
Intravenous immunoglobulins (IVIg) in childhood immune thrombocytopenia: towards personalized medicine—a narrative review
Introduction
Intravenous immunoglobulins (IVIg) treatment is an effective first-line therapy in immune thrombocytopenia (ITP). ITP is divided into three phases, starting with newly diagnosed ITP (≤3 months), persistent ITP (>3 months) and chronic ITP (≥12 months) (1). We introduced the term transient ITP to differentiate ITP that resolved within 3 months (2). Notably, childhood ITP has different disease characteristics than adult ITP, featuring a more severe bleeding pattern, and a high rate of post-infectious and self-limiting cases (3). In children, guidelines favor observation, whereas ITP in adults with low platelet counts is suggested to be treated (4). This review focuses on the efficacy of IVIg and its possible working mechanisms in childhood ITP, although the treatment is also efficacious in adult ITP (5).
The treatment of childhood ITP with IVIg was coined in 1980 by Imbach and colleagues, who showed the effective treatment of thrombocytopenia by IVIg in acute and chronic ITP (6,7). Together with the emergent pathophysiological role of anti-platelet antibodies in ITP (8-11), the successful immunotherapy of ITP with IVIg marked the transition of the disease’s definition from ‘idiopathic’ to ‘immune’ thrombocytopenia (Imbach; personal communication). Although the exact working mechanism remains unknown (Table 1), the prevailing hypothesis is that IVIg treatment leads to the blocking of IgG Fc receptors (FcγR) on phagocytes, as well as clearance of pathological anti-platelet IgG antibodies by saturation of the neonatal IgG Fc receptor (FcRn) (12,29-31). A comprehensive discussion of IVIg working mechanisms is provided elsewhere (32-34).

Full table
Mucosal bleeding occurs in 40% of children with newly diagnosed ITP (35,36). During 1-year follow-up with observation only, clinically significant bleeding occurs in 13% of children (35). Previous registry data indicated a low risk of moderate or severe bleeding, but it needs to be taken into account that >70% of these children were treated, and they reflect a higher proportion of chronic ITP patients that are known to have less severe bleeding episodes (37,38). Most moderate and severe bleeding episodes occur in patients with a platelet count ≤20×109/L (37), but the platelet count is considered poor predictor of the ITP bleeding severity (39-41).
Many cases of childhood ITP resolve spontaneously (36,42), and the current clinical managements of ITP favors a ‘watchful waiting’ strategy for ITP. Spontaneous recovery, i.e., transient ITP, may more likely in younger children, boys, those with a preceding infection, mucosal bleeding, and an abrupt disease onset (43-46). The medical treatment of ITP is considered in case of moderate or severe bleeding, reduced health-related quality of life (HRQoL), and individual patient circumstances (e.g., potential for injury, psychosocial factors). Thus, treatment may aim to reduce or prevent clinically significant bleeding, provide rescue therapy in acute bleeding episodes, reduce fatigue, or improve the quality of life. In Europe, corticosteroids and IVIg are the preferred first-line treatment options for ITP. IVIg is the suggested treatment in The Netherlands and endorsed by the Joint Working Group (JWG) of the German, Austrian and Swiss hematological societies updated recommendations in 2018 (47). IVIg specifically leads to a rapid resolution of thrombocytopenia within few days (1,48), whereas corticosteroid responses are less rapid (49,50). The 2019 updated ASH guideline favors corticosteroids over IVIg, yet this recommendation is based on assessment of the literature for the recovery outcome at 6 and 12 months after the treatment (4). Evidently, short- and long-term effects and treatment goals need to be balanced. In this review, we discuss the short- and long-term effects of IVIg treatment in childhood ITP, and discuss recent findings that may allow the targeting of IVIg to those patients who benefit the most. We present the following article in accordance with Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/aob-20-59).
Short-term effects of IVIg
IVIg treatment leads to increases in platelet count within a few days of the administration (6,51). Compared to patients that are managed with observation, this increase in platelet count is displayed for up to 1-to-3 months after treatment (35). A number of comparative randomized controlled trials (RCTs) show a significantly higher ITP recovery rate after IVIg treatment on this short-term perspective, compared to no treatment or corticosteroids (35,49,50,52). In these RCT, IVIg also led to a more rapid recovery of platelet counts than corticosteroids. Across the trials, the effectiveness of IVIg to resolve thrombocytopenia varied markedly between 50–95%, depending on the specific inclusion and exclusion criteria as well as outcome criteria for response that were only standardized in 2009 (1). In the largest and most recent trial that used the current definitions and included patients with newly diagnosed ITP within 72 hours of diagnosis, 68% of children exhibited a complete response with platelets ≥100×109/L 1 week after IVIg therapy (35). When we assessed individual patient data, we observed several distinct longitudinal platelet count trajectories of patients with initial complete responses that were either sustained or not, and a group of patients that showed very low platelet responses or no platelet response at all (Figure 1). According to the standardized response criteria, where two timepoints are used to assess the response to treatment (1), these data showed that a response at week 1 and month 1 was fully associated with a long-term favorable disease outcome, whereas the other response groups showed more variable longitudinal platelet responses and a higher rate of persistent and chronic ITP (Figure 1).
