
In vitro detection and removal of direct oral anticoagulants from patient plasma specimens
DOAC detection in urine
In 2019, DOASENSE GmbH, Heidelberg Germany released the DOAC Dipstick®, a urine test strip that detects and distinguishes between the therapeutic direct thrombin (factor IIa) inhibitor (DTI) dabigatran and the therapeutic anti-activated factor X (anti-Xa) inhibitors apixaban, edoxaban, and rivaroxaban (1). The strip is embossed with four chemical pads that provide qualitative analyses—in order from top to bottom; DTI dabigatran detection pad (manufacturer’s designation is ‘pad 4’); anti-factor Xa inhibitors apixaban, rivaroxaban, or edoxaban detection pad (‘pad 3’); an inert pad that highlights potential interfering urinary pigments (‘pad 2’); and a qualitative creatinine measurement pad that identifies potential renal insufficiency (‘pad 1’) (Figure 1). Pads 4 and 3 each provide a matrix where specific enzymes and substrates respectively directed against DTIs and anti-Xa inhibitors are immobilized. Pigments detectable on pad 2, such as certain drugs, protein, urobilinogen, bilirubin, or hemoglobin, may interfere with color interpretation. Pad 1 detects potential renal insufficiency that may reduce test sensitivity. The device is unaffected by unfractionated heparin, low molecular weight heparin, and fondaparinux.
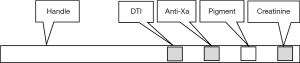
After dipping in freshly excreted urine for 2–3 seconds, the strips are placed on a horizontal surface. The pads develop color reduction endpoints within 10 minutes that the operator compares visually to a color chart, provided (Figure 2). If both DOAC test pads (top two) show a positive result, the test is invalid because it is unlikely that a patient has been treated with both DOAC types. The DOAC Dipstick®, which received its CE mark in 2018, is cleared for professional use in Europe and is recommended for speedy point of care DOAC detection in emergent overdose-related hemorrhage.
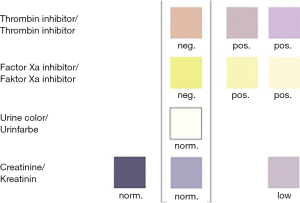
Approximately 80% of plasma dabigatran, 33% of edoxaban or rivaroxaban, and 25% of apixaban is cleared by the kidney. The direct anti-Xa anticoagulant betrixaban is 93% cleared by the liver and is unlikely to be detectable in all but the most concentrated urine. When renal clearance is inadequate, as indicated by a color-negative creatinine pad, DOAC detection by this method is unreliable. The lower limit for detection of creatinine is 0.25 g/L, equivalent to approximately 30 mL/minute creatinine clearance.
Reaction pad visual sensitivity is approximately 95 ηg/mL for DTIs and anti-Xa inhibitors. DOAC patients typically have values above 200 ηg/mL in urine, exceeding the corresponding levels in plasma, due to drug accumulation in urine.
In 2020, Harenberg et al. reported DOAC Dipstick® clinical efficacy data using urine specimens from 880 patients from 18 centers that included 451 who were receiving apixaban, edoxaban, or rivaroxaban and 429 who were receiving dabigatran (2). Of these, 391 with non-valvular atrial fibrillation (NVAF) were treated with dabigatran while 17 had venous thromboembolic disease (VTE). Conversely, 287 with NVAF and 136 with VTE were treated with anti-Xa inhibitors. There were 49 with additional indications, 21 taking dabigatran, and 28 who were administered anti-Xa inhibitors. Test strip results were compared to liquid chromatography-tandem mass spectrometry (LC-MS/MS) results. The lower limit of LC-MS/MS detection was 4 ng/mL for all DOACs; however, a limit of 30 ng/mL was established as the threshold for positivity using the strips. Tables 1-3 illustrate the clinical efficacy of the reagent pads.
Table 1
| DOAC | N | Median, ηg/mL | 5th percentile, ηg/mL | 95th percentile, ηg/mL |
|---|---|---|---|---|
| Apixaban | 170 | 648 | 89 | 3,213 |
| Edoxaban | 131 | 8,785 | 417 | 71,203 |
| Rivaroxaban | 150 | 1,903 | 248 | 8,160 |
| Dabigatran | 429 | 4,206 | 515 | 21,642 |
DOAC, direct oral anticoagulant.
