Dormant mechanisms reveal the clinical significance of tumor dormancy: a narrative review
Introduction
When tumor cells enter a growth stagnation period (G0/G1) due to cell growth pressure or lack of necessary growth factors in the new environment, this state of quiescence is called tumor dormancy (1-3). Tumor dormancy is an unstable state, which can re-enter the proliferative state under the appropriate environment (4). If tumor cells are in a dormant state, the patients can maintain the survival state with tumor and the tumor is stable. If tumor cells are transformed from dormancy to activated proliferation, the metastasis is formed and the tumor progresses. In order to control malignant tumors, there are two main ways to control dormant tumor cells: the first is to make it dormant for a long time, and the second is to completely eradicate dormant tumor cells, to put an end to the source of tumor recurrence.
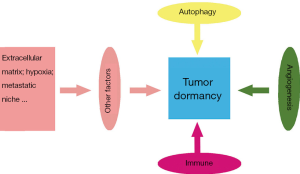
More and more scientists are engaged in the research of tumor dormancy, and have proposed a lot of possible mechanisms for maintaining tumor dormancy (5-11). However, tumor dormancy is still an unstable state, which is difficult to maintain for a long time (4,12). Because of the low proliferation characteristics of dormant tumor cells, it is insensitive to conventional radiotherapy and chemotherapy (13). Thus, it is difficult to completely eradicate dormant tumor cells. We need to find novel mechanisms to maintain tumor dormancy or novel targets to eradicate dormant tumor cells. A growing focus has been paid to autophagy, angiogenesis and tumor immune editing as mechanisms that dictate tumor cell dormancy (14-16). This review emphasizes on the regulation of autophagy, angiogenesis and tumor immune editing on tumor dormancy, and derives its clinical significance through these mechanisms (17,18). The study of the above mechanisms can effectively minimize the risk of cancer recurrence (19) (Figure 1). We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/aob-20-46).
Autophagy promotes the survival of tumor dormant cells
Autophagy is an adaptive response of cells to adverse environment and lack of nutrition. In this process, cells eat their own organelles, so as to ensure proper energy balance and to recycle dysfunctional organelles and macromolecules (31). During the process of malignant transformation, autophagy has determinant effects enabling cell survival. Autophagy can also be activated in tumor cells and causes tumor cells to resist anoikis, which leads to the survival of tumor cells. Severe nutritional deficiency can strongly induce intracellular autophagy make cells reach a new energy balance, and thus enter a reversible dormancy state. When the tissue microenvironment changes, such as tumor cells regain sufficient nutrition or 6-biphosphatase-3 increases expression, cells will be activated and proliferated again. Autophagy can, in addition to starvation, be activated by myriad physiological stress stimuli, which includes hypoxia, high temperature, hormonal stimulation, endoplasmic reticulum stress or pharmacological agents, to name just a few. This good pressure tolerance given to tumor cells by autophagy is beneficial for resting cells to wait for the opportunity to form metastatic foci in distant organs. Autophagy has been proposed to play a key role in tumor cell dormancy, although few studies have addressed this hypothesis experimentally (32-39).
It is demonstrated in mouse and human 3D in vitro and in vivo preclinical models of dormancy that dramatic metastatic burden and decreasing cell survival can be owing to pharmacologic or genetic inhibition of autophagy in dormant cells. Damaged mitochondria and reactive oxygen species (ROS) accumulated within the cells can impairs the survival of dormant cancer cells significantly, while the accumulation is usually produced by the pharmacological autophagy inhibition, in which different autophagy inhibitors, such as 3-methyladenine, bafilomycin and hydroxy CQ are administrated. Autophagy related gene 7 (ATG7) is necessary for autophagy activation. Knockout of autophagy related gene 7 (ATG7) can prevent P-body clearance and MET (mesenchymal-epithelial transition), inhibit the growth of metastatic tumor. Therefore, autophagy may trigger the signal pathway in dormant cells by changing the microenvironment, which promotes the survival of dormant cancer cells and the recurrence of metastatic tumors (39,40). Besides ROS and ATG7, there might be other vulnerability autophagy checkpoints to consider, particularly in the context of chemoresistance in colorectal cancer, liver cancer, brain tumors, and melanoma (41-44).
Ras homologue member I (ARHI) is an inhibitor of the PI3K-AKT-mTOR cascade. ARHI encoded protein is expressed in human breast, ovary and other tissues, while down-regulated or deleted in breast cancer, ovarian cancer and other tumor tissues, suggesting that it is related to the occurrence and development of these tumors. It has been reported that the re-expression of ARHI leads to autophagy death. This may be related to the increase of oxidative stress, the decrease of ATP/ADP and the decrease of mitochondrial function caused by the expression of ARHI. When the cell grows as a xenograft in mice, it keeps the cell dormant. When the level of ARHI in dormant cells decrease, the xenograft grows rapidly. However, regrowth of xenografted tumors can be reduced significantly upon reduction of ARHI levels, by means of chloroquine that inhibited ARHI-induced autophagy, through which a conclusion could be reached that autophagy contributed to the survival of dormant cells. Through further analysis, it was suggested that by treating growth factors [insulin-like growth factor-1 (IGF-1), macrophage colony-stimulating factor (M-CSF)], angiogenic factors [vascular endothelial growth factor (VEGF), interleukin-8 (IL-8)], and matrix proteins found in xenografts to cultured human ovarian cancer cells in which ARHI had been re-expressed, autophagic cell death was reduced. Therefore, ARHI can induce autophagic cell death, but can also promote tumor dormancy in the presence of factors that promote survival in the cancer microenvironment. This characteristic of ARHI gene is expected to be the key point of inducing tumor cell dormancy and anti-tumor metastasis and recurrence (45-50).