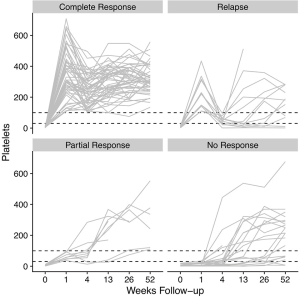
Beyond the normalization of platelet counts, the RCT data shows that IVIg treatment, compared to no treatment, leads to a reduction of clinically significant bleeding symptoms (35). After the administration of IVIg in 100 children with newly diagnosed ITP, only one child exhibited Buchanan grade 3 and grade 4/5 bleeding that necessitated treatment during a full year follow-up. In comparison, among 100 children that were observed, 13 children displayed bleeding episodes that necessitated treatment, of which three episodes with grade 3 bleeding and ten with grade 4/5 bleeding. In the same study, treatment with IVIg led to adverse reactions with nausea, vomiting or headache in four patients and allergic reactions in one patient (among 100 treated children). Interestingly, the reduction of bleeding symptoms by IVIg did not translate to a measurably improved HRQoL as compared to observation (53), as measured by the child- and ITP-specific Pediatric Quality of Life Inventory (PedQL) and the Kids’ ITP Tools (KIT) questionnaires (54,55). For context, it needs to be considered that 32% of patients did not respond to the therapy, yet all treated children were exposed to the impact of medical care on a child, as well as the potential of side effects of IVIG (35).
IVIg also has a role in the emergency treatment of severe and life-threatening bleedings and is at times combined with methylprednisolone, platelet transfusions (47), or even splenectomy.
In summary, IVIg is an effective treatment in newly diagnosed ITP to rapidly resolve thrombocytopenia and reduce clinically significant bleeding. For emergency and rescue treatment, IVIg leads to a more rapid response than corticosteroids alone.
Long-term effects of IVIg
Several cohort studies and an early meta-analysis (nine studies) indicated that children who developed chronic ITP were less often treated with IVIg (56). This led to the hypothesis that IVIg may, potentially by some immunomodulatory mechanism, prevent the development of chronic ITP. In the Intercontinental Cooperative ITP Study Group (ICIS) Registry I data, the same association of IVIg with less chronic ITP was observed (57). The aggregated data from the latest meta-analysis (14 studies) suggested that IVIg led to a reduced chance to develop chronic ITP with an odds ratio of 0.71 (95% CI, 0.52–0.97) (44). Importantly, a challenge to the interpretation of these observational data is the potential bias by confounding by indication. For instance, physicians may be inclined to treat children who show more severe bleeding symptoms (which is associated with self-limiting ITP).
Given this uncertainty concerning the potential utility of IVIg to modulate ITP disease courses, our research team set up an almost nationwide RCT in 60 hospitals in the Netherlands to compare observation versus IVIg treatment for the primary efficacy outcome of the the incidence of chronic ITP [the Treatment With or Without IVIg for Kids With ITP trial (TIKI)]. Between 2009 to 2015, 200 children with newly diagnosed ITP were enrolled at pediatric departments within 72 hours of diagnosis (35). All children were followed for 1 year after the diagnosis. At the 1-year follow-up, 10/100 (10%) IVIg-treated children developed chronic ITP versus 12/100 (12%) children who were observed [relative risk (RR), 0.83; 95% CI, 0.38–1.84]. Notably, the definition of chronic ITP was changed by the International Working Group during the trial’s recruitment phase from a platelet count <150×109/L at 6 months to <100×109/L at 12 months (1). According to the old definition, at the 6-month follow-up, 18 IVIg-treated children exhibited chronic ITP versus 28 children who were observed (RR, 0.64; 95% CI, 0.38–1.08). Of note, recovery to a platelet count ≥100×109/L at 6 months follow-up was almost similar between the trial arms, 84% and 78% for the IVIg and observation arms, respectively (RR, 1.07; 95% CI, 0.94–1.23). This platelet threshold correlates well with subsidence of bleeding symptoms.