Table 2
| LC MS/MS | N | DOAC Dipstick® | |
|---|---|---|---|
| Pos | Neg | ||
| Positive | 452 | 435 | 17 |
| Negative | 428 | 7 | 421 |
| Sum | 880 | 442 | 438 |
DOAC, direct oral anticoagulant; LC-MS/MS, liquid chromatography-tandem mass spectrometry.
Table 3
| LC MS/MS | N | DOAC Dipstick® | |
|---|---|---|---|
| Pos | Neg | ||
| Positive | 429 | 427 | 2 |
| Negative | 451 | 3 | 448 |
| Sum | 880 | 430 | 450 |
DOAC, direct oral anticoagulant; LC-MS/MS, liquid chromatography-tandem mass spectrometry.
Comparison to the LC-MS/MS results in all arms generated a P value of <0.05 (Table 4). Receiver operating characteristic curves reported c-values of 0.989 with the anti-Xa inhibitors and 0.995 with the DTI inhibitor. There was no variation in the visual evaluations of the dipstick pads among the study centers.
Table 4
| Parameter | Anti-Xa inhibitor pad | DTI pad | |||
|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | ||
| Sensitivity | 0.962 | 0.941; 0.978 | 0.995 | 0.983; 0.999 | |
| Specificity | 0.984 | 0.967; 0.993 | 0.991 | 0.978; 0.998 | |
| Accuracy | 0.973 | 0.960; 0.982 | 0.993 | 0.985; 0.998 | |
| PPV | 0.961 | 0.939; 0.977 | 0.996 | 0.984; 0.999 | |
| NPV | 0.984 | 0.968; 0.994 | 0.991 | 0.976; 0.998 | |
CI, confidence interval; DTI, direct thrombin inhibitor; PPV, positive predictive value; NPV, negative predictive value.
DOACs interfere with clot-based and chromogenic substrate assays
DOACs prolong all clot-based coagulation assays and falsely reduce results reported from chromogenic substrate assays that employ factor Xa as a reactant (Table 5) (3-5). Medical laboratory scientists also employ clot-based and chromogenic substrate assays to measure DOAC plasma activity. The clinical in vivo DOAC reversal agents idarucizumab (Praxbind®, Boehringer Ingelheim International GmbH, Ingelheim am Rhein, Germany) and andexanet alpha (Anexxa®, Portola Pharmaceuticals, Inc. South San Francisco, USA) are manufactured to manage the in vivo hemorrhage associated with DOAC overdose. Although these could also in theory be used to abrogate in vitro test interference, their expense and limited availability hampers such application. Thus, researchers have searched for alternative in vitro methods to remove or neutralize DOACs as means to perform routine and special coagulation assays, in particular, lupus anticoagulant (LA) testing, where DOACs are present.
Table 5
| DOAC interference |
Results vary by instrument and reagent and by DOAC plasma levels. PT, prothrombin time; PTT, partial thromboplastin time (also known as activated partial thromboplastin time—APTT), TT, thrombin time; DRVVT, dilute Russell viper venom time; LAC, lupus anticoagulant; APCR, activated protein C resistance ratio.
DOAC-Stop®
DOAC-Stop® (DS, Haematex Research, Hornsby, Australia) provides an activated charcoal (carbon) tablet designed to remove or neutralize the highest likely clinical concentrations of dabigatran, apixaban, rivaroxaban and edoxaban while exerting minimal change to non-DOAC plasmas (6,7). The operator adds one 18 mg adsorbent tablet to 0.5–1.5 mL citrated plasma, gently mixes for 5 minutes, centrifuges for 2 minutes at 2,000 g to precipitate the tablet-bound DOACs and removes the supernatant for subsequent laboratory testing.
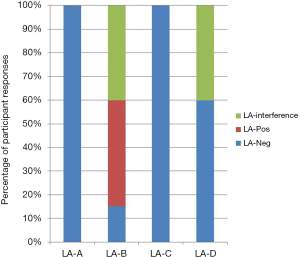
Because DOACs are employed as VTE therapy, there often arises the need for LA profiling in DOAC treated patients. Favaloro et al. added a therapeutic level of rivaroxaban to pooled normal plasma (PNP) and treated aliquots with Anexxa® and DS (8). Aliquots were lyophilized and distributed internationally as a supplementary exercise through the Royal College of Pathologists of Australasia Quality Assurance Program (RCPAQAP). Of the 92 participating laboratories, 82 completed and returned LA-PTT-and DRVVT-based profiles. The results are summarized in Figure 3. As expected, the rivaroxaban treated sample generated a false LAC with most methods. The authors furthermore reported that DS effectively neutralized the false LA ratios induced by rivaroxaban. In vitro Anexxa® also neutralized the LA ratio but failed to shorten clotting times, necessitating further testing. A subsequent study of similar design evaluated rivaroxaban induced interference in APCR testing and found full correction by DS and partial correction using Anexxa®. In this study, rivaroxaban generated false positive APCR results for only a minority of APCR-tests (9). A third study used a similar strategy to evaluate rivaroxaban-induced interference in factor VIII and IX assays. In this study, 55% and 95% of laboratories, respectively, reported abnormal FIX and FVIII levels for the rivaroxaban sample whereas DS corrected values in 100% of FIX and 86% of FVIII specimens. For Anexxa®, 59% of laboratories reported abnormal FVIII levels and 18% reported abnormal FIX results subsequent to neutralization (10).