However, it has been reported that autophagy defects are associated with tumorigenesis. Because in human breast cancer, ovarian cancer and prostate cancer, autophagy regulatory factor BECLIN 1 is a single allele deletion, while BECLIN 1 (+/−) mice are prone to tumor. Autophagy stimulation maintains cellular adaptability by maintaining protein and organelle quality control. Inhibition of DNA damage and genomic instability, as well as limiting necrosis-related inflammation may play a key role in cancer prevention (51-54).
Individualized anti-tumor therapy by inhibiting autophagy of tumor cells is a hot topic in current research. The mammalian target of rapamycin (mTOR) is now perceived as sensor that functions as something like a coordinator manipulating the balance between growth and autophagy to contend with physiological conditions and environmental stress at cellular level. mTOR is a serine/threonine protein kinase that could be subsumed under the phosphatidylinositol kinase-related kinase (PIKK) family (55). Against which the mTOR inhibitor everolimus displays potent antitumor activity in patients with metastatic disease by impeding autophagy and tumor dormancy onset (18,56,57). PI3K/AKT/mTOR pathway is altered in germline and somatic tissues downstream PTEN and this could be exploited as actionable mutations or to stratify patients treated with approved inhibitors (e.g., sirolimus, PI3K inhibitors, etc.) (58). Copper deprivation reduces ATP level and increases mitochondrial ROS level, which caused an increase in cell autophagy and rendered cancer cells in a dormant state. Copper chelator tetra thiomolybdate and autophagy inhibitor CQ can prevent tumor cells from entering dormant state and kill tumor cells effectively (59).
Interestingly, some studies also suggest that cancer may be controlled by inducing autophagy. Such as protein restriction mimetic resveratrol induces autophagy by limiting calories, which makes tumor cells dormant for a long time period (60). Thus, in this period the patients can maintain the survival state with tumor and the tumor is stable. Tumor dormancy is temporary and difficult to maintain for a long time. Therefore, through inducing autophagy is less effective than inhibiting autophagy in controlling the tumor, since the former tries to cause the tumor cell to dormant for a long time while the latter causes the tumor cell death.
Inhibition of angiogenesis can lead to tumor dormancy
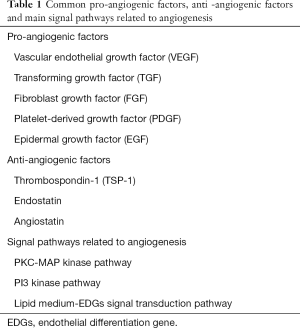
Angiogenesis is a sign of tumor growth and progression, and tumor growth is vessel-dependent (13). Angiogenesis is a complex multi-step physiological process that occurs throughout life both in normal tissues and in disease. It is tightly regulated by the balance between pro-angiogenic and anti-angiogenic factors (Table 1). The angiogenic switch has been identified as the key step during tumor progression. The angiogenic switch mainly depends on the disruption of pro-angiogenic factors and anti-angiogenic factors equilibrium. The expression of pro-angiogenic genes increases due to physiological stimuli, such as hypoxia or oncogene activation and tumor suppressor mutations as the mass grows. In favor of pro-angiogenic factors and on the recruitment of circulating endothelial progenitors (CEPs) can activate the angiogenic switch and promote the formation of new blood vessels. The notion that angiogenic endothelial cells could communicate signals to tumor cells raises questions about the possibility of achieving tumor dormancy by counteracting angiogenesis (61-66). On the other hand, blood vessels can deliver nutrients and oxygen to the tumor microenvironment (67). The implementation of anti-angiogenesis can change the tumor microenvironment, so as to prevent the growth and metastasis of tumor, and induce a dormant state in the tumor cells (68). It is worth mentioning that the isolation of angiogenic factors lead to the hypothesis of inhibiting angiogenesis to block vessel formation and result in tumor dormancy (69,70). Involvement of angiogenesis in dormancy is key for the regulation of tumor growth in mice (71-73).
Full table
Further studies have shown that dormant tumor cells can secrete higher levels of thrombospondin, which has a strong inhibition effect on angiogenesis. Complex interactions between tumor cells and endothelial cells can control tumor awakening from dormancy. Angiogenesis plays an important role in tumor dormancy (74). Vascular dormancy is considered to be a kind of tumor dormancy. Vascular dormancy can inhibit the proliferation and angiogenesis of endothelial cells, regulate the balance between angiogenic factors and anti-angiogenic factors, make tumor cells withdraw from the growth cycle, maintain the resting state, and significantly inhibit tumor recurrence and metastasis.
It has been found that angiogenic related genes are the most functional genes in tumor cell dormancy. Platelet reactive protein, an endogenous angiogenic inhibitor, as well as angiostatin and endothelial inhibin binding protein, were found to be up-regulated in all detected dormant tumor cell lines. The increased expression of platelet reactive protein in non-angiogenic cells is mediated by the activation of PI3K/c-Myc pathway. To sum up, PI3K-mediated thrombospondin can inhibit tumor angiogenesis and inhibit tumor proliferation and metastasis (75). It has also been found that microRNAs, mediated by nano-carriers involved in osteosarcoma tumor-host interaction can inhibit osteosarcoma angiogenesis and induce tumor dormancy. These microRNAs include: miR-34a, miR-93 and miR-200c, which prevent tumor progression by reducing the mRNA levels of genes critical to tumor angiogenesis and cancer progression in general (76). Additionally, vitamin E succinate inhibits melanoma angiogenesis and promotes melanoma dormancy (77).