Our trial was powered to detect a reduction of the rate of chronic ITP with an estimated incidence of 25% with observation. Using the old chronic ITP definition at the 6 months follow-up, we observed 28% at that timepoint, and the trial was thus not underpowered. Due to the change in the definition of chronic ITP, however, we observed that only 12% of children developed chronic ITP according to the new definition at the 1-year follow-up. Although it is theoretically possible that IVIg reduces this rate to 10% (2% absolute risk reduction, 17% RR reduction), the data show that IVIg has no large effect to reduce the incidence of chronic ITP. To conclusively test the presence of such a minor effect, a very large trial population would be required. Thus, the current data presumably represent the best available evidence for the foreseeable future. Of note, the inclusion of patients at the time of diagnosis in general pediatric departments led to a comparatively young study population (median age ~3.5 years, 25% were above 7 years old). It could be considered that in this setting the incidence of chronic ITP was too low and the potential effect of IVIg to prevent chronic ITP could not be sufficiently shown. However, this is not supported by the trial’s data, as we actually observed that an age at diagnosis above 6 years is associated with reduced IVIg responsiveness (58), as discussed below.
Altogether, the RCT data clearly show that IVIg treatment does not change the incidence of chronic ITP.
Reconciling the short-term efficacy of IVIg with absence of efficacy on long-term recovery
How is the observation of the efficacy of IVIg on the short-term to be combined with absence of efficacy on recovery on the long term? Imbach and colleagues observed in their RCT in 1985: “at least two prognostic subgroups of childhood ITP [are suggested] and corticosteroids and [IVIg] do not have the same effects in the subgroup requiring longer therapy” (52). Similarly, in TIKI, the incidence of chronic ITP patients in IVIg non-responders was also markedly increased (59). One explanation is that patients who initially recovered show subsequent relapse. However, this is only incidentally observed. The other possibility is that IVIg is efficacious in patients who would otherwise spontaneously recover during observation. In other words, early treatment with IVIg (without relapse) may differentiate to some degree early in the disease course whether a child would develop persistent or chronic ITP, or not. Realizing this, IVIg could be targeted to those patients who benefit the most. With an indication of who would respond to IVIg and who would not, the expected outcome of emergency IVIg treatment or case-by-case therapy could be determined and considered in the clinical decision making. Thus, we studied if we could identify a set of clinical and biological parameters that allow the identification of patients who would respond to IVIg, versus those who would not.
Individualization of IVIg therapy
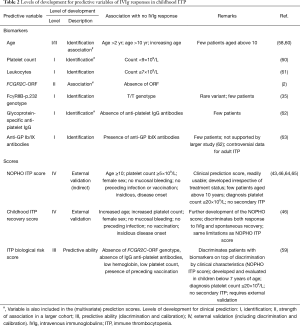
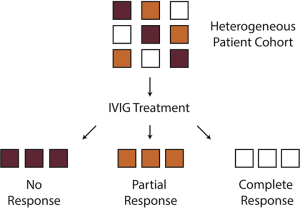
Different levels of evidence need to be considered to individualize IVIg therapy. Disease models such as mouse models can be used to propose mechanisms than can subsequently be clinically evaluated. To target IVIg therapy to specific patients, clinical and biological markers of the response to IVIg need to be identified (association), and the individual or combined predictive value of the markers needs to be assessed (prediction; Table 2). Moreover, for clinical use, the identification of specific patient characteristics is used to explain and predict treatment responses before any treatment is administered (Figure 2). Of note, this also allows a more detailed dissection of the pathophysiology of ITP in specific disease subgroups.
Full table
Proposed markers associated with IVIg response
While the exact working mechanisms of IVIg remain unresolved, multiple mechanisms should be investigated (32,33). Mouse models of ITP show a critical role of the only myeloid inhibitory Fc-receptor FcγRIIB for response to IVIg (17,20,66). This receptor has a loss-of-function allele with a switch from the p.232I>T genotype that excludes the receptor from lipid rafts (67,68). Intriguingly, in the IVIg arm of the TIKI trial, none of the three patients with the FcγRIIB-p.232T/T genotype showed a response to IVIg (35). However, homozygosity for the p.232T allele is rare and cannot explain all non-responses to IVIg.
Next to these mouse data, only limited clinical data is available to understand which patient responds to IVIg and who will not (Table 2). Evidently, the determination of who responds to IVIg is the inverse of identifying patients who do not respond to IVIg. In one study, the non-response to IVIg was associated with an age above 2 years and a platelet count below 9×109/L (60). One more study found that IVIg non-responders had more often a leukocyte count ≤7×109/L (61). The increased expression of IFN-γ in peripheral blood mononuclear cells was found by another study to be associated with non-response to IVIg (69). There is controversial data on the presence of anti-platelet glycoprotein Ib/IX IgG antibodies and response to IVIg (63,70,71). In the TIKI study, only 11/176 (6%) patients showed anti-GP Ib/IX antibodies using state-of-the-art detection, and eight of these eleven patients had anti-platelet antibodies directed to multiple antigens (62). Intriguingly, among a total of 12 IVIg-patients with anti-platelet IgG antibodies directed towards GP IIb/IIIa, Ib/IX or V, we observed an almost full response to IVIg (62), suggesting that IVIg may be particularly effective when IgG antibodies are present. As mentioned above, we observed that children above 6 years of age showed a reduced response rate to IVIg (58). Remarkably, compared to 69% in the whole cohort, only 29% of 14 patients aged above 10 years showed a complete response to IVIg, and none of six patients aged above 12 years exhibited a complete response to IVIg (58).
