The treatment of immune thrombocytopenia (ITP)—focus on thrombopoietin receptor agonists
Introduction
While thrombocytopenia is a common consultation for the hematologist, immune thrombocytopenia (ITP) still remains a reasonably uncommon explanation. ITP has been defined as an isolated platelet count less than 100,000/µL with no other explanation (1). It affects 2–4 per 100,000 individuals annually with an overall prevalence of about 10/100,000 individuals (2,3). It is probably much more common than we realize in that many patients with modest thrombocytopenia of 50,000–100,000/µL often go unreported and certainly many individuals with platelet counts between 100,000/µL and the standard normal of 150,000/µL have some autoimmune pathogenesis despite the finding that only 6.9% (95% CI: 4.0–12.0%) progress to ITP (4).
Although 18% of patients with platelet counts under 10,000/µL are asymptomatic (5), bleeding is the main risk in patients with ITP. Patients with ITP often tolerate a degree of thrombocytopenia which in other situations will result in major bleeding. This probably reflects the young, large, hyperfunctional platelets which is reflected in the normal bleeding time seen in ITP patients (6).
While bleeding remains the main concern in patients with ITP, two other clinical aspects of ITP warrant attention. The first is that many patients with ITP have a reduced health-related quality of life (HRQoL). While this is often hard to assess and quantify, there is a clear relationship between thrombocytopenia and fatigue (7,8). Although the underlying cause remains unclear, it has been suggested that circulating cytokines may be involved (9). Treatment of ITP improves the patient’s HRQoL including fatigue with thrombopoietin receptor agonists (TPO-RA) probably having a greater magnitude of effect (7,10). The second important aspect is that ITP is also a prothrombotic disorder, like all other disorders of increased platelet turnover (11-14). In a well-designed Danish registry study, 391 chronic ITP patients were compared with a matched reference cohort of 3,128 patients without ITP (13). Overall rates of venous thromboembolism were 5.32 (95% CI: 2.86–9.89) per 1,000 patient years for ITP patients versus 2.04 (95% CI: 1.45–2.87) per 1,000 years for the controls, a 2.65 (95% CI: 1.27–5.50) fold increase; thromboses occurred even at very low platelet counts. Sarpatwari (14) compared 1,070 ITP patients with 4,280 patients without ITP (matched for age, gender, primary care practice) in a UK General Practice Research Database and found that after a median of 47.6 months (range, 3.0–192.5 months) of follow-up ITP patients had adjusted hazard ratios of 1.58 (95% CI: 1.01–2.48), 1.37 (95% CI: 0.94–2.00), and 1.41 (95% CI: 1.04–1.91) for venous, arterial, and combined (arterial and venous) thromboembolic events, respectively, compared with controls. The event rates increased as the platelet count decreased. The pathophysiology of the thrombosis remains unclear.
The clinical course of ITP is often variable but a staging scheme for the disease has been created wherein the first 3 months are considered newly diagnosed ITP, months ≥3–12 are considered persistent ITP and after 12 months the disease is considered chronic ITP (1). This staging scheme is convenient for disease classification and may have some relationship to the underlying pathophysiology. In general, adult ITP patients have a very low rate of spontaneous remission without therapy estimated to be between 0.9% (15) and 10.3% (9/87) (16) whereas patients in the newly diagnosed and persistent phase may have a remission rate with therapy of up to 32% (17). Recent data also show that epitope spreading may be occurring as the disease progresses (18). There remains much interest as to whether more aggressive therapy with rituximab (19), recombinant thrombopoietin (20) or mycophenolate (MMF) (21) in the acute and persistent phase might mitigate the emergence of more chronic disease.
With the introduction of TPO–RA, rituximab and fostamatinib, therapeutic options for treating ITP have evolved from our prior standard care of corticosteroids and splenectomy. These have culminated in a number of new guidelines for treating ITP (22-25). In particular, I will rely upon the recent American Society of Hematology (ASH) Guidelines (22) and the International Consensus (IC) Group Report (23) in this review. In reference to these guidelines, it is important to note two things. The first is that guidelines are not mandates for therapy but as described by the WHO are “intended to assist providers and recipients of healthcare and other stakeholders to make informed decisions. A recommendation provides information about what policymakers, healthcare providers or patients should do” (26). The second is that despite the claim to be “evidence-based”, they are almost entirely expert opinion. For example, all 11 adult recommendations of the ASH Guidelines are “very low certainty of the evidence” and only 2 recommendations were “strong” (patients with platelet counts over 30,000/µL rarely require therapy, corticosteroids should not be used more than 6 weeks). There are simply not enough randomized controlled trials comparing head-to-head different treatment algorithms for ITP.
The purpose of this review is to help understand the diagnosis and treatment of adult ITP recognizing the limitations of these guidelines and available clinical data. In so doing, I will assess the available evidence and explain how I apply it in my clinical practice in over 2,000 adult ITP patients. In using the personal pronoun, it should be recognized that some of what I relate below is my own personal clinical practice which may not be fully “evidence-based”.
The pathophysiology of ITP
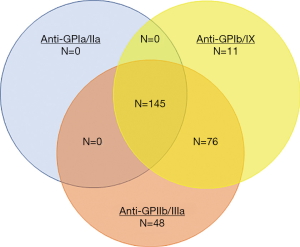
The pathophysiology of ITP has undergone much investigation in recent decades and is a disease of both increased platelet destruction as well as a disease of inappropriately low platelet production. The historical studies of Harrington (27) and others (28-31) showed that the infusion of plasma or blood products from patients with ITP caused a decline in platelet counts in healthy recipients with eventual identification that antiplatelet antibodies played a major role in this disorder (28,32,33). Indeed, modern methods detected antiplatelet antibody on the surface of 280 (69%) of 360 ITP patient platelet samples. Multiple antibodies against multiple antigens were common: of these 280, 145 (52%) had antibodies against all 3 major platelet antigens, GPIIb/IIIa, GPIb/IX, and GPIa/IIa; few had antibodies only against GPIb/IX (3.9%) or GPIa/IIa (0%) (Figure 1).