In addition to the above-mentioned factors that inhibit angiogenesis, there are also a large number of factors that promote angiogenesis in the human body. Neutrophil-derived MMP-9 was required for 14,15-EET to induce angiogenesis during the growth of dormant micrometastases. 14,15-EET could induce neutrophilic infiltration in metastatic lesions and the conversion of neutrophil function, thus triggering the growth of minimal dormant metastases. 14,15-EET promotes the growth of dormant micrometastases in a dose-dependent manner (78). Tissue factor (TF) is a risk factor for metastasis, and in mouse models, TF drives metastasis in a coagulation-dependent manner (79). In hypercoagulable state, TF increased, and tumor dormancy was affected by TFs. It has been found that the microenvironment orchestrated by TF expression drives permanent changes in the phenotype, gene-expression profile, DNA copy number, and DNA methylation state of the tumor cells that escape from dormancy. TF can promote tumor angiogenesis and transform tumor dormant cells into active proliferation, which leads to the recurrence of malignant tumor (80). Immature dendritic cells also can exert proangiogenic effects when infiltrating the tumor microenvironment (81).
Based on the above mechanisms, a large number of anti-tumor angiogenic drugs are introduced into clinic, such as: recombinant human endostatin injection, bevacizumab, apatinib, etc. Anti-tumor targeted therapy with anti-angiogenic drugs and their targets that block nutrient availability is a pillar of cancer treatment. This constitutes an opportunity to provide some evidence and/or propose new hypothesis about the mechanisms of resistance and efficacy in the context of target tissue, vessel and immune system, which is the following topic. For example, anti-VEGF monoclonal antibody reduced vascular density and tumor growth in mice bearing xenografts of glioblastoma multiforme, leiomyosarcoma and rhabdomyosarcoma (82).Another example is the efficiency of metronomic chemotherapy in lung cancer, leading to anti-tumor and anti-angiogenic effects without causing toxicity (83). However, the effect of their clinical application is not as satisfactory as that shown in animal experiments, which indicates that tumor angiogenesis is a complex process regulated by many factors and multiple signal pathways. Blocking a certain signal pathway alone cannot completely prevent the formation of tumor blood vessels. We need to further study the effect of anti-tumor angiogenic drugs promoting tumor dormancy.
Tumor dormancy is a clinical phenomenon related to immune equilibrium in the process of tumor immune editing
Tumor immune editing is a process in which the immune system controls tumor growth and forms tumor immunogenicity, including three stages: elimination, equilibrium and escape (84). Tumor dormancy is a clinical phenomenon related to immune equilibrium in the process of tumor immune editing (85) (Figure 2). When certain changes occur in some tumor cells, resulting in the inability of the immune system to fully recognize and remove them, they survive in the body. Because the immune system still has a certain monitoring function, these tumor cells do not proliferate rapidly into clinically visible lesions, resulting in tumor dormancy. Dormant cells have genetic instability, and a variety of mutations occur. After a period of accumulation, the cells are finally transformed into another phenotype, thus avoiding the attack of the immune system and rapid proliferation, leading to immune escape. Maintaining the tumor immunity in the equilibrium period will lead to tumor dormancy, tumor growth inhibition, and will not cause harm to the body for the time being (89).
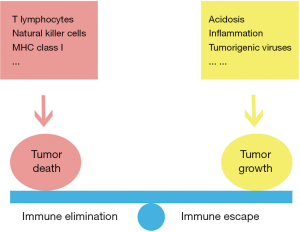
The immune system does a good job in promoting their permanent dormancy, through restraining disseminated cancer cells. After antitumor therapy, some tumor cells become senescent, a process known as therapy-induced senescence (TIS). The senescence-associated secretory phenotype (SASP) is a hallmark of TIS. Some senescent tumor cells can be regarded as dormant tumor cells, which can be restored to proliferate when the immune system is weakened (90-96). With excellent ability of recognizing intracellular antigens which are expressed by all tumor cell types, T lymphocytes functions significantly in maintaining immune equilibrium with metastatic dormant cells. The decreased duration of dormancy and shortened mean time for B cell lymphoma recurrence on the spleen has close relationship with the depletion of the CD8+ T cells, which could be found in a mouse model of tumor dormancy. This also suggests that via production of interferon (IFN)-γ, CD8+ T cells can facilitate the induction and maintenance of the state of dormancy. Avoidance of micro angiogenesis and promotion of hypoxic-induced dormancy could be realized by CXCL9 and CXCL10 produced by CD4+ T cells. A cytotoxic T lymphocyte (CTL) response could be elicited by the natural killer (NK) cells due to its activator function. The fact that a long period of dormancy in vitro and in vivo is included in perforin which was secreted by NK, has been observed. CTLs and NK cells can work cooperatively to eradicate cancer cells with or without class I major histocompatibility complex (MHC-I), and thus minimize the chance of immune evasion and metastasis. As a complementary to CTLs, NK cells kill tumor cells without self-markers by releasing perforin and granzyme B into the targeted cells. Immunotherapeutic interventions in metastatic dormancy may help to control or eradicate cancer disease (94,97-102).