Proposed markers predictive of IVIg response
The pro-inflammatory open reading frame variant of the human myeloid Fc-receptor FcγRIIC (FCGR2C-ORF) has been associated with susceptibility to ITP (72). We observed that the FCGR2C-ORF variant also discriminates favorable responses to IVIg (2). Interestingly, we found that the variant also discriminates patients who show a timely spontaneous recovery, suggesting that indeed the same biological parameters are associated with recovery and IVIg response. Thus, pending further validation, this marker could be useful to differentiate responses to IVIg treatment.
We have recently developed the Childhood ITP Recovery Score (http://itprecoveryscore.org), an externally validated multivariate prediction score that gives a probability for transient ITP based on the clinical characteristics age, sex, platelet count at diagnosis, mucosal bleeding, preceding infection and vaccination, and disease onset (Table 2) (46). Intriguingly, over the 1-year follow-up in TIKI, the score differentiates by the same criteria both favorable spontaneous recovery as well as response to IVIg. Again, this emphasizes that the same patient characteristics are associated with IVIg response and recovery. During validation, the score classified 63% of patients as ‘high chance of recovery’ and among these, 85%, 90% and 95% had recovered by 3-, 6- and 9-month follow-up (46). Patients with intermediate or low chance of recovery had a significantly lower recovery rate at these follow-up visits. Although a degree of uncertainty remains, the Childhood ITP Recovery Score is a readily applicable, externally validated decision support tool that may be a good indicator of the ITP prognosis.
Given the availability of biological markers that could be added to a multivariate prediction model, a logical next step is to extending the clinical characteristics (Childhood ITP Recovery Score) into a more extensive score that could potentially better predict disease outcomes. Thus, we have recently used the targeted assessment of genetic variants and immune characteristics to predict IVIg responses for a biological risk score (59). A combination of five biomarkers (Table 2), including the FCGR2C-ORF variant and IgG anti-platelet antibodies, was predictive of IVIg responses. The score detected non-responses to IVIg with a sensitivity of 0.91 (95% CI, 0.81–1.00) and a specificity of 0.67 (95% CI, 0.53–0.80). This implied that a response to IVIg was usually observed when it was predicted, with a predictive value of 0.91 (95% CI, 0.82–1.00). Importantly, predicting outcomes together with both molecular markers and clinical characteristics worked significantly better than any of them alone. An improved discrimination was achieved for children with the most uncertainty, i.e., low and intermediate chance of recovery. Beyond platelet responses, the score also predicted bleeding outcomes. The biomarker-based prediction needs to take into account the variation in biomarkers during normal child development and the heterogeneity in clinical presentation of ITP at various ages, and this was solved here by adjusting all numerical variables for age, and limiting the population to the children up to 7 years old.
Taken together, we now have individual patient markers available that are either associated with response to IVIg, or have already been shown to predict responses to IVIg. In the future, pending further validation and stratified treatment studies, these determinants could be used to direct IVIg therapy to patients who can respond to IVIg. For those who are expected to show no response, alternative or adjunctive treatment schemes could be beneficial for short- and long-term treatment goals.
Clinical implications
Through the research of the past two decades, there is now better data available about the effects of IVIg therapy and its potential role in clinical management. The potential uses of IVIg therapy comprise the prevention of chronic ITP, effect on HRQoL, and stopping/prevention of bleeding. To briefly review, we now have dependable data showing that IVIg treatment does not prevent the development chronic disease when administered to patients with newly diagnosed ITP. There is no evidence to substantiate a favorable effect of IVIg treatment on HRQoL in newly diagnosed childhood ITP patients, and further research is necessary to evaluate potential HRQoL benefits for subgroups. Bleeding episodes can be effectively and rapidly stopped with IVIg treatment. Moreover, bleedings can be prevented with IVIg therapy, although we only have a preliminary estimate for the magnitude of the reduction of bleeding events (effect size).