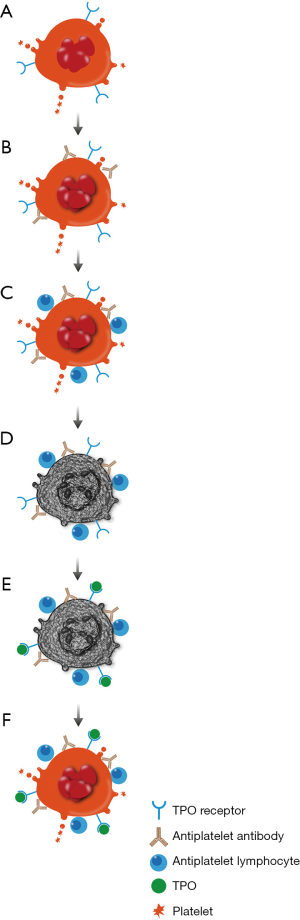
Other studies have shown that platelet production is inhibited to various degrees in patients with ITP. Platelet kinetic studies have shown a normal or reduced rate of platelet production from megakaryocytes (34,35). Indeed, the plasma from 12 of 18 ITP patients inhibited in vitro megakaryocyte growth by 26–95% (36). T cells may play a role directly in attacking platelets or megakaryocytes and alterations in T regulatory lymphocytes may also open a window for loss of immune tolerance (37). Megakaryocytes in the bone marrow have been shown to undergo apoptosis (38). Figure 2 summarizes these effects on megakaryocytes.
How to diagnose ITP
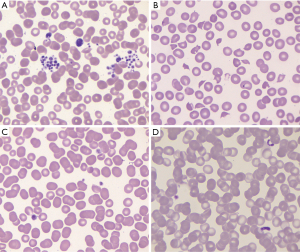
The diagnosis of ITP is a clinical diagnosis. By definition it is based upon the platelet count being less than 100,000/µL (1) usually with no other cytopenias or clear causation such as chronic liver disease. The first step in any thrombocytopenia evaluation is to perform a thorough review of the peripheral blood smear to assess for platelet clumping (pseudothrombocytopenia) (Figure 3A), to exclude other white cell abnormalities indicative of an underlying lymphoproliferative or myeloproliferative disorder, and especially, to exclude schistocytes (Figure 3B) indicative of a much more life-threatening thrombotic microangiopathy. Platelets in ITP patients are often large and well granulated, the “megathrombocyte“ (Figure 3C) described by Karpatkin (39), and are routinely associated with an increased mean platelet volume (MPV) (40); circulating “proplatelets” may also be seen (Figure 3D).
After review of the peripheral blood smear, our evaluation is then focused on distinguishing patients with primary ITP from those who have some other underlying condition and for whom the ITP is therefore secondary. While this may not alter how we treat the ITP, it is important to identify those patients with underlying lymphoproliferative disorders, HIV or hepatitis C infection. The initial evaluation should include testing for HIV, hepatitis C and routine chemistries including liver function tests. I do not routinely test for Helicobacter pylori except in patients from Japan or other countries where this may be a more common etiology for ITP; Helicobacter pylori infection is an uncommon cause of ITP in the United States (41). In addition, we routinely test for anti-cardiolipin antibodies, lupus anticoagulant, anti-thyroid peroxidase and antinuclear antibodies to help establish whether there is a potential risk for thrombosis and to help predict outcome. ITP patients commonly have an “autoimmune predilection” and we have shown that antithyroid peroxidase antibody positivity was associated with a lower probability of remission (OR, 0.26; 95% CI: 0.09–0.79; P=0.017); lupus anticoagulant positivity was associated with a higher rate of thrombosis (OR, 8.92; 95% CI: 1.94–40.95; P=0.005) as was antinuclear antibody positivity (P=0.001) (42). We also check the LDH and direct antiglobulin tests; the former correlates with disease activity (43) and the latter is done to exclude a possible Evans syndrome. Serum protein electrophoresis and quantitative immunoglobulin levels are performed to assess for immune deficiency syndromes.
The final evaluation of our ITP patients is to send a serum thrombopoietin level and a direct antiplatelet antibody test. The latter helps confirm the diagnosis particularly if the thrombopoietin level is in the normal range under 100 pg/mL (44). In patients with a thrombopoietin level over 500 pg/mL who have no antiplatelet antibody, we have concern about the underlying diagnosis of ITP. The thrombopoietin level is also helpful in predicting response to TPO receptor agonist. In general, the greater the TPO level rises above the upper limit of normal of 100 pg/mL, the lower the response to a TPO-RA. At these elevated levels of TPO, romiplostim appears to be more active than eltrombopag; using ROC analysis, TPO thresholds of ≤136 pg/mL for eltrombopag and ≤209 pg/mL for romiplostim optimally discriminated between responders and non-responders (45). At thrombopoietin levels greater than 400 pg/mL, less than 10% of patients respond to TPO-RA; in such patients one should query the underlying diagnosis and probably not use a TPO-RA.
To us the best confirmation of the diagnosis of ITP is a consistent response to basic ITP therapies such as corticosteroids or IVIG. We like to classify ITP patients as “responders” or “non-responders” to corticosteroid/IVIG with the latter usually more difficult to treat. Given the clinical nature of the diagnosis of ITP, in patients who demonstrate minimal response to corticosteroid/IVIG, the underlying diagnosis should be reconsidered. In line with all recent published guidelines (22,23), we have rarely needed to do bone marrow biopsies in patients with ITP and certainly do not mandate them in patients over 65. Bone marrow examination is only performed in patients for whom there is been no demonstrated response to standard ITP therapy such as IVIG and corticosteroids or in those with additional unexplained cytopenias.
Who requires treatment
Many patients with ITP do not require any therapy but do well simply with observation. In the minimally symptomatic patient with a platelet count over 20,000/µL and with no other risk factors, we will simply follow. Initially this means checking a platelet count every week or 2 for several months to assess stability. If patients remain in this range without dipping below 20,000/µL, we then check every several months for 1 year and by mutual agreement thereafter once or twice a year. Such observation patients are informed of the risks of bleeding and signs thereof. They are given an around-the-clock telephone number to call should symptoms arise and subsequent treatment be indicated by clinical symptoms or if the platelet count drops substantially below 20,000/µL. They also usually have standing orders for a CBC at a local laboratory and have a small supply of prednisone at home.
While the platelet count is a reasonable guide for treatment decisions, it is certainly not the only variable to be considered. Active bleeding is obviously a situation mandating therapy for patients with a platelet count under 30,000/µL. Unfortunately, the definition of “active” varies considerably but for us means having a drop in hematocrit or having hematuria, hematemesis, epistaxis (>20 minutes), or hematochezia; modest bruising or petechiae is not necessarily a reason to treat. Other risk factors to be taken into consideration are age, level of activity of the patient, need for procedures, need for anticoagulation. For example, our 20,000/µL platelet count threshold is in line with current guidelines but might be elevated to 30,000/µL in patients who require a single anticoagulant drug or to 50,000/µL in patients who need two forms of antithrombotic therapy such as aspirin and warfarin.
Whether to admit a patient to the hospital or not is often a difficult decision but is primarily based on symptoms and platelet count as well as our prior knowledge of that patient. Patients who are actively bleeding obviously require immediate care and hospital admission. In general, new patients presenting with platelet counts below 10,000/µL are admitted to the hospital if only to monitor platelet counts until stability is ascertained. The recent ASH Guidelines favored hospital admission for newly diagnosed patients with platelet counts under 20,000/µL (22). Patients who are well-known to us are occasionally admitted for active bleeding with most of their care being accomplished as an outpatient.