A variety of situations can lead to immune escape. The immune system destroys tumor cells by recognizing antigenic determinants. When the antigenic determinants of a small number of tumor cells change, their immunogenicity changes, MHC-I lose expression. T lymphocytes cannot recognize tumor cells, and immune escape occurs. Through suppressing activation, proliferation and cytokine expression effects on T cells and inhibiting the phagocytosis of tumor-associated macrophages (TAMs), the PD-1/PD-L1 signaling pathway can limit the functions of T effector cells, NK cells, dendritic cells, TAMs, etc. So as to promote the possibility of tumor immune escape (103). Localized lactic acidosis has a strong immunosuppressive effect and mediates an immune escape of tumors (104). Macrophage migration inhibitory factor (MIF) is a pleiotropic cytokine that plays a key role in cancer. MIF is upregulated in neuroblastoma tissues and cell lines and it contributes to neuroblastoma aggressiveness and immune-escape (105). Inflammatory mediators and inflammatory cells in the inflammatory microenvironment promote the transformation of normal cells to cancer cells in the early stage of cancer, promote the growth and development of cancer cells, and induce tumor immune escape (106). Vascular endothelial growth factor C (VEGFC), an activator of lymph angiogenesis, is newly identified as an immunomodulator which can regulate the immune system so that tumor cells more easily escape immune surveillance (107). Many tumorigenic viruses can also cause immune escape. Epstein-Barr virus (EBV) miRNAs can inhibit the expression and presentation of viral antigens, inhibit immune activation and immunotoxicity, assisting host cells to escape from immunity, and providing conditions for further immortalized tumorigenesis of the host cells (108). In organ transplant cases, because the donor has occult cancer, the recipient can develop cancer in the transplanted organ over a period of months to years after systemic immunosuppression. The only survivor was a recipient with discontinued immunosuppression. These findings suggest that cells prone to tumor development might be in a dormant state, and dormant cells are activated when the receptor’s immunity decreases and triggers immune escape (109-111). In patients with immune escape, the incidence and recurrence rate of cancer are higher. Therefore, we need to avoid immune escape as far as possible to prevent dormant tumor cells from re-entering the state of proliferation.
All other currently available cancer therapies are toxic with off-target effects, whereas immune cells could establish memory against dormant tumor antigens such as mutated tumor antigens, and keep them dormant for the lifetime of an individual (112). Therefore, the immune mechanisms controlling cancer progression have been the focus of intensive research. Through the study of immune mechanism, a large number of immune checkpoint inhibitors poured into the clinic. Antibodies that block the interaction between PD-1 and ligand PD-L1 have achieved remarkable clinical success in cancer therapy, and immunotherapy has become a hot spot in cancer therapy. However, a large gap exists between clinical efficacy and theory, and only a small proportion of patients show a lasting response to immunotherapy. Moreover, the degree of efficacy of checkpoint inhibitors is also varies widely among different tumor types. The extreme complexity, robustness, and plasticity of the immune system might raise many challenges, but it also leaves us with reasons for optimism as we anticipate the impact of breakthroughs in our fundamental understanding of immunology (113-116).
Conclusions
The important limitations of tumor dormancy research are the lack of appropriate experimental models and consistent and rich sources of dormant tumor cells. Dormancy is a hidden state, so it is difficult to observe and study directly. Although a variety of mechanisms have been proposed that may affect tumor dormancy, it is actually only a tip of the iceberg so far (75,117). The mechanism of tumor dormancy is so complex and diverse, and the field of tumor dormancy is just like a vast universe, which requires people to continue to explore.
By studying the effects of autophagy, angiogenesis and immunity on tumor dormancy, a large number of new drugs and treatments have been adopted into the clinic. At present, most drugs and treatments take effects mainly through two aspects: maintaining tumor dormancy or preventing tumor cells from entering dormancy during chemoradiation. However, simply interfering with one of the factors cannot achieve satisfactory results, since tumor dormancy is affected by a number of factors. At present, with the lack of biomarkers for tumor dormant cells, it is impossible to formulate a reasonable personalized treatment plan by monitoring tumor dormant cells effectively. Besides, the lack of therapeutic targets for tumor dormant cells also makes it difficult to completely eradicate tumor dormant cells. Should the two mentioned difficulties overcome in the future, we can better prolong the disease-free survival (DFS) of patients and improve the prognosis of patients.