If a decision is made to treat, an estimation of the expected response can be obtained with clinical characteristics, using the Childhood ITP Recovery Score. This score showed a similar discrimination of recovery in patients who were treated with IVIg and in patients who were observed. In patients predicted to have a high chance of recovery, 85% will exhibit a full recovery 3 months after the diagnosis, as compared to 69% and 32% of patients with an intermediate or low chance of recovery. Moreover, in patients with a low chance of recovery, additional or alternative treatments, closer clinical monitoring, or screening for autoimmune diseases could potentially be considered.
Discussion
In ITP, IVIg resolves thrombocytopenia and leads to a reduction of clinically significant bleeding, which is balanced by adverse events (side effects) in few patients. IVIg is effective on short term, but not superior to observation over the long term (≥6 months). Given its efficacy, IVIg remains the main stay of treatment for emergency and rescue treatment, and should be considered next to corticosteroids when rapid platelet responses are desired. The relative place of IVIg compared to TPO receptor agonists for short-term treatment in newly diagnosed ITP is unknown but currently being investigated. Individualization of IVIg therapy to a select group of patients who experience a significant disease burden and may respond well to IVIg—based on clinical and biological parameters—could be an interesting future strategy that minimizes costs and risks and maximizes benefits. In this setting, the effect of IVIg treatment for patient-reported outcomes and HRQoL should be closely studied. Similarly, it remains interesting to investigate if the same factors that associate with favorable response to IVIg also associate with responses to corticosteroids. Finally, the development of extended, multivariate prediction models that include biological markers could allow the improved targeting of treatments in childhood ITP.
Acknowledgments
Funding: This work was funded by a grant from the Landsteiner Foundation for Blood Transfusion Research (LSBR) and a doctoral stiped by the Studienstiftung des Deutschen Volkes to DES.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (John W. Semple and Rick Kapur) for the series “Treatment of Immune Thrombocytopenia (ITP)” published in Annals of Blood. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/aob-20-59
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob-20-59). The series “Treatment of Immune Thrombocytopenia (ITP)” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood 2009;113:2386-93. [Crossref] [PubMed]
- Schmidt DE, Heitink-Pollé KMJ, Laarhoven AG, et al. Transient and chronic childhood immune thrombocytopenia are distinctly affected by Fc-γ receptor polymorphisms. Blood Adv 2019;3:2003-12. [Crossref] [PubMed]
- Despotovic JM, Grimes AB. Pediatric ITP: is it different from adult ITP? Hematology Am Soc Hematol Educ Program 2018;2018:405-11. [Crossref] [PubMed]
- Neunert C, Terrell DR, Arnold DM, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv 2019;3:3829-66. [Crossref] [PubMed]
- Newland AC, Treleaven JG, Minchinton RM, et al. High-dose intravenous IgG in adults with autoimmune thrombocytopenia. Lancet 1983;1:84-7. [Crossref] [PubMed]
- Imbach P, Barandun S, d'Apuzzo V, et al. High-dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura in childhood. Lancet 1981;1:1228-31. [Crossref] [PubMed]
- Imbach P. The clinical translation of intravenous immunoglobulin from substitution to immunomodulation. In: Imbach P. editor. Antibody therapy. Cham: Springer; 2018:1-11.
- Harrington WJ, Minnich V, Hollingsworth JW, et al. Demonstration of a thrombocytopenic factor in the blood of patients with thrombocytopenic purpura. J Lab Clin Med 1951;38:1-10. [PubMed]
- Harrington WJ, Sprague CC, Minnich V, et al. Immunologic mechanisms in idiopathic and neonatal thrombocytopenic purpura. Ann Intern Med 1953;38:433-69. [Crossref] [PubMed]
- Shulman NR, Marder VJ, Weinrach RS. Similarities between known antiplatelet antibodies and the factor responsible for thrombocytopenia in idiopathic purpura. Physiologic, serologic and isotopic studies. Ann N Y Acad Sci 1965;124:499-542. [Crossref] [PubMed]