What is initial treatment of ITP
Corticosteroids are the standard initial treatment for patients with ITP worldwide. This is emphasized in all current guidelines with IVIG being added in cases of severe bleeding or need for procedures. Dexamethasone and prednisone are the standard corticosteroid regimens used in ITP. The recent meta-analysis of the several clinical trials comparing dexamethasone with prednisone (46) showed that at day 14 an overall response (platelet count over 30,000/µL) was obtained by 79% of patients receiving dexamethasone and 59% of those on prednisone, suggesting a faster relative response rate (RR) with dexamethasone (RR: 1.22, 95% CI: 1.00–1.49, P=0,048). However, at 6 months the overall response rate was no different [54% with dexamethasone versus 43% with prednisone (RR 1.16, 95% CI: 0.79–1.71; P=0.44).]
We rarely use dexamethasone for several reasons. Many of our patients are over 70 and have had untoward psychological effects on dexamethasone. Moreover, we have found that many patients who receive dexamethasone as initial therapy in the hospital for severe thrombocytopenia have a very encouraging rise (sometimes within days) but often require readmission to the hospital a week or two later because of the transient response. Our standard corticosteroid regimen is prednisone usually at a fixed dose of 60 mg a day with subsequent decrements by 10–20 mg a day tapering over the next 8 weeks. As recommended in current guidelines, it is always our goal to get most patients off prednisone within 8 weeks (22,23). There is a small subset of patients who do quite well at prednisone doses of 2.5 to 5 mg a day and for whom the long-term benefit of this simple therapy outweighs the very small complications of long-term steroid use at this dose (45).
Since we do not use corticosteroids for more than 8 weeks, we do not use any concurrent medications to mitigate bone mineralization problems but many patients are placed on an antacid regimen for GI protection. Only those who might be on prednisone 20 mg or greater for more than a month are placed on Pneumocystis carinii prophylaxis with either trimethoprim-sulfamethoxazole or atovaquone.
During initial therapy with prednisone, responders are discharged from the hospital at platelet counts stably above 10,000/mL and platelet counts are monitored once or twice a week through local laboratories. Prednisone doses are adjudicated by weekly phone call or electronic messaging.
Corticosteroids have several interesting effects in ITP. Prednisone reduces platelet clearance when given to volunteers receiving antiplatelet antibody from ITP patients, thought to be due to reducing phagocytosis by the reticuloendothelial system (31). But platelet kinetic studies show that while prednisone has no effect on the rate of platelet destruction in chronic ITP patients it markedly improves the rate of platelet production. After the administration of prednisone the rate of platelet production rose from 25±17×109 platelets/L/day up to 57±19×109 platelets/L/day (normal: 41±5×109 platelets/L/day) (35). Lastly there is a poorly understood interaction between platelets and endothelial cells. In thrombocytopenic patients the endothelium becomes thin and fenestrated with fewer gap junctions between endothelial cells (47,48). This may be the etiology of petechiae in thrombocytopenic patients. Soon after administrating prednisone, these endothelial defects are almost completely reversed, consistent with the clinical finding that petechiae often resolve well before the platelet count rises.
There is emerging data that suggests that upfront therapy may increase the rate of response and potentially mitigate the evolution to chronic disease.
- A meta-analysis of 5 studies (19) adding rituximab to standard of care in the newly diagnosed patients showed an increased rate of complete responses (platelets >100,000/µL; 47% versus 33%, P=0.002), but there was no difference in partial response (platelets >30,000/µL; 58% vs. 47%, P=0.11), bleeding (9.2% vs. 5.2%, P=0.44) or infections (20% vs. 1%, P=0.17). The authors concluded that “evidence for sustained responses beyond 6–12 months is limited.” The ASH Guidelines do not recommend upfront rituximab.
- Recently, a randomized prospective controlled study looked at the addition of 14 days of daily recombinant human thrombopoietin (rhTPO) to dexamethasone therapy in newly diagnosed ITP patients (20). The study showed that those receiving rhTPO had higher response rates at day 14 (89.0% vs. 66.7%, P<0.001), at month 6 (51.0% vs. 36.5%, P=0.02), and a slightly higher treatment-free remission rate thereafter (46.0% vs. 32.3%, P=0.043). However, rhTPO is licensed in few countries outside of China.
- The other new addition to the upfront therapies is the recent, as yet unpublished study by Bradbury (21) in which mycophenolate mofetil (MMF) was added to standard corticosteroid treatment. After a median follow-up of 18 months (12 months minimal follow-up) only 22% (11/59) of those on MMF compared with 44% (27/61) of those on corticosteroids alone experienced treatment failure (defined as a platelet count less than 30,000/µL and a need for second line therapy), a major reduction in progression (HR 0.41; 95% CI: 0.21–0.80, P=0.0064). This study is handicapped by the fact that only 44% of the corticosteroid arm showed treatment failure (usually this is about 80%) and the finding that those patients on MMF had a reduced HRQoL.
Other than corticosteroids, IVIG and in severe situations the early administration of a TPO-RA, we do not routinely use other therapy in the treatment of the newly diagnosed patient. The recent data on mycophenolate may alter this approach.
What if the patient becomes corticosteroid dependent or is a non-responder to corticosteroids
While over 80% of ITP patients initially respond to corticosteroids, probably only 20% maintain an adequate platelet count once corticosteroids are stopped (15); this rate is probably even lower currently given the reduced duration of time over which corticosteroids should be administered. As corticosteroids are tapered in our patients, when new bleeding arises or the platelet count drops below 20,000/µL, we usually hold the prednisone taper, reassess indications for therapy, and usually begin other medical therapy to maintain a hemostatic platelet count.
It is important to note that by truncating the duration of the corticosteroid course, we arrive earlier at the need to initiate other medical therapies despite the fact that except for romiplostim, they are licensed only for chronic ITP. This has been well recognized by both the ASH Guidelines and IC Report where the decision to begin alternative medical therapy is often at the end of the third month from disease diagnosis. Excellent clinical data exist [mostly with romiplostim (49) and eltrombopag (50)] showing that the TPO-RA are as effective in early ITP (under 1 year duration) as they are in chronic ITP (≥1 year duration).