Because Xa is the target coagulation factor for the anti-Xa inhibitor DOACs, in LA testing centers there is concern for DOAC interference with the DRVVT, which is based on Xa inactivation. Slavik et al. collected 60 DOAC patient specimens, 20 each treated with apixaban, dabigatran and rivaroxaban (11). Samples were split and pipetted into 500 µL aliquots. DS tablets were halved, and the 9 mg half-tablets were used to treat the 500 µL test arm specimens using the developers’ recommendation. The untreated arm served as controls. Both arms were assayed for all three DOACs using LC MS/MS. The authors reported that DOAC-Stop® eliminated dabigatran from 99.5%, rivaroxaban from 97.9%, and apixaban from 97.1% of participants’ plasmas. Residual concentrations did not exceed 2.7 ng/mL for dabigatran, 10.9 ng/mL for rivaroxaban, or 13.0 ng/mL for apixaban, levels that do not affect coagulation.
Rivaroxaban and apixaban have been shown to prolong the PT and PTT in a DOAC and reagent-dependent manner, to cause the one-stage clot-based factor VIII assays to under-report, and to generate false positive DRVVT screen/confirm ratios. Platton and Hunt collected plasmas from 20 patients taking rivaroxaban and 20 taking apixaban, plus control plasmas from 20 patients being tested for LA who were receiving no anticoagulant therapy (12). Their findings are summarized in Table 6. The investigators concluded that DS results were valid, provided the operator was aware of reagent and coagulometer variabilities and initial DOAC concentrations.
Table 6
| PT in rivaroxaban patients: DS normalized 17 out of 20 prolonged samples, two of the three remaining sample results became prolonged upon treatment |
LA, lupus anticoagulants; DS, DOAC-Stop®; PTT, partial thromboplastin time; DRVVT, dilute Russell viper venom time.
Calibrated automated thrombography (CAT) is a thrombin generation-based global hemostasis assay. Kopatz et al. spiked PNP with apixaban, dabigatran, edoxaban or rivaroxaban with and without DS and performed CAT using 5 ρM tissue factor as the agonist (13). DS restored thrombin generation, but the treated plasmas demonstrated increased thrombin generation compared to controls. The investigators assayed the natural coagulation pathway inhibitors antithrombin, total and free protein S, and tissue factor pathway inhibitor and found that tissue factor pathway inhibitor was decreased from a mean of 10.5 ηg/mL to 8.4 ηg/mL, which may have accounted for the increased thrombin generation. They also assayed fibrinogen and found there was no difference between control and DS-treated PNP. The investigators also speculated that DS normalization could help identify a therapeutic DOAC when interpreting an uncertain CAT response.
A 2019 study examined DS effect on LA testing on plasmas from 75 VTE patients receiving DOACs (14). There were 50 patients on rivaroxaban, 20 on dabigatran and five receiving apixaban. Six were diagnosed with antiphospholipid syndrome and disqualified from data collection. Investigators collected fasting whole blood specimens 2–28 h after DOAC administration. PTT- and DRVVT-based LA testing was performed at baseline and after DS neutralization. All treated PTT-based LA test results were negative for LA. Remaining results are summarized in Table 7.
Table 7
| DOAC | N | Controls | PTT screen | DRVVT ratio >1.2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-DS, ηg/mL | Post-DS, ηg/mL | Pre-DS, s | Post-DS, s | Pre-DS, n | Post-DS, n | ||||
| All DOACs | 69 | 87 | 8 | 63.7 | 42.7 | 35 | 6 | ||
| Apixaban | 5 | 14 | 0.4 | 44.5 | 44.5 | 0 | 0 | ||
| Rivaroxaban | 46 | 124 | 15 | 64.1 | 43.8 | 31 | 4 | ||
| Dabigatran | 18 | 45 | <5 | 66.9 | 41.0 | 4 | 2 | ||
PTT, partial thromboplastin time; DRVVT, dilute Russell viper venom time; DS, DOAC-Stop®; DOAC, direct oral anticoagulant.
