wilhelm J, et al. Platelet-activating protamine-heparin-antibodies lead to higher protamine demand in patients undergoing cardiac surgery. J Thorac Cardiovasc Surg 2015;150:967-73.e1. [Crossref] [PubMed]
- Wadowski PP, Felli A, Schiferer A, et al. Argatroban in Thrombocytopenic Patients Sensitized to Circulating Protamine-Heparin Complexes. J Cardiothorac Vasc Anesth 2017;31:1779-83. [Crossref] [PubMed]
Cite this article as: Zlamal J, Bakchoul T. Acquired immune thrombocytopenia: an update on pathophysiology, diagnosis and management. Ann Blood 2018;3:45.