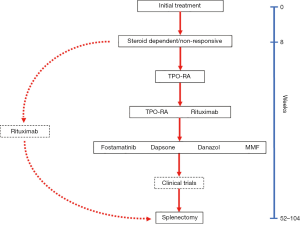
Since I eschew the concept of lines of therapy, recognizing that many “first-line” therapies such as prednisone are often used much later in the disease course (and some later line therapies like TPO-RA may be used in initial therapy), I will stick with the concept of “subsequent” or “alternative“ medical therapies which are available to treat ITP. As described in Figure 4, our most common subsequent medical therapy is a TPO-RA given their high rates of response, ease of availability and low rates of adverse effects; TPO-RA are used in preference over rituximab given the recent ASH Guidelines (22). In some patients with other underlying autoimmune disorders, increased risk of thrombosis, or concurrent hemolytic anemia we will instead use rituximab despite its lower response rate and shorter duration of effect.
The TPO-RA have markedly changed the approach to treating ITP. In clinical trials they have shown response rates as high as 93%, onset of action often in 7 to 14 days, minimal side effects, a sustained duration of effect (51,52) and improved HRQoL (7,10,17,53-58). The abundant clinical trials data have been well summarized in excellent reviews for romiplostim (59,60), eltrombopag (61,62) and avatrombopag (63) and I will not replicate those efforts here. Instead, I would like to review the available TPO-RA in the context of how we employ them in our current practice.
Our general practice is to start a TPO-RA and then gradually taper the patient off the remaining corticosteroids over the next 4 weeks. Most patients can be subsequently maintained on the TPO-RA alone. A few require a small daily dose of corticosteroids, usually prednisone 2.5–5 mg, which provides excellent synergy with TPO-RA (45). Our choice of TPO-RA largely depends upon the clinical situation and the patient’s insurance. In hospitalized patients, we usually start romiplostim and continue as an outpatient as long as the patient is comfortable with the regimen, eventually switching some to an oral agent. Although not commonly self-administered at home in the United States, most of our patients can administer romiplostim at home after discussion with their insurer and training the patient. Home administration has been shown to be as effective as that by a healthcare provider (64). In non-hospitalized patients with appropriate insurance, we prefer to start an oral TPO-RA either avatrombopag or eltrombopag. As discussed below, we currently favor avatrombopag given its single pill size, lack of need to check liver function tests, lack of dietary restrictions (65) and increased potency (66).
For all patients on TPO-RA our goal is a platelet count of 50,000–250,000/µL; as discussed further below, patients with platelet counts consistently over 200,000/µL should be assessed for remission by planned dose reduction. Since thrombocytopenia is the major risk in ITP patients, we do not find it appropriate to hold any TPO-RA if the platelet count rises over 400,000/µL as suggested in the prescribing information. Holding a TPO-RA can precipitate a rebound thrombocytopenia where the platelet count may drop to life-threatening levels a week or two later. In one romiplostim study, 13% of subjects were found to drop the platelet count below their prior baseline levels when romiplostim was withheld (57). Instead, we simply decrease the TPO-RA dose by 50–66%. It is also important to note that with the TPO-RA there may be what I call a “first dose effect“ where the platelet count rises considerably 1 or 2 weeks after the first dose only to decline later to lower levels with no change in drug dose (54). This is probably due to the effect of the TPO-RA on rapidly reversing the apoptosis that is occurring in the expanded pool of bone marrow megakaryocytes which results in a surge of platelet production as that cohort of megakaryocytes is processed into functional platelets (Figure 2). This rise in platelet count should not result in a dose alteration in the first 2 weeks of treatment since the platelet count will usually decline in subsequent weeks if the initial dose is maintained. Furthermore, self-limited events like infection may drop the platelet count transiently while a corticosteroid injection may raise it transiently; in those situations we do not modify the TPO-RA dose. Once a patient attains a stable target platelet count on a stable dose of TPO-RA, platelet counts are checked monthly. Frequent platelet count measurements and frequent dosage changes often cause cycling of the platelet count which is distressing to both the patient and the caregiver. For some patients a wider platelet count target of 50,000–400,000/µL needs to be considered. If a responding patient needs to come off therapy, dosage should be gradually weaned over several weeks with close observation of platelet counts to avoid producing a rebound thrombocytopenia.
There are 4 major concerns in using TPO-RA in ITP patients:
- Rebound thrombocytopenia occurs in some patients in whom TPO-RA are abruptly stopped. This has been discussed above.
- Bone marrow fibrosis has been shown to occur in animals given recombinant thrombopoietin or romiplostim (67) and was completely reversed upon drug discontinuation (68). In a 3-year prospective bone marrow examination study in 169 romiplostim-treated ITP patients, only 7 (5.3%) increased their Bauermeister reticulin score by 2 grades and only 2 (1.5%) developed collagen fibrosis. Although all 9 patients experienced no hematologic problems, the reticulin disappeared in the 3 patients who stopped treatment and had a repeat bone marrow biopsy (69). A 2-year prospective study in 162 eltrombopag-treated patients, showed only a small increase in reticulin staining (MF-1 on the WHO scale of MF-0 to MF-3) from 6% prior to treatment to 11% at 2 years; only 6/93 (6.3%) developed collagen fibrosis over 2 years and it was as likely to disappear as to persist with drug continuation. Neither study suggested serial bone marrow biopsies need to be done on ITP patients treated with TPO–RA.
- Anti-TPO antibodies developed in healthy subjects given a first generation recombinant thrombopoietin, rHuPEG–MGDF; antibodies formed against the recombinant molecule and cross-reacted with endogenous thrombopoietin creating thrombocytopenia (70). We have recently analyzed samples from 958 patients in 13 clinical trials with romiplostim. At baseline, 3.7% had antibodies that bound with romiplostim and 0.1% had antibodies that neutralized romiplostim; after treatment with romiplostim, 8.3% and 0.4% of patients had a positive test for binding or neutralizing antibodies, respectively. None of these patients experienced a clinical loss of platelet count response and no patients had antibodies that neutralized endogenous TPO (71). In post-marketing analysis of 184 samples submitted to assess the presence of neutralizing antibodies in patients who have had an insufficient response or loss of response to romiplostim, only 1 patient was found to have a neutralizing antibody against the drug. Antidrug antibodies have not formed against either avatrombopag or eltrombopag.