Acknowledgments
Funding: This work was supported by grants from Ministry of Science and Technology of China, the Natural Science Foundation of China (81172537, 81272900, 81772828), Scientific and Technological Project of Guangdong Province (2016A020216028).
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/aob-20-46
Peer Review File: Available at http://dx.doi.org/10.21037/aob-20-46
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob-20-46). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Butturini E, Carcereri de Prati A, Boriero D, et al. Tumor Dormancy and Interplay with Hypoxic Tumor Microenvironment. Int J Mol Sci 2019;20:4305. [Crossref] [PubMed]
- Pavan Grandhi TS, Potta T, Nitiyanandan R, et al. Chemomechanically engineered 3D organotypic platforms of bladder cancer dormancy and reactivation. Biomaterials 2017;142:171-85. [Crossref] [PubMed]
- Davis JE Jr, Kirk J, Ji Y, et al. Tumor Dormancy and Slow-Cycling Cancer Cells. Adv Exp Med Biol 2019;1164:199-206. [Crossref] [PubMed]
- Muzes G, Sipos F. Metastatic Cell Dormancy and Re-activation: An Overview on Series of Molecular Events Critical for Cancer Relapse. Anticancer Agents Med Chem 2017;17:472-82. [Crossref] [PubMed]
- Axelrod HD, Valkenburg KC, Amend SR, et al. AXL Is a Putative Tumor Suppressor and Dormancy Regulator in Prostate Cancer. Mol Cancer Res 2019;17:356-69. [Crossref] [PubMed]
- Liu Y, Lv J, Liu J, et al. STAT3/p53 pathway activation disrupts IFN-β-induced dormancy in tumor-repopulating cells. J Clin Invest 2018;128:1057-73. [Crossref] [PubMed]
- Jia Q, Yang F, Huang W, et al. Low Levels of Sox2 are required for Melanoma Tumor-Repopulating Cell Dormancy. Theranostics 2019;9:424-35. [Crossref] [PubMed]
- Luo XL, Deng CC, Su XD, et al. Loss of MED12 Induces Tumor Dormancy in Human Epithelial Ovarian Cancer via Downregulation of EGFR. Cancer Res 2018;78:3532-43. [Crossref] [PubMed]
- Liu Y, Lv J, Liang X, et al. Fibrin Stiffness Mediates Dormancy of Tumor-Repopulating Cells via a Cdc42-Driven Tet2 Epigenetic Program. Cancer Res 2018;78:3926-37. [Crossref] [PubMed]
- Liu Q, Chen F, Hou L, et al. Nanocarrier-Mediated Chemo-Immunotherapy Arrested Cancer Progression and Induced Tumor Dormancy in Desmoplastic Melanoma. ACS Nano 2018;12:7812-25. [Crossref] [PubMed]
- Lenk L, Pein M, Will O, et al. The hepatic microenvironment essentially determines tumor cell dormancy and metastatic outgrowth of pancreatic ductal adenocarcinoma. Oncoimmunology 2017;7:e1368603 [Crossref] [PubMed]
- Wilkie KP, Hahnfeldt P. Tumor-immune dynamics regulated in the microenvironment inform the transient nature of immune-induced tumor dormancy. Cancer Res 2013;73:3534-44. [Crossref] [PubMed]
- Endo H, Inoue M. Dormancy in cancer. Cancer Sci 2019;110:474-80. [Crossref] [PubMed]
- Goddard ET, Bozic I, Riddell SR, et al. Dormant tumour cells, their niches and the influence of immunity. Nat Cell Biol 2018;20:1240-9. [Crossref] [PubMed]
- Manjili MH. The premise of personalized immunotherapy for cancer dormancy. Oncogene 2020;39:4323-30. [Crossref] [PubMed]
- Vera-Ramirez L. Cell-intrinsic survival signals. The role of autophagy in metastatic dissemination and tumor cell dormancy. Semin Cancer Biol 2020;60:28-40. [Crossref] [PubMed]
- Páez D, Labonte MJ, Bohanes P, et al. Cancer dormancy: a model of early dissemination and late cancer recurrence. Clin Cancer Res 2012;18:645-53. [Crossref] [PubMed]
- Rossari F, Zucchinetti C, Buda G, et al. Tumor dormancy as an alternative step in the development of chemoresistance and metastasis - clinical implications. Cell Oncol (Dordr) 2020;43:155-76. [Crossref] [PubMed]
- Osisami M, Keller ET. Mechanisms of Metastatic Tumor Dormancy. J Clin Med 2013;2:136-50. [Crossref] [PubMed]
- Kai F, Drain AP, Weaver VM. The Extracellular Matrix Modulates the Metastatic Journey. Dev Cell 2019;49:332-46. [Crossref] [PubMed]
- Barkan D, Green JE, Chambers AF. Extracellular matrix: a gatekeeper in the transition from dormancy to metastatic growth. Eur J Cancer 2010;46:1181-8. [Crossref] [PubMed]
- Pradhan S, Sperduto JL, Farino CJ, et al. Engineered In Vitro Models of Tumor Dormancy and Reactivation. J Biol Eng 2018;12:37. [Crossref] [PubMed]
- Jahanban-Esfahlan R, Seidi K, Manjili MH, et al. Tumor Cell Dormancy: Threat or Opportunity in the Fight against Cancer. Cancers (Basel) 2019;11:1207. [Crossref] [PubMed]
- Cappariello A, Rucci N. Tumour-Derived Extracellular Vesicles (EVs): A Dangerous "Message in A Bottle" for Bone. Int J Mol Sci 2019;20:4805. [Crossref] [PubMed]
- Pradhan S, Slater JH. Tunable hydrogels for controlling phenotypic cancer cell states to model breast cancer dormancy and reactivation. Biomaterials 2019;215:119177 [Crossref] [PubMed]
- Hoppe-Seyler K, Bossler F, Lohrey C, et al. Induction of dormancy in hypoxic human papillomavirus-positive cancer cells. Proc Natl Acad Sci U S A 2017;114:E990-8. [Crossref] [PubMed]
- Nobre AR, Entenberg D, Wang Y, et al. The Different Routes to Metastasis via Hypoxia-Regulated Programs. Trends Cell Biol 2018;28:941-56. [Crossref] [PubMed]