- Cines DB, Schreiber AD. Immune thrombocytopenia. Use of a Coombs antiglobulin test to detect IgG and C3 on platelets. N Engl J Med 1979;300:106-11. [Crossref] [PubMed]
- Debré M, Bonnet MC, Fridman WH, et al. Infusion of Fc gamma fragments for treatment of children with acute immune thrombocytopenic purpura. Lancet 1993;342:945-9. [Crossref] [PubMed]
- Kuitwaard K, de Gelder J, Tio-Gillen AP, et al. Pharmacokinetics of intravenous immunoglobulin and outcome in Guillain-Barré syndrome. Ann Neurol 2009;66:597-603. [Crossref] [PubMed]
- Levy Y, Sherer Y, Ahmed A, et al. Autoantibody level modification in adult patients with idiopathic thrombocytopenic purpura following intravenous immunoglobulin treatment. Nat Immun 1998;16:207-14. [Crossref] [PubMed]
- Hansen RJ, Balthasar JP. Effects of intravenous immunoglobulin on platelet count and antiplatelet antibody disposition in a rat model of immune thrombocytopenia. Blood 2002;100:2087-93. [Crossref] [PubMed]
- Tackenberg B, Jelcic I, Baerenwaldt A, et al. Impaired inhibitory Fcgamma receptor IIB expression on B cells in chronic inflammatory demyelinating polyneuropathy. Proc Natl Acad Sci U S A 2009;106:4788-92. [Crossref] [PubMed]
- Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science 2001;291:484-6. [Crossref] [PubMed]
- Ephrem A, Chamat S, Miquel C, et al. Expansion of CD4+CD25+ regulatory T cells by intravenous immunoglobulin: a critical factor in controlling experimental autoimmune encephalomyelitis. Blood 2008;111:715-22. [Crossref] [PubMed]
- De Groot AS, Moise L, McMurry JA, et al. Activation of natural regulatory T cells by IgG Fc-derived peptide "Tregitopes". Blood 2008;112:3303-11. [Crossref] [PubMed]
- Siragam V, Crow AR, Brinc D, et al. Intravenous immunoglobulin ameliorates ITP via activating Fc gamma receptors on dendritic cells. Nat Med 2006;12:688-92. [Crossref] [PubMed]
- Fokkink WJR, Selman MHJ, Dortland JR, et al. IgG Fc N-glycosylation in Guillain-Barré syndrome treated with immunoglobulins. J Proteome Res 2014;13:1722-30. [Crossref] [PubMed]
- Le Pottier L, Sapir T, Bendaoud B, et al. Intravenous immunoglobulin and cytokines: focus on tumor necrosis factor family members BAFF and APRIL. Ann N Y Acad Sci 2007;1110:426-32. [Crossref] [PubMed]
- Basta M, Van Goor F, Luccioli S, et al. F(ab)'2-mediated neutralization of C3a and C5a anaphylatoxins: a novel effector function of immunoglobulins. Nat Med 2003;9:431-8. [Crossref] [PubMed]
- Rossi F, Kazatchkine MD. Antiidiotypes against autoantibodies in pooled normal human polyspecific Ig. J Immunol 1989;143:4104-9. [PubMed]
- Dietrich G, Kazatchkine MD. Normal immunoglobulin G (IgG) for therapeutic use (intravenous Ig) contain antiidiotypic specificities against an immunodominant, disease-associated, cross-reactive idiotype of human anti-thyroglobulin autoantibodies. J Clin Invest 1990;85:620-5. [Crossref] [PubMed]
- Semple JW, Kim M, Lazarus AH, et al. Gamma-globulins prepared from sera of multiparous women bind anti-HLA antibodies and inhibit an established in vivo human alloimmune response. Blood 2002;100:1055-9. [Crossref] [PubMed]
- Viard I, Wehrli P, Bullani R, et al. Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science 1998;282:490-3. [Crossref] [PubMed]
- Schaub A, von Gunten S, Vogel M, et al. Dimeric IVIG contains natural anti-Siglec-9 autoantibodies and their anti-idiotypes. Allergy 2011;66:1030-7. [Crossref] [PubMed]
- Clarkson SB, Kimberly RP, Valinsky JE, et al. Blockade of clearance of immune complexes by an anti-Fc gamma receptor monoclonal antibody. J Exp Med 1986;164:474-89. [Crossref] [PubMed]
- Bleeker WK, Teeling JL, Hack CE. Accelerated autoantibody clearance by intravenous immunoglobulin therapy: studies in experimental models to determine the magnitude and time course of the effect. Blood 2001;98:3136-42. [Crossref] [PubMed]
- Crow AR, Song S, Semple JW, et al. IVIg inhibits reticuloendothelial system function and ameliorates murine passive-immune thrombocytopenia independent of anti-idiotype reactivity. Br J Haematol 2001;115:679-86. [Crossref] [PubMed]
- Schwab I, Nimmerjahn F. Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nat Rev Immunol 2013;13:176-89. [Crossref] [PubMed]
- Nagelkerke SQ, Kuijpers TW. Immunomodulation by IVIg and the role of Fc-gamma receptors: classic mechanisms of action after all? Front Immunol 2015;5:674. [Crossref] [PubMed]