- Increased thrombosis has been a concern in all TPO-RA trials given the underlying hypercoagulable state associated with ITP. Available data from prospective, placebo-controlled studies with romiplostim show that the overall rate of thromboembolic events was the same in those treated with romiplostim [n=994; 5.5/100 patient-years (pt-yr); 95% CI: 4.4–6.8] compared with those on standard of care (SOC)/placebo [n=138; 5.5/100 pt-yr; 95% CI: 2.0–11.9] (72). Serious thrombotic/thromboembolic events were also no different but were handicapped by very low numbers of events overall: romiplostim with 4.0/100 pt-yr (95% CI: 3.1–5.2) versus placebo/SOC with 1.8/100 pt-yr (95% CI: 0.2–6.6). Our analysis of 9 published trials with romiplostim showed that thrombotic events occurred 3 times more frequently in those over age 60 than in younger patients but were unrelated to the platelet count (49). Similar low rates of thrombosis were seen in eltrombopag ITP trials with again no relation to the platelet count (10). However in studies with eltrombopag in chronic hepatitis C infection, thromboembolic events rates were higher in those treated with eltrombopag (5.8/100 pt-yr) compared with those in the placebo arm (1.9/100 pt-yr). Our own experience seems to be that there are more thromboembolic events occurring in ITP patients since we started using TPO-RA. As suggested by others, we do not think that this risk has been fully assessed for ITP patients (62). Most clinical trials excluded patients with a history of active or chronic thromboembolic disease. In our practice, we exercise considerable caution in our ITP patients who have a history of thromboembolism, antiphospholipid antibody syndrome or active autoimmune diseases. This risk factor clearly requires more evaluation.
It is important to review the individual nuances of using each of the TPO-RA (Table 1):
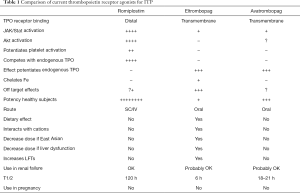
Full table
- Romiplostim is FDA approved for the treatment of thrombocytopenia in adult patients with ITP who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy with the restriction for chronic disease having been recently removed (73). The recommended starting dose is 1 mcg/kg given weekly with dose escalation by 1 mcg/kg every week up to a maximum of 10 mcg/kg until a platelet count over 50,000/µL is obtained (73). That is an inadequate starting dose and too slow a dose escalation. We usually start at either 3 mcg/kg or 5 mcg/kg weekly with a dose escalation of 2 mcg/kg every 1–2 weeks until the target platelet count is attained (54). The average (SD) dose of romiplostim in an open label phase 3 trial in adults was 3.9 (2.1) mcg/kg (54). In an analysis of 1,111 patients in multiple phase III trials, the mean (SD) weekly romiplostim dose was 4.4 (3.4) mcg/kg in nonsplenectomized patients and slightly higher at 4.9 (4.0) mcg/kg for splenectomized patients (74). Romiplostim has a half-life of about 120 hours (75) and is optimally administered every week (57); less frequent dosing often results in great fluctuations in platelet counts. Finally, romiplostim is supplied in vials labeled as 125, 250 and 500 mcg but contain an overfill amount of 230, 375, and 625 mcg, respectively, that can be used to avoid waste.
- Eltrombopag is FDA approved for the treatment of thrombocytopenia in adult patients with chronic immune thrombocytopenia (ITP) who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy (76). This drug is available in pill sizes of 12.5, 25, 50 and 75 mg and oral suspensions of 12.5 and 25 mg. Per the prescribing information, the standard starting dose is 50 mg/day except in some patients of Asian ancestry or those with liver dysfunction in whom lower doses are suggested. The starting dose is then titrated up to the maximum of 75 mg a day or down to lower doses to achieve target platelet counts. We find this to be a very arduous scheme often necessitating multiple prescriptions for different doses to be written and vetted through insurance. Instead, we have developed a different program for eltrombopag in which all patients (except those with liver dysfunction or of Asian ancestry) are started on 75 mg once a day (77). When an adequate platelet count is obtained, we will simply decrease the dosing frequency from daily to every second or third day to obtain an adequate platelet count, consistent with the pharmacodynamics of this drug (77-80). Over two thirds of our eltrombopag patients are currently receiving eltrombopag only 2 or 3 times a week (77). Abnormal liver function tests probably occur in about 10% of all patients, are mild in nature, and usually resolve even with continuation of the drug (10). The concern that eltrombopag promotes cataracts has been put to rest recently (81). Eltrombopag is a potent iron chelator and rare patients might develop iron deficiency (82). The major impediment to using eltrombopag is the dietary restrictions; calcium and other cations neutralize the drug and it must be ingested at least 2 hours before and 4 hours after any meal, medication or dietary supplement containing such items (76). This seemingly modest restriction often presents a major barrier to patients not able to comply with the diet.
- Avatrombopag is FDA approved for adult patients with chronic ITP who have had an insufficient response to a previous treatment (83). It is available only as a 20 mg tablet and per the prescribing information treatment is initiated at a dose of 20 mg daily with subsequent doses titrated per the platelet count from dose level 1 (20 mg weekly) up to dose level 6 (40 mg daily) (83). There are no dietary restrictions in its use (prescribing information states it should be taken with food), no potential for significant liver function abnormalities requiring monitoring and it does not appear to chelate iron (84-86). In the initial clinical study, a platelet count response over 50,000/µL was seen in 80% of those receiving a dose of 20 mg daily and in none of those on placebo; in an extension study, a little over half of the patients were able to maintain that response on over 75% of their subsequent visits (56). In the small pivotal phase III study, patients randomized to avatrombopag attained a platelet count over 50,000/µL on a median of 12.4 weeks of the 24-week study versus 0 weeks for those on placebo (P=0.0001) (87). In both studies a platelet count over 50,000/µL was obtained by the eighth day of therapy in over two thirds of patients; while this appears to be a more rapid response then with the other TPO-RA, it may reflect a difference in the starting dose used in these clinical trials. Our only major reservation with avatrombopag is the relative paucity of data from clinical studies or real-world evidence
What to do if a patient fails a TPO-RA
Although TPO-RA have been the backbone of our treatment algorithm, some patients have minimal or no response, develop side effects of the treatment (e.g., headaches most commonly), or lack insurance coverage. In some patients with an inadequate response to TPO-RA, as mentioned above, a low dose of prednisone may synergize greatly and provide an adequate platelet count. In 50-80% of others, switching to another TPO-RA will be successful (88,89).
For those failing TPO-RA or not able to receive it, there are a number of other medical options.
Rituximab is usually our next medical therapy although not FDA approved for this indication. In adults this provides an overall response rate of about 57% declining to about 38% at year 1 and 21% by year 5 (90). When responding patients lose their response, retreatment often works. There is some suggestion that women under age 40 obtain more long-term remissions at 48 months and 72 months (47% and 47%, respectively) then other patients (33% and 25%, respectively) (91), but this has not been supported by all studies (92). While our standard dose of rituximab is 375 mg/m2 weekly for 4 weeks, 1000 mg given every 2 weeks has been shown to be equally effective (93); even lower doses show effect (94). The major adverse events with rituximab are well-known and include infusion reactions and delayed neutropenia (95). Infectious complications are surprisingly rare in ITP patients; progressive multifocal leukoencephalopathy (PML) is exceptionally rare and I am aware of only 2 such patients, one of which was mine who had received extensive immunosuppression for other autoimmune diseases (96). The major complication of rituximab is that it prevents successful vaccination for at least 6 months (97-99).