Apixaban and rivaroxaban concentrations were measured using the chromogenic anti-Xa assay with specific calibrators, while dabigatran levels were determined using the plasma-diluted thrombin time (DTT) assay (Hemoclot Thrombin Inhibitor, Hyphen Biomed, Neuville-sur-Oise, France). DS completely removed dabigatran and reduced rivaroxaban and apixaban by 98% and 92.3% respectively, P<0.05. DRVVT ratios were computed as normalized (patient/standard plasma clotting time) screen/confirm ratios. DOAC interference in 97.3% of DRVVT results yielded ratios exceeding 1.2, generating a false positive LA report. DS led to reduction of ratios to below 1.2 in most cases.
Favresse et al. performed a series of thrombophilia-related assays on 135 DOAC-treated patients including 38 who were administered apixaban, 40 dabigatran, 15 edoxaban, and 42 rivaroxaban; plus 20 control plasmas (15). They assayed pre- and post-DS levels of dabigatran using the ecarin chromogenic assay (STA-ECA-II, Diagnostica Stago, Asnieres-sur-Seine, France) and apixaban, edoxaban, and rivaroxaban using the chromogenic anti-Xa assay (STA-liquid anti-Xa, Diagnostica Stago, Asnieres-sur-Seine, France). The post-DS levels were all below the limit of quantification of the corresponding DOAC assays. DS treatment overcame the effect of DOACs on PTT-LA (Diagnostica Stago, Asnieres-sur-Seine, France), DRVVT screen, and DRVVT confirm tests. False-positive results were observed in 75% of PTT-LA tests but fell to zero after DS treatment regardless of DOAC type. Although statistically significant differences post-DS were also observed for activated protein C resistance ratio (all but rivaroxaban), protein S (dabigatran), and antithrombin (apixaban and edoxaban) the differences between pre- and post-DS fell within the reference change value interval, indicating no clinical impact.
DOAC-Remove®
DOAC-Remove® (DR, 5-Diagnostics, Heuberg, Switzerland) is an activated charcoal tablet designed to adsorb in vitro DOACs in a manner similar to DS. One tablet is added to 1 mL of plasma, mixed gently for 10 minutes at ambient temperature, and centrifuged for 2 minutes at 2,500 g, 20 °C. Supernatant plasma is removed and centrifuged again to remove residual activated charcoal.
Jourdi et al. collected plasmas from three facilities where patients were being tested for LA; 49 who were taking apixaban, 48 rivaroxaban, 24 dabigatran and 30 on no anticoagulant (16). Pre-and post-DR treatment DOAC levels were measured using the chromogenic anti-Xa assay for apixaban and rivaroxaban and the DTT assay (Hemoclot Thrombin Inhibitor, Hyphen Biomed, Neuville-sur-Oise, France) for dabigatran. The investigators also measured 28 randomly selected plasma samples from within the study population for pre- and post-DOAC levels by high-performance liquid chromatography tandem mass spectrometry (HPLC-MS/MS). HPLC-MS/MS concentrations were reduced to <5 ng/mL in 5 out of 10 apixaban, 8 out of 10 rivaroxaban, and 7 out of 8 dabigatran samples.
The investigators performed DRVVT screen and confirm assays on all samples using STA-Staclot dRVVT Screen® and Confirm® (Diagnostica Stago, Asnières-sur-Seine, France) in one center, and LAC Screening® and LAC Confirmation® (Siemens Diagnostics, Saint-Denis, France) in the other two. They performed DRVVT screen/confirm assays on pre- and post-DR samples on all patient specimens. DR treatment did not change DRVVT screen/confirm results in the non-DOAC patients. DOAC interference was corrected in 76% of apixaban, 85% of rivaroxaban, and 95% of dabigatran patients, after DR treatment. The authors documented comparable results independent of reagent/analyzer system.
The investigators recommend DR for all rivaroxaban samples and all positive apixaban and dabigatran samples. However, residual DOAC interference may not be ruled out in cases of persisting DRVVT positives in apixaban and dabigatran samples results after treatment with DR.