- Crow AR, Song S, Siragam V, et al. Mechanisms of action of intravenous immunoglobulin in the treatment of immune thrombocytopenia. Pediatr Blood Cancer 2006;47:710-3. [Crossref] [PubMed]
- Heitink-Pollé KMJ, Uiterwaal CSPM, Porcelijn L, et al. Intravenous immunoglobulin vs observation in childhood immune thrombocytopenia: a randomized controlled trial. Blood 2018;132:883-91. [Crossref] [PubMed]
- Rosthøj S, Hedlund-Treutiger I, Rajantie J, et al. Duration and morbidity of newly diagnosed idiopathic thrombocytopenic purpura in children: a prospective Nordic study of an unselected cohort. J Pediatr 2003;143:302-7. [Crossref] [PubMed]
- Neunert CE, Buchanan GR, Imbach P, et al. Severe hemorrhage in children with newly diagnosed immune thrombocytopenic purpura. Blood 2008;112:4003-8. [Crossref] [PubMed]
- Neunert CE, Buchanan GR, Imbach P, et al. Bleeding manifestations and management of children with persistent and chronic immune thrombocytopenia: data from the Intercontinental Cooperative ITP Study Group (ICIS). Blood 2013;121:4457-62. [Crossref] [PubMed]
- Buchanan GR, de Alarcon PA, Feig SA, et al. Acute idiopathic thrombocytopenic purpura--management in childhood. Blood 1997;89:1464-5; author reply 1466. [Crossref] [PubMed]
- Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood 2010;115:168-86. [Crossref] [PubMed]
- van Bladel ER, Laarhoven AG, van der Heijden LB, et al. Functional platelet defects in children with severe chronic ITP as tested with 2 novel assays applicable for low platelet counts. Blood 2014;123:1556-63. [Crossref] [PubMed]
- Imbach P, Kuhne T, Müller D, et al. Childhood ITP: 12 months follow-up data from the prospective registry I of the Intercontinental Childhood ITP Study Group (ICIS). Pediatr Blood Cancer 2006;46:351-6. [Crossref] [PubMed]
- Edslev PW, Rosthøj S, Treutiger I, et al. A clinical score predicting a brief and uneventful course of newly diagnosed idiopathic thrombocytopenic purpura in children. Br J Haematol 2007;138:513-6. [Crossref] [PubMed]
- Heitink-Pollé KMJ, Nijsten J, Boonacker CWB, et al. Clinical and laboratory predictors of chronic immune thrombocytopenia in children: a systematic review and meta-analysis. Blood 2014;124:3295-307. [Crossref] [PubMed]
- Bennett CM, Neunert C, Grace RF, et al. Predictors of remission in children with newly diagnosed immune thrombocytopenia: data from the Intercontinental Cooperative ITP Study Group Registry II participants. Pediatr Blood Cancer 2018;65:e26736-7. [Crossref] [PubMed]
- Schmidt DE, Wendtland Edslev P, Heitink-Pollé KMJ, et al. A clinical prediction score for transient versus persistent childhood immune thrombocytopenia. J Thromb Haemost 2021;19:121-30. [Crossref] [PubMed]
- Matzdorff A, Meyer O, Ostermann H, et al. Immune thrombocytopenia - current diagnostics and therapy: recommendations of a Joint Working Group of DGHO, ÖGHO, SGH, GPOH, and DGTI. Oncol Res Treat. Oncol Res Treat 2018;41:1-30. [Crossref] [PubMed]
- Neunert C, Lim W, Crowther M, et al. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood 2011;117:4190-207. [Crossref] [PubMed]
- Blanchette VS, Luke B, Andrew M, et al. A prospective, randomized trial of high-dose intravenous immune globulin G therapy, oral prednisone therapy, and no therapy in childhood acute immune thrombocytopenic purpura. J Pediatr 1993;123:989-95. [Crossref] [PubMed]
- Blanchette V, Imbach P, Andrew M, et al. Randomised trial of intravenous immunoglobulin G, intravenous anti-D, and oral prednisone in childhood acute immune thrombocytopenic purpura. Lancet 1994;344:703-7. [Crossref] [PubMed]
- Imbach P, Jungi T, Barandun S, et al. Intravenous immunoglobulin in immune thrombocytopenia. Lancet 1983;2:577-8. [Crossref] [PubMed]
- Imbach P, Wagner HP, Berchtold W, et al. Intravenous immunoglobulin versus oral corticosteroids in acute immune thrombocytopenic purpura in childhood. Lancet 1985;2:464-8. [Crossref] [PubMed]
- Heitink-Pollé KM, Haverman L, Annink KV, et al. Health-related quality of life in children with newly diagnosed immune thrombocytopenia. Haematologica 2014;99:1525-31. [Crossref] [PubMed]
- Klaassen RJ, Blanchette V, Burke TA, et al. Quality of life in childhood immune thrombocytopenia: international validation of the kids' ITP tools. Pediatr Blood Cancer 2013;60:95-100. [Crossref] [PubMed]