Fostamatinib is a newer agent with a unique mechanism of action. It inhibits syk kinase thereby reducing macrophage destruction of platelets. In the two major studies with this agent, a stable platelet count response (at least 4 of 6 biweekly platelet counts >50,000/µL in the absence of rescue medication on study weeks 14–24) occurred in 18% of fostamatinib-treated patients and in 2% of those on placebo, P=0.0003 (100). However, the overall response (at least one platelet count >50,000/µL in the first 12 weeks of the study) was 43% for fostamatinib and 14% for placebo with a median time to the first response of about 2 weeks. This 24-week study was markedly handicapped by the ability of patients on both arms to enter the open label phase of the study early; 60% of the fostamatinib patients and 84% of placebo patients left the randomized portion of the study after 12 weeks due to lack of response. A recent post-hoc assessment of these 2 placebo-controlled studies and an extension phase study showed a response rate (at least 1 platelet count greater >50,000/µL) in 54% (79/145) of subjects but in 78% (25/32) of those treated second-line versus 48% (54/113) if third-line or higher. Platelet counts “consistently” >50,000/µL were seen in 50% (16/32) of second line patients and only 30% (34/113) of those in third or higher lines of treatment (101). These modest response rates and the rather high rates of diarrhea, hypertension and nausea have limited our enthusiasm for this drug.
Mycophenolate mofetil is a useful agent in ITP but has not been well studied until recently. MMF is a product of Penicillium fungi that was discovered in 1893 based upon its antibacterial properties. It is converted to its active form, mycophenolic acid, which then inhibits inosine monophosphate dehydrogenase, an enzyme necessary for purine synthesis primarily in lymphocytes and thereby inhibits DNA synthesis of T and B cells. The recent unpublished data by Bradbury (21) suggest a significant effect in newly-diagnosed ITP (vide supra). There are no randomized, prospective studies in other settings but a large number of retrospective analyses showed that doses of 1,000–2,000 mg/day provided response rates of 40–80% with complete response rates of 30–50% in “refractory” patients (102-105). Adverse events were uncommon and the drug was well-tolerated. We commonly employ MMF as a “steroid sparing“ treatment in patients who have demonstrated a prednisone response. We start at a dose of 500 mg twice a day and if tolerated increase that to 1,000 mg twice a day; if no response is seen after 2 months, the drug is discontinued.
Dapsone has a long history of use in chronic ITP. It is yet another steroid sparing option in responsive ITP patients. Although its exact mechanism of action is unknown, it is a strong oxidant and causes a limited degree of red cell hemolysis which may block macrophage consumption of platelets. In prospective studies, response rates of 50% at one month were reported with durable response rates of 21% (106-110). We have found that a dose of 100 mg a day often provides an additional 20,000/µL increase in platelet count in many patients with marginal response to other agents. Care needs to be taken to make certain that patients do not have glucose-6-phosphate dehydrogenase deficiency since this drug may cause rapid red cell hemolysis (111).
Danazol is another steroid sparing medication like dapsone and MMF which is helpful in some ITP patients. It is a modified steroid molecule that binds to many steroid receptors including androgen and glucocorticoid receptors with modest effect. An oral dose of 200–800 mg/day works slowly to provide a response rate of 38% at 1 month and a durable response rate of 57% (112-118). Its androgenic properties are not welcome in female patients and it may elevate liver function tests in others.
What if the patient fails multiple medical therapies
Refractory ITP has been defined as patient with failed splenectomy (1). This definition is certainly outdated and needs to be replaced since many patients will never accept splenectomy as an option. Our concept of “refractory” is a patient who has failed two or more medical therapies after treatment with corticosteroids/IVIG whether splenectomized or not. Recent studies have shown that the more lines of therapy a patient has failed, the more “refractory” the patient is to subsequent medical therapy (101). Furthermore, we profile refractory patients as to whether they have ever responded to anything especially corticosteroids/IVIG or whether they have had no response to anything so far. The former we regarded as being “responsive” patients while the latter “nonresponsive patients” we think require further assessment as to whether they actually have ITP and reassess the indications for therapy. Most such non-responsive patients require a bone marrow examination and probably should be reassessed as to the causation the thrombocytopenia. While additional medical ITP therapies can be entertained, this non-responsive population clearly needs the indications for therapy justified.
In refractory patients, we will always reassess the need for therapy. Many patients can live a reasonably normal life at platelet counts under 20,000/µL with only occasional need for corticosteroids or antifibrinolytic agents. If therapy is required, we will often use combinations of treatments such as TPO-RA along with MMF, dapsone, danazol, or low-dose prednisone. If rituximab has not been used, it is highly recommended. Although combinations of two different TPO-RA or a TPO-RA along with fostamatinib can be considered, we find the expense of such combined therapies to be prohibitive. In emergency situations, vincristine can be added. Clinically stable patients should be assessed for clinical trials or splenectomy.
What is the role of clinical trials
Refractory patients and sometimes patients earlier in the disease course might benefit from exposure to novel agents that are not yet approved by regulatory bodies. There are a wide variety of new agents being developed (Table 2) which include inhibitors of the neonatal Fc receptor (119), Bruton kinase inhibitors, anti-CD38 molecules, and immunoproteasome inhibitors.
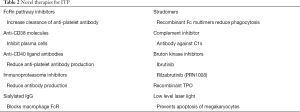
Full table
When is splenectomy appropriate
We rarely recommend splenectomy in adult patients except as a last measure or because of patient preference. Patient preference is important and discussed further below. We have found that patients in a number of critical occupations including military staff and airline pilots might have work prohibitions if they have the possibility of worsening of ITP. Such patients as well as those who are fatigued from medical therapy should be assessed for splenectomy. As mentioned above, it is not easy to predict the response rate for splenectomy. The response rate of about 80% (120,121) in older studies is strongly biased with patients who had splenectomy in the first months of their disease; these newly diagnosed or persistent patients might have done equally well had they been given medical therapy. In general, we think it is important when splenectomy is raised to consider the advantages and disadvantages of the procedure recognizing that once the spleen is removed, patients are left with a persistent hematologic and immune defect, i.e., asplenia, which should not be considered a treatment-free state. Per the recent ASH Guidelines, we usually recommend a course of rituximab prior to the procedure hoping to provide a nonsurgical option (Figure 4). Although radiolabeled platelet spleen scans have been suggested to guide treatment (122), they are not currently available in North America.
Does ITP ever go into remission
A key aspect in caring for ITP patients is to have a mutual understanding of the terms “response”, “remission” and “cure”. To me, a cure of ITP is the complete absence of any disease manifestations or therapy for at least 5 years, normal platelet count, and if available, no detectable antiplatelet antibody. After treatment, only 10/228 (4.3%) of our patients met this strict criteria (18).