- Endo H, Okami J, Okuyama H, et al. The induction of MIG6 under hypoxic conditions is critical for dormancy in primary cultured lung cancer cells with activating EGFR mutations. Oncogene 2017;36:2824-34. [Crossref] [PubMed]
- Montagner M, Sahai E. In vitro Models of Breast Cancer Metastatic Dormancy. Front Cell Dev Biol 2020;8:37. [Crossref] [PubMed]
- Capulli M, Hristova D, Valbret Z, et al. Notch2 pathway mediates breast cancer cellular dormancy and mobilisation in bone and contributes to haematopoietic stem cell mimicry. Br J Cancer 2019;121:157-71. [Crossref] [PubMed]
- Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell 2011;147:728-41. [Crossref] [PubMed]
- Lu Z, Luo RZ, Lu Y, et al. The tumor suppressor gene ARHI regulates autophagy and tumor dormancy in human ovarian cancer cells. J Clin Invest 2008;118:3917-29. [Crossref] [PubMed]
- Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell 2010;140:313-26. [Crossref] [PubMed]
- Flynn AB, Schiemann WP. Autophagy in breast cancer metastatic dormancy: Tumor suppressing or tumor promoting functions? J Cancer Metastasis Treat 2019;5:43. [PubMed]
- Gewirtz DA. Autophagy, senescence and tumor dormancy in cancer therapy. Autophagy 2009;5:1232-4. [Crossref] [PubMed]
- Sosa MS, Bragado P, Debnath J, et al. Regulation of tumor cell dormancy by tissue microenvironments and autophagy. Adv Exp Med Biol 2013;734:73-89. [Crossref] [PubMed]
- Katheder NS, Khezri R, O'Farrell F, et al. Microenvironmental autophagy promotes tumour growth. Nature. 2017;541:417-20. [Crossref] [PubMed]
- La Belle Flynn A, Calhoun BC, Sharma A, et al. Autophagy inhibition elicits emergence from metastatic dormancy by inducing and stabilizing Pfkfb3 expression. Nat Commun 2019;10:3668. [Crossref] [PubMed]
- Vera-Ramirez L, Vodnala SK, Nini R, et al. Autophagy promotes the survival of dormant breast cancer cells and metastatic tumour recurrence. Nat Commun 2018;9:1944. [Crossref] [PubMed]
- Shinde A, Hardy SD, Kim D, et al. Spleen Tyrosine Kinase-Mediated Autophagy Is Required for Epithelial-Mesenchymal Plasticity and Metastasis in Breast Cancer. Cancer Res 2019;79:1831-43. [Crossref] [PubMed]
- Ojha R, Leli NM, Onorati A, et al. ER Translocation of the MAPK Pathway Drives Therapy Resistance in BRAF-Mutant Melanoma. Cancer Discov 2019;9:396-415. [Crossref] [PubMed]
- Moeckel S, LaFrance K, Wetsch J, et al. ATF4 contributes to autophagy and survival in sunitinib treated brain tumor initiating cells (BTICs). Oncotarget 2019;10:368-82. [Crossref] [PubMed]
- Huang X, Gan G, Wang X, et al. The HGF-MET axis coordinates liver cancer metabolism and autophagy for chemotherapeutic resistance. Autophagy 2019;15:1258-79. [Crossref] [PubMed]
- Ou J, Peng Y, Yang W, et al. ABHD5 blunts the sensitivity of colorectal cancer to fluorouracil via promoting autophagic uracil yield. Nat Commun 2019;10:1078. [Crossref] [PubMed]
- Sutton MN, Yang H, Huang GY, et al. RAS-related GTPases DIRAS1 and DIRAS2 induce autophagic cancer cell death and are required for autophagy in murine ovarian cancer cells. Autophagy 2018;14:637-53. [Crossref] [PubMed]
- Ornelas A, McCullough CR, Lu Z, et al. Induction of autophagy by ARHI (DIRAS3) alters fundamental metabolic pathways in ovarian cancer models. BMC Cancer 2016;16:824. [Crossref] [PubMed]
- Washington MN, Suh G, Orozco AF, et al. ARHI (DIRAS3)-mediated autophagy-associated cell death enhances chemosensitivity to cisplatin in ovarian cancer cell lines and xenografts. Cell Death Dis 2015;6:e1836 [Crossref] [PubMed]
- Lu Z, Baquero MT, Yang H, et al. DIRAS3 regulates the autophagosome initiation complex in dormant ovarian cancer cells. Autophagy 2014;10:1071-92. Erratum in: Autophagy 2014 Aug;10(8):1482. [Crossref] [PubMed]
- Zhong C, Shu M, Ye J, et al. Oncogenic Ras is downregulated by ARHI and induces autophagy by Ras/AKT/mTOR pathway in glioblastoma. BMC Cancer 2019;19:441. [Crossref] [PubMed]
- Lu Z, Yang H, Sutton MN, et al. ARHI (DIRAS3) induces autophagy in ovarian cancer cells by downregulating the epidermal growth factor receptor, inhibiting PI3K and Ras/MAP signaling and activating the FOXo3a-mediated induction of Rab7. Cell Death Differ 2014;21:1275-89. [Crossref] [PubMed]
- Zhou H, Yuan M, Yu Q, et al. Autophagy regulation and its role in gastric cancer and colorectal cancer. Cancer Biomark 2016;17:1-10. [Crossref] [PubMed]
- Xu HD, Qin ZH. Beclin 1, Bcl-2 and Autophagy. Adv Exp Med Biol 2019;1206:109-26. [Crossref] [PubMed]
- Chen N, Karantza-Wadsworth V. Role and regulation of autophagy in cancer. Biochim Biophys Acta 2009;1793:1516-23. [Crossref] [PubMed]
- Yang ZJ, Chee CE, Huang S, et al. Autophagy modulation for cancer therapy. Cancer Biol Ther 2011;11:169-76. [Crossref] [PubMed]
- Jung CH, Ro SH, Cao J, et al. mTOR regulation of autophagy. FEBS Lett 2010;584:1287-95. [Crossref] [PubMed]
- Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 2012;366:520-9. [Crossref] [PubMed]
- Hadji P, Coleman R, Gnant M. Bone effects of mammalian target of rapamycin (mTOR) inhibition with everolimus. Crit Rev Oncol Hematol 2013;87:101-11. [Crossref] [PubMed]