- Varni JW, Seid M, Knight TS, et al. The PedsQL 4.0 Generic Core Scales: sensitivity, responsiveness, and impact on clinical decision-making. J Behav Med 2002;25:175-93. [Crossref] [PubMed]
- Beck CE, Nathan PC, Parkin PC, et al. Corticosteroids versus intravenous immune globulin for the treatment of acute immune thrombocytopenic purpura in children: a systematic review and meta-analysis of randomized controlled trials. J Pediatr 2005;147:521-7. [Crossref] [PubMed]
- Tamminga R, Berchtold W, Bruin M, et al. Possible lower rate of chronic ITP after IVIG for acute childhood ITP an analysis from registry I of the Intercontinental Cooperative ITP Study Group (ICIS). Br J Haematol 2009;146:180-4. [Crossref] [PubMed]
- Schmidt DE, Wendtland Edslev P, Heitink-Pollé KMJ, et al. Age at diagnosis shapes the prognosis of childhood immune thrombocytopenia. medRxiv 2021. doi:
10.1101/2020.06.09.20125385 . - Schmidt DE, Heitink-Pollé KMJ, Mertens B, et al. Biological stratification of clinical disease courses in childhood immune thrombocytopenia. J Thromb Haemost 2021; Epub ahead of print. [Crossref] [PubMed]
- Higashide Y, Hori T, Yoto Y, et al. Predictive factors of response to IVIG in pediatric immune thrombocytopenic purpura. Pediatr Int 2018;60:357-61. [Crossref] [PubMed]
- Morimoto Y, Yoshida N, Kawashima N, et al. Identification of predictive factors for response to intravenous immunoglobulin treatment in children with immune thrombocytopenia. Int J Hematol 2014;99:597-602. [Crossref] [PubMed]
- Schmidt DE, Heitink-Pollé KMJ, Porcelijn L, et al. Anti-platelet antibodies in childhood immune thrombocytopenia: prevalence and prognostic implications. J Thromb Haemost 2020;18:1210-20. [Crossref] [PubMed]
- Fu L, Ma J, Cheng Z, et al. Platelet-specific antibodies and differences in their expression in childhood immune thrombocytopenic purpura predicts clinical progression. Pediatr Investig 2019;2:230-5. [Crossref] [PubMed]
- Donato H, Picón A, Martinez M, et al. Demographic data, natural history, and prognostic factors of idiopathic thrombocytopenic purpura in children: a multicentered study from Argentina. Pediatr Blood Cancer 2009;52:491-6. [Crossref] [PubMed]
- Revel-Vilk S, Yacobovich J, Frank S, et al. Age and duration of bleeding symptoms at diagnosis best predict resolution of childhood immune thrombocytopenia at 3, 6, and 12 months. J Pediatr 2013;163:1335-9.e1-2.
- Pagan JD, Kitaoka M, Anthony RM. Engineered sialylation of pathogenic antibodies in vivo attenuates autoimmune disease. Cell. 2018;172:564-77.e13. [Crossref] [PubMed]
- Floto RA, Clatworthy MR, Heilbronn KR, et al. Loss of function of a lupus-associated FcgammaRIIb polymorphism through exclusion from lipid rafts. Nat Med 2005;11:1056-8. [Crossref] [PubMed]
- Nagelkerke SQ, Schmidt DE, de Haas M, et al. Genetic variation in low-to-medium-affinity Fcγ receptors: functional consequences, disease associations, and opportunities for personalized medicine. Front Immunol 2019;10:2237. [Crossref] [PubMed]
- Mouzaki A, Theodoropoulou M, Gianakopoulos I, et al. Expression patterns of Th1 and Th2 cytokine genes in childhood idiopathic thrombocytopenic purpura (ITP) at presentation and their modulation by intravenous immunoglobulin G (IVIg) treatment: their role in prognosis. Blood 2002;100:1774-9. [Crossref] [PubMed]
- Go RS, Johnston KL, Bruden KC. The association between platelet autoantibody specificity and response to intravenous immunoglobulin G in the treatment of patients with immune thrombocytopenia. Haematologica 2007;92:283-4. [Crossref] [PubMed]
- Peng J, Ma SH, Liu J, et al. Association of autoantibody specificity and response to intravenous immunoglobulin G therapy in immune thrombocytopenia: a multicenter cohort study. J Thromb Haemost 2014;12:497-504. [Crossref] [PubMed]
- Breunis WB, van Mirre E, Bruin M, et al. Copy number variation of the activating FCGR2C gene predisposes to idiopathic thrombocytopenic purpura. Blood 2008;111:1029-38. [Crossref] [PubMed]
Cite this article as: Schmidt DE, Heitink-Pollé KMJ, Bruin MCA, de Haas M. Intravenous immunoglobulins (IVIg) in childhood immune thrombocytopenia: towards personalized medicine—a narrative review. Ann Blood 2021;6:3.