Most of the time we are talking about response and remission. Most ITP patients do well and have a response to some therapy and many will come off therapy completely. Data by Sailer (123) showed that in patients not undergoing splenectomy, 61% attained a platelet count off therapy over 100,000/µL and 86% over 30,000/µL by year 5 from diagnosis. Recent data showed that approximately 32% of patients diagnosed in the first months of ITP and treated with romiplostim achieved a remission off therapy by 1 year (17). We have shown that 12/43 (20%) of patients with chronic ITP treated with a TPO receptor agonist for more than 6 months come off that therapy with most maintaining a normal platelet count (124). There is considerable interest as to whether treatment itself modifies the disease course (125) rather than simply providing a hemostatic platelet count which allows time for the underlying autoimmune process to mitigate.
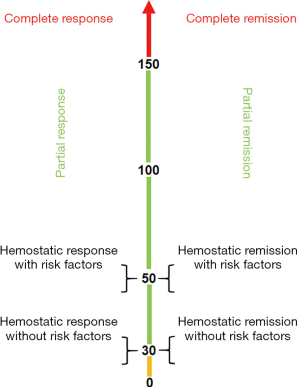
I would like to suggest that the definitions of response and remission should be reassessed. As proposed in Figure 5, the categories remain a function of the platelet count: a hemostatic response/remission is a platelet count ≥30,000/µL in those without risk factors and ≥50,000/µL in those with risk factors (such as anticoagulation, age, prior bleeding events); a partial response/remission is a platelet count greater than 30,000/µL (and 20,000/µL over baseline) up to a platelet count of 149,999/µL; a complete response/remission is a platelet count ≥150,000/µL. The main distinction between response and remission is a time component which I propose to be 6 months. In addition, each of these response/remission categories needs to be modified as to whether it is being maintained by some therapy or maintenance-free.
Let me suggest some examples. A patient who has undergone a splenectomy and now has a platelet count of 175,000/µL for over 6 months is considered to have a maintained complete remission. A patient who has been given rituximab and now has a platelet count of 165,000/µL for 7 months has a maintenance-free complete remission. A patient who is on a TPO-RA and now has a platelet count of 45,000/µL for 3 months has a maintained partial response.
What is the role of the patient in this process
The role of the patient in choosing the ITP therapy has been emphasized in recent guidelines (22,23). Both emphasized the need to include patient “values and preferences” in decision making. However, neither guideline tells what is meant by “values and preferences”. In Table 3, I have listed those which I commonly use. Over time of the disease, these values and preferences may vary considerably as does the control of the decision-making process between doctor and patient. The frightened, newly diagnosed patient has different preferences than the more experienced later ITP patient. The newly diagnosed patient is often dependent solely upon the physician for making strong recommendations and will invariably choose a medical therapy over surgery. Later in the disease course, the balance of control shifts and the patient’s values and preferences and quality of life play a greater role in treatment decisions than just the worry about the platelet count.

Full table
In treating patients who are more complicated and more refractory, decisions about patient safety at low platelet counts and treatment goals need considerable attention. What I have found to be an important tool is to go to the microscope and show the patient their platelets. For those who are living at a reasonably low platelet count of say 10,000–15,000/µL, it is reassuring to show them that their platelets are large and hypergranular and that this is the justification for why I have felt comfortable not encouraging more aggressive treatments.
Summary
It has been my attempt to provide an overview of the clinical care of the adult patient with ITP. In so doing I have tried to substantiate our clinical practice in caring for this patient population with the available research studies and guidelines. What is described above is our approach to the typical ITP patient at different potential stages in their disease. In making these recommendations I am certainly cognizant of the fact that not all drugs are available to all patients even in the United States. The expense and co-pays for many therapies such as TPO-RA are often prohibitive to the approach we have had the luxury of using in recent years. I have also had to ignore many other aspects of treating the ITP population such as ITP patients with pregnancy, secondary thrombocytopenia such as those with CLL or hepatitis C, those on anticoagulants, those who have had a thrombotic event and pediatric patients.
My overall treatment recommendations in ITP are as follows:
- Many ITP patients do not need treatment;
- Initial treatment is corticosteroids, preferably prednisone, and if severe bleeding, IVIG;
- Splenectomy works but should be delayed at least 12 to 24 months. Patients should be informed of the increased rate of venous thromboembolism and infection and should be appropriately vaccinated before undergoing surgery;
- Not all adult ITP patients will become/remain chronic;
- Give medical therapy a chance before splenectomy;
- Rituximab occasionally gives long-term treatment-free response;
- TPO-RA are highly effective;
- Low rate of adverse effects;
- Improve Health-related Quality of Life;
- May not need to “be forever”; over half come off therapy within 2 years;
- Fostamatinib may be considered in more refractory cases;
- Don’t forget danazol, azathioprine, dapsone, mycophenolate, cyclosporine;
- A large number of very promising new ITP treatments (Bruton kinase inhibitors, neonatal Fc receptor inhibitors, anti-CD 38 antibodies) are being developed and patients should be offered access to these trials.