- Yehia L, Ngeow J, Eng C. PTEN-opathies: from biological insights to evidence-based precision medicine. J Clin Invest 2019;129:452-64. [Crossref] [PubMed]
- Yu Z, Zhou R, Zhao Y, et al. Blockage of SLC31A1-dependent copper absorption increases pancreatic cancer cell autophagy to resist cell death. Cell Prolif 2019;52:e12568 [Crossref] [PubMed]
- Ferraresi A, Titone R, Follo C, et al. The protein restriction mimetic Resveratrol is an autophagy inducer stronger than amino acid starvation in ovarian cancer cells. Mol Carcinog 2017;56:2681-91. [Crossref] [PubMed]
- Kazerounian S, Lawler J. Integration of pro- and anti-angiogenic signals by endothelial cells. J Cell Commun Signal 2018;12:171-9. [Crossref] [PubMed]
- Shaked Y, McAllister S, Fainaru O, et al. Tumor dormancy and the angiogenic switch: possible implications of bone marrow- derived cells. Curr Pharm Des 2014;20:4920-33. [Crossref] [PubMed]
- Natale G, Bocci G. Does metronomic chemotherapy induce tumor angiogenic dormancy? A review of available preclinical and clinical data. Cancer Lett 2018;432:28-37. [Crossref] [PubMed]
- Indraccolo S. Insights into the regulation of tumor dormancy by angiogenesis in experimental tumors. Adv Exp Med Biol 2013;734:37-52. [Crossref] [PubMed]
- Mira A, Morello V, Cespedes MV, et al. Stroma-derived HGF drives metabolic adaptation of colorectal cancer to angiogenesis inhibitors. Oncotarget 2017;8:38193-213. [Crossref] [PubMed]
- Baeriswyl V, Christofori G. The angiogenic switch in carcinogenesis. Semin Cancer Biol 2009;19:329-37. [Crossref] [PubMed]
- Moserle L, Amadori A, Indraccolo S. The angiogenic switch: implications in the regulation of tumor dormancy. Curr Mol Med 2009;9:935-41. [Crossref] [PubMed]
- Ghajar CM, Peinado H, Mori H, et al. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol 2013;15:807-17. [Crossref] [PubMed]
- Bagley RG. Commentary on Folkman: "Tumor Angiogenesis Factor Cancer Res 2016;76:1673-4. [Crossref] [PubMed]
- Folkman J, Merler E, Abernathy C, et al. Isolation of a tumor factor responsible for angiogenesis. J Exp Med 1971;133:275-88. [Crossref] [PubMed]
- Indraccolo S, Stievano L, Minuzzo S, et al. Interruption of tumor dormancy by a transient angiogenic burst within the tumor microenvironment. Proc Natl Acad Sci U S A 2006;103:4216-21. [Crossref] [PubMed]
- Giuriato S, Ryeom S, Fan AC, et al. Sustained regression of tumors upon MYC inactivation requires p53 or thrombospondin-1 to reverse the angiogenic switch. Proc Natl Acad Sci U S A 2006;103:16266-71. [Crossref] [PubMed]
- Holmgren L, O'Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med 1995;1:149-53. [Crossref] [PubMed]
- Indraccolo S, Minuzzo S, Masiero M, et al. Cross-talk between tumor and endothelial cells involving the Notch3-Dll4 interaction marks escape from tumor dormancy. Cancer Res 2009;69:1314-23. [Crossref] [PubMed]
- Almog N, Ma L, Raychowdhury R, et al. Transcriptional switch of dormant tumors to fast-growing angiogenic phenotype. Cancer Res 2009;69:836-44. [Crossref] [PubMed]
- Tiram G, Segal E, Krivitsky A, et al. Identification of Dormancy-Associated MicroRNAs for the Design of Osteosarcoma-Targeted Dendritic Polyglycerol Nanopolyplexes. ACS Nano 2016;10:2028-45. [Crossref] [PubMed]
- Malafa MP, Fokum FD, Smith L, et al. Inhibition of angiogenesis and promotion of melanoma dormancy by vitamin E succinate. Ann Surg Oncol 2002;9:1023-32. [Crossref] [PubMed]
- Luo J, Feng XX, Luo C, et al. 14,15-EET induces the infiltration and tumor-promoting function of neutrophils to trigger the growth of minimal dormant metastases. Oncotarget 2016;7:43324-36. [Crossref] [PubMed]
- Versteeg HH. Tissue Factor: Old and New Links with Cancer Biology. Semin Thromb Hemost 2015;41:747-55. [Crossref] [PubMed]
- Magnus N, Garnier D, Meehan B, et al. Tissue factor expression provokes escape from tumor dormancy and leads to genomic alterations. Proc Natl Acad Sci U S A 2014;111:3544-9. [Crossref] [PubMed]
- Fainaru O, Almog N, Yung CW, et al. Tumor growth and angiogenesis are dependent on the presence of immature dendritic cells. FASEB J 2010;24:1411-8. [Crossref] [PubMed]
- Kim KJ, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 1993;362:841-4. [Crossref] [PubMed]
- Qin RS, Zhang ZH, Zhu NP, et al. Enhanced antitumor and anti-angiogenic effects of metronomic Vinorelbine combined with Endostar on Lewis lung carcinoma. BMC Cancer 2018;18:967. [Crossref] [PubMed]
- Teng MW, Vesely MD, Duret H, et al. Opposing roles for IL-23 and IL-12 in maintaining occult cancer in an equilibrium state. Cancer Res 2012;72:3987-96. [Crossref] [PubMed]
- Romero I, Garrido C, Algarra I, et al. T lymphocytes restrain spontaneous metastases in permanent dormancy. Cancer Res 2014;74:1958-68. [Crossref] [PubMed]
- Baxevanis CN, Perez SA. Cancer Dormancy: A Regulatory Role for Endogenous Immunity in Establishing and Maintaining the Tumor Dormant State. Vaccines (Basel) 2015;3:597-619. [Crossref] [PubMed]
- Quezada SA, Peggs KS, Simpson TR, et al. Shifting the equilibrium in cancer immunoediting: from tumor tolerance to eradication. Immunol Rev 2011;241:104-18. [Crossref] [PubMed]
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011;331:1565-70. [Crossref] [PubMed]