In a preliminary report of the “Carbon in Vitro Anticoagulant Removing” (CAVIAR) study, Valaize et al. collected 24 plasmas from patients on DOACs at various concentrations; 11 taking rivaroxaban, 8 taking apixaban and 5 administered dabigatran (17). The residual DOAC concentrations after DR treatment were below the lower limit of quantitation in all but one rivaroxaban sample whose starting concentration was 400 ng/mL and whose residual concentration was 31 ng/mL. Before DR treatment, all rivaroxaban samples were reported as LA-positive whereas the apixaban or dabigatran samples DRVVT results reported as LA-negative. After DR, all rivaroxaban samples but one reported as LA-negative. Apixaban and dabigatran remained LA-negative.
The investigators also performed pre- and post-DR DRVVT screen/confirm assays on six LA-positive non-DOAC (control) patient plasmas. DR did not change the non-DOAC DRVVT results.
DOAC Filters
In a preliminary study, Bouvy et al. compared the efficacy of two DOAC Filter devices, DP-Filter® (Universite De Namur, Belgium) and DOAC Filter® (Diagnostica Stago, Asnières-sur-Seine, France) to DS (18). For DP-Filter® the operator dispenses 500 µL of plasma to the top, seals, and vortexes the device. After a 5-minute room temperature incubation, the device is centrifuged at 200 g and the filtrate collected for testing. For the DOAC Filter® the operator assembles the device, dispenses 600 µL to the top, centrifuges 15 minutes at 300 g and collects the filtrate in the removable microtainer.
The investigators spiked PNP with dabigatran, rivaroxaban or apixaban at 0, 125, 250, and 500 ηg/mL. DOAC recovery was measured on a STARMax2 analyzer (Diagnostica Stago, Asnières-sur-Seine, France) using the chromogenic anti-Xa assay for rivaroxaban and apixaban, or ecarin chromogenic substrate assay for dabigatran. They also analyzed PT and PTT.
The three devices reduced all rivaroxaban and dabigatran concentrations to below the limits of detection. DS and DP-Filter® reduced all apixaban concentrations to below the detection limit. The DOAC Filter® was unable to eliminate apixaban at a concentration over 250 ng/mL All three devices restored normal PT and PTT, however the DP-Filter® shortened the PT under every condition. The DS procedure has the shortest turnaround time at 7 minutes but in this study occasionally left a visible residue. DOAC Filter® required the fewest steps. DS and DP-Filter® required the smallest sample volume.
Summary
Medical laboratory scientists must daily detect and classify DOACs in patients, often in emergent clinical situations. They must subsequently perform clot-based or chromogenic assays that define patients’ hemostatic status. Clinical studies confirm the DOAC Dipstick® may be used on urine to accurately detect and distinguish between the DTI dabigatran and the anti-Xa inhibitor class, which includes apixaban, rivaroxaban, and edoxaban. However, levels identified in urine do not necessarily inform on co-existing plasma levels. Within defined parameters, four products, DOAC-Stop®, DOAC-Remove®, DOAC Filter®, and DP-Filter® apply various mechanisms to remove DOACs in order to produce an essentially DOAC-free plasma, in which in nearly all cases, parameters become unaffected by the DOAC and can be accurately measured. By judiciously selecting and applying these devices and observing their limitations, the operator may then report clot-based and chromogenic substrate results with reasonable confidence.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Emmanuel J. Favaloro) for the series “Anticoagulant and antithrombotic therapy: globally applied according to local geographical selection criteria” published in Annals of Blood. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob-20-16). The series “Anticoagulant and antithrombotic therapy: globally applied according to local geographical selection criteria” was commissioned by the editorial office without any funding or sponsorship. Prof. GAF reports personal fees from Precision BioLogic Inc., Dartmouth, NS, personal fees from BioMedica Diagnostics, Inc. Windsor, NS, personal fees from Michigan State University, personal fees from Rutgers University, outside the submitted work; in all cases there is no relationship to products discussed in this article. Mr. DLM reports personal fees from DiaPharma, Inc., that have no relationship with the submitted work. The products described here are not produced nor are they in competition with DiaPharma, BioMedica Diagnostics, Inc., or Precision BioLogic Inc. products and neither company provided funding or information for this article.