Acknowledgments
The views expressed in this review have been formulated over the past 40 years by interactions with my ITP patients, subjects on my ITP trials and my many colleagues working in this area. To my patients, I owe my thanks for always allowing me to help improve their lives and share their fears. To my clinical trial subjects, I am grateful for their persistence and enthusiasm to try novel therapies whether subsequently effective and not. Finally, I am indebted to my many colleagues including Hanny Al-Samkari, James Bussel, Douglas Cines, Nichola Cooper, Terry Gernsheimer, Waleed Ghanima, Bertrand Godeau, Craig Kessler, Howard Liebman, Mark Michel, Adrian Newland, Drew Provan and John Semple who have provided challenging ideas, interesting questions and long-lasting friendships. I thank Dr. Drew Provan for helping with the artistry in Figure 2.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (John W. Semple and Rick Kapur) for the series “Treatment of Immune Thrombocytopenia (ITP)” published in Annals of Blood. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob-21-23). The series “Treatment of Immune Thrombocytopenia (ITP)” was commissioned by the editorial office without any funding or sponsorship. Dr. Kuter reports other from Protalex, personal fees and other from Bristol-Myers Squibb, personal fees and other from Rigel, personal fees and other from Bioverativ, other from Agios, personal fees and other from Syntimmune, personal fees and other from Principia, other from Alnylam, personal fees from ONO, personal fees from Pfizer, personal fees from 3SBios, personal fees from Eisai, personal fees from GlaxoSmithKline, personal fees from Genzyme, personal fees from Shire, personal fees and non-financial support from Amgen, personal fees from Shionogi, personal fees from MedImmune, personal fees and other from Novartis, personal fees from Alexion, personal fees and other from Argenx, personal fees from Zafgen, personal fees from Fujifilm, personal fees from Kyowa Kirin, personal fees and other from Takeda, personal fees from Platelet Disorders Support Group, personal fees and other from UCB, personal fees and other from Immunovant, outside the submitted work. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood 2009;113:2386-93. [Crossref] [PubMed]
- Christiansen CF, Bahmanyar S, Ghanima W, et al. Chronic immune thrombocytopenia in Denmark, Sweden and Norway: The Nordic Country Patient Registry for Romiplostim. EClinicalMedicine 2019;14:80-7. [Crossref] [PubMed]
- Schoonen WM, Kucera G, Coalson J, et al. Epidemiology of immune thrombocytopenic purpura in the General Practice Research Database. Br J Haematol 2009;145:235-44. [Crossref] [PubMed]
- Stasi R, Amadori S, Osborn J, et al. Long-term outcome of otherwise healthy individuals with incidentally discovered borderline thrombocytopenia. PLoS Med 2006;3:e24 [Crossref] [PubMed]
- Neylon AJ, Saunders PW, Howard MR, et al. Clinically significant newly presenting autoimmune thrombocytopenic purpura in adults: a prospective study of a population-based cohort of 245 patients. Br J Haematol 2003;122:966-74. [Crossref] [PubMed]
- Harker LA, Slichter SJ. The bleeding time as a screening test for evaluation of platelet function. N Engl J Med 1972;287:155-9. [Crossref] [PubMed]
- Kuter DJ, Mathias SD, Rummel M, et al. Health-related quality of life in nonsplenectomized immune thrombocytopenia patients receiving romiplostim or medical standard of care. Am J Hematol 2012;87:558-61. [Crossref] [PubMed]
- Newton JL, Reese JA, Watson SI, et al. Fatigue in adult patients with primary immune thrombocytopenia. Eur J Haematol 2011;86:420-9. [Crossref] [PubMed]
- Gudbrandsdottir S, Ghanima W, Nielsen CH, et al. Effect of thrombopoietin-receptor agonists on circulating cytokine and chemokine levels in patients with primary immune thrombocytopenia (ITP). Platelets 2017;28:478-83. [Crossref] [PubMed]
- Cheng G, Saleh MN, Marcher C, et al. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6-month, randomised, phase 3 study. Lancet 2011;377:393-402. [Crossref] [PubMed]
- Aledort LM, Hayward CP, Chen MG, et al. Prospective screening of 205 patients with ITP, including diagnosis, serological markers, and the relationship between platelet counts, endogenous thrombopoietin, and circulating antithrombopoietin antibodies. Am J Hematol 2004;76:205-13. [Crossref] [PubMed]
- Bennett I, Forssen U, Enger V, et al. Risk of thrombotic events among patients with chronic idiopathic thrombocytopenia purpura (ITP). Haematologia 2008;93:125.
- Severinsen MT, Engebjerg MC, Farkas DK, et al. Risk of venous thromboembolism in patients with primary chronic immune thrombocytopenia: a Danish population-based cohort study. Br J Haematol 2011;152:360-2. [Crossref] [PubMed]
- Sarpatwari A, Bennett D, Logie JW, et al. Thromboembolic events among adult patients with primary immune thrombocytopenia in the United Kingdom General Practice Research Database. Haematologica 2010;95:1167-75. [Crossref] [PubMed]
- Pizzuto J, Ambriz R. Therapeutic experience on 934 adults with idiopathic thrombocytopenic purpura: Multicentric Trial of the Cooperative Latin American group on Hemostasis and Thrombosis. Blood 1984;64:1179-83. [Crossref] [PubMed]
- Stasi R, Stipa E, Masi M, et al. Long-term observation of 208 adults with chronic idiopathic thrombocytopenic purpura. Am J Med 1995;98:436-42. [Crossref] [PubMed]
- Newland A, Godeau B, Priego V, et al. Remission and platelet responses with romiplostim in primary immune thrombocytopenia: final results from a phase 2 study. Br J Haematol 2016;172:262-73. [Crossref] [PubMed]
- Al-Samkari H, Rosovsky RP, Karp Leaf RS, et al. A modern reassessment of glycoprotein-specific direct platelet autoantibody testing in immune thrombocytopenia. Blood Adv 2020;4:9-18. [Crossref] [PubMed]
- Chugh S, Darvish-Kazem S, Lim W, et al. Rituximab plus standard of care for treatment of primary immune thrombocytopenia: a systematic review and meta-analysis. Lancet Haematol 2015;2:e75-81. [Crossref] [PubMed]
- Yu Y, Wang M, Hou Y, et al. High-dose dexamethasone plus recombinant human thrombopoietin vs high-dose dexamethasone alone as frontline treatment for newly diagnosed adult primary immune thrombocytopenia: A prospective, multicenter, randomized trial. Am J Hematol 2020;95:1542-52. [Crossref] [PubMed]
- Bradbury CA GR, Pell J, Breheny K, Kandiyali R, Ingram J, Thomas I, Bagot C, Hill Q. HaemSTAR Collaborators, Cooper N. LBA-2 A Multicentre Randomised Trial of First Line Treatment Pathways for Newly Diagnosed Immune Thrombocytopenia: Standard Steroid Treatment Versus Combined Steroid and Mycophenolate. the Flight Trial. Blood 2020;136.
- Neunert C, Terrell DR, Arnold DM, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv 2019;3:3829-66. [Crossref] [PubMed]
- Provan D, Arnold DM, Bussel JB, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv 2019;3:3780-817. [Crossref] [PubMed]
- Matzdorff A, Meyer O, Ostermann H, et al. Immune Thrombocytopenia - Current Diagnostics and Therapy: Recommendations of a Joint Working Group of DGHO, OGHO, SGH, GPOH, and DGTI. Oncol Res Treat 2018;41:1-30. [Crossref] [PubMed]
- Kashiwagi H, Kuwana M, Hato T, et al. Reference guide for management of adult immune thrombocytopenia in Japan: 2019 Revision. Int J Hematol 2020;111:329-51. [Crossref] [PubMed]
WHO Handbook for Guidelines Development 2012 . Available online: https://apps.who.int/iris/bitstream/handle/10665/75146/9789241548441_eng.pdf- Harrington WJ, Minnich V, Hollingsworth JW, et al. Demonstration of a thrombocytopenic factor in the blood of patients with thrombocytopenic purpura. J Lab Clin Med 1951;38:1-10. [PubMed]
- Shulman NR, Marder VJ, Aledort LM, et al. Isoantibodies against Platelets and Leukocytes. Bibl Haematol 1964;19:439-47. [PubMed]
- Shulman NR, Marder VJ, Weinrach RS. Comparison of Immunologic and Idiopathic Thrombocytopenia. Trans Assoc Am