- Mittal D, Gubin MM, Schreiber RD, et al. New insights into cancer immunoediting and its three component phases--elimination, equilibrium and escape. Curr Opin Immunol 2014;27:16-25. [Crossref] [PubMed]
- Bellovin DI, Das B, Felsher DW. Tumor dormancy, oncogene addiction, cellular senescence, and self-renewal programs. Adv Exp Med Biol 2013;734:91-107. [Crossref] [PubMed]
- Toso A, Revandkar A, Di Mitri D, et al. Enhancing chemotherapy efficacy in Pten-deficient prostate tumors by activating the senescence-associated antitumor immunity. Cell Rep 2014;9:75-89. [Crossref] [PubMed]
- Di Mitri D, Toso A, Chen JJ, et al. Tumour-infiltrating Gr-1+ myeloid cells antagonize senescence in cancer. Nature 2014;515:134-7. [Crossref] [PubMed]
- Simova J, Sapega O, Imrichova T, et al. Tumor growth accelerated by chemotherapy-induced senescent cells is suppressed by treatment with IL-12 producing cellular vaccines. Oncotarget 2016;7:54952-64. [Crossref] [PubMed]
- Triana-Martínez F, Loza MI, Domínguez E. Beyond Tumor Suppression: Senescence in Cancer Stemness and Tumor Dormancy. Cells 2020;9:346. [Crossref] [PubMed]
- Saleh T, Tyutynuk-Massey L, Cudjoe EK Jr, et al. Non-Cell Autonomous Effects of the Senescence-Associated Secretory Phenotype in Cancer Therapy. Front Oncol 2018;8:164. [Crossref] [PubMed]
- Saleh T, Tyutyunyk-Massey L, Murray GF, et al. Tumor cell escape from therapy-induced senescence. Biochem Pharmacol 2019;162:202-12. [Crossref] [PubMed]
- Romero I, Garrido F, Garcia-Lora AM. Metastases in immune-mediated dormancy: a new opportunity for targeting cancer. Cancer Res 2014;74:6750-7. [Crossref] [PubMed]
- Wang HF, Wang SS, Huang MC, et al. Targeting Immune-Mediated Dormancy: A Promising Treatment of Cancer. Front Oncol 2019;9:498. [Crossref] [PubMed]
- Rezvani K, Rouce RH. The Application of Natural Killer Cell Immunotherapy for the Treatment of Cancer. Front Immunol 2015;6:578. [Crossref] [PubMed]
- Newick K, O'Brien S, Moon E, et al. CAR T Cell Therapy for Solid Tumors. Annu Rev Med 2017;68:139-52. [Crossref] [PubMed]
- Ferrari de Andrade L, Tay RE, Pan D, et al. Antibody-mediated inhibition of MICA and MICB shedding promotes NK cell-driven tumor immunity. Science 2018;359:1537-42. [Crossref] [PubMed]
- Zhang P, Zhai Y, Cai Y, et al. Nanomedicine-Based Immunotherapy for the Treatment of Cancer Metastasis. Adv Mater 2019;31:e1904156 [Crossref] [PubMed]
- Zhou J, Tang Z, Gao S, et al. Tumor-Associated Macrophages: Recent Insights and Therapies. Front Oncol 2020;10:188. [Crossref] [PubMed]
- Siska PJ, Singer K, Evert K, et al. The immunological Warburg effect: Can a metabolic-tumor-stroma score (MeTS) guide cancer immunotherapy? Immunol Rev 2020;295:187-202. [Crossref] [PubMed]
- Cavalli E, Ciurleo R, Petralia MC, et al. Emerging Role of the Macrophage Migration Inhibitory Factor Family of Cytokines in Neuroblastoma. Pathogenic Effectors and Novel Therapeutic Targets? Molecules 2020;25:1194. [Crossref] [PubMed]
- Xie Q, Li F, Fang L, et al. The Antitumor Efficacy of beta-Elemene by Changing Tumor Inflammatory Environment and Tumor Microenvironment. Biomed Res Int 2020;2020:6892961 [Crossref] [PubMed]
- Qin T, Xia J, Liu S, et al. Clinical importance of VEGFC and PD-L1 co-expression in lung adenocarcinoma patients. Thorac Cancer 2020;11:1139-48. [Crossref] [PubMed]
- Li W, He C, Wu J, et al. Epstein barr virus encodes miRNAs to assist host immune escape. J Cancer 2020;11:2091-100. [Crossref] [PubMed]
- MacKie RM, Reid R, Junor B. Fatal melanoma transferred in a donated kidney 16 years after melanoma surgery. N Engl J Med 2003;348:567-8. [Crossref] [PubMed]
- Matser YAH, Terpstra ML, Nadalin S, et al. Transmission of breast cancer by a single multiorgan donor to 4 transplant recipients. Am J Transplant 2018;18:1810-4. [Crossref] [PubMed]
- Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011;365:725-33. [Crossref] [PubMed]
- Manjili MH. Tumor Dormancy and Relapse: From a Natural Byproduct of Evolution to a Disease State. Cancer Res 2017;77:2564-9. [Crossref] [PubMed]
- Baxevanis CN, Fortis SP, Perez SA. The balance between breast cancer and the immune system: Challenges for prognosis and clinical benefit from immunotherapies. Semin Cancer Biol 2021;72:76-89. [Crossref] [PubMed]
- Boire A, Coffelt SB, Quezada SA, et al. Tumour Dormancy and Reawakening: Opportunities and Challenges. Trends Cancer 2019;5:762-5. [Crossref] [PubMed]
- Wu TD, Madireddi S, de Almeida PE, et al. Peripheral T cell expansion predicts tumour infiltration and clinical response. Nature 2020;579:274-8. [Crossref] [PubMed]
- Greil R, Hutterer E, Hartmann TN, et al. Reactivation of dormant anti-tumor immunity - a clinical perspective of therapeutic immune checkpoint modulation. Cell Commun Signal 2017;15:5. [Crossref] [PubMed]
- Marlow R, Honeth G, Lombardi S, et al. A novel model of dormancy for bone metastatic breast cancer cells. Cancer Res 2013;73:6886-99. [Crossref] [PubMed]
Cite this article as: Chang Y, Chen J. Dormant mechanisms reveal the clinical significance of tumor dormancy: a narrative review. Ann Blood 2021;6:15.