Ethical Statement: The authors are accountable for all aspects of the manuscript and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Harenberg J, Schreiner R, Hetjens S, et al. Detecting Anti-IIa and Anti-Xa Direct Oral Anticoagulant (DOAC) Agents in Urine using a DOAC Dipstick. Semin Thromb Hemost 2019;45:275-84. [Crossref] [PubMed]
- Harenberg J, Beyer-Westendorf J, Crowther M, et al. Accuracy of a Rapid Diagnostic Test for the Presence of Direct Oral Factor Xa or Thrombin Inhibitors in Urine-A Multicenter Trial. Thromb Haemost 2020;120:132-40. [Crossref] [PubMed]
- Gosselin RC, Adcock DM, Douxfils J. An update on laboratory assessment for direct oral anticoagulants (DOACs). Int J Lab Hematol 2019;41:33-9. [Crossref] [PubMed]
- Gessoni G, Valverde S, Valle L, et al. Effect of dabigatran on a prothrombinase-based assay for detecting activated protein C resistance: an ex vivo and in vitro study in normal subjects and factor V Leiden carriers. Blood Transfus 2017;15:562-7. [PubMed]
- Gosselin R, Grant RP, Adcock DM. Comparison of the effect of the anti-Xa direct oral anticoagulants apixaban, edoxaban, and rivaroxaban on coagulation assays. Int J Lab Hematol 2016;38:505-13. [Crossref] [PubMed]
- Exner T, Michalopoulos N, Pearce J, et al. Simple method for removing DOACs from plasma samples. Thromb Res 2018;163:117-22. [Crossref] [PubMed]
- Exner T, Rigano J, Favaloro EJ. The effect of DOACs on laboratory tests and their removal by activated carbon to limit interference in functional assays. Int J Lab Hematol 2020;42:41-8. [Crossref] [PubMed]
- Favaloro EJ, Gilmore G, Arunachalam S, et al. Neutralising rivaroxaban induced interference in laboratory testing for lupus anticoagulant (LA): A comparative study using DOAC Stop and andexanet alfa. Thromb Res 2019;180:10-9. [Crossref] [PubMed]
- Favaloro EJ, Gilmore G, Bonar R, et al. Laboratory testing for activated protein C resistance: rivaroxaban induced interference and a comparative evaluation of andexanet alfa and DOAC Stop to neutralise interference. Clin Chem Lab Med 2020;58:1322-31. [Crossref] [PubMed]
- Favaloro EJ, Gilmore G, Bonar R, et al. Reducing the effect of DOAC interference in laboratory testing for factor VIII and factor IX: A comparative study using DOAC Stop and andexanet alfa to neutralize rivaroxaban effects. Haemophilia 2020;26:354-62. [Crossref] [PubMed]
- Slavik L, Jacova J, Friedecky D, et al. Evaluation of the DOAC-Stop Procedure by LC-MS/MS Assays for Determining the Residual Activity of Dabigatran, Rivaroxaban, and Apixaban. Clin Appl Thromb Hemost 2019;25:1076029619872556 [Crossref] [PubMed]
- Platton S, Hunt C. Influence of DOAC Stop on coagulation assays in samples from patients on rivaroxaban or apixaban. Int J Lab Hematol 2019;41:227-33. [Crossref] [PubMed]
- Kopatz WF, Brinkman HJM, Meijers JCM. Use of DOAC Stop for elimination of anticoagulants in the thrombin generation assay. Thromb Res 2018;170:97-101. [Crossref] [PubMed]
- Ząbczyk M, Kopytek M, Natorska J, et al. The effect of DOAC-Stop on lupus anticoagulant testing in plasma samples of venous thromboembolism patients receiving direct oral anticoagulants. Clin Chem Lab Med 2019;57:1374-81. [Crossref] [PubMed]
- Favresse J, Lardinois B, Sabor L, et al. Evaluation of the DOAC-Stop® Procedure to Overcome the Effect of DOACs on Several Thrombophilia Screening Tests. TH Open 2018;2:e202-e209. [Crossref] [PubMed]
- Jourdi G, Delrue M, Stepanian A, et al. Potential usefulness of activated charcoal (DOAC remove®) for dRVVT testing in patients receiving Direct Oral AntiCoagulants. Thromb Res 2019;184:86-91. [Crossref] [PubMed]
- Valaize J, Julien D, Borgel A, et al. Does in-vitro addition of activated charcoal allow lupus anticoagulant testing with dRVVT in plasma of patients treated with DOAC? The CAVIAR study. Poster 215; 2018 European Congress on Thrombosis and Haemostasis; Marseille, France.
- Bouvy C, Evrard J, Siriez R, et al. Removal of DOACs from plasma: performance comparison and pre-analytical considerations of three different devices. Poster 220; 2018 European Congress on Thrombosis and Haemostasis; Marseille, France.
Cite this article as: McGlasson DL, Fritsma GA. In vitro detection and removal of direct oral anticoagulants from patient plasma specimens. Ann Blood 2020;5:25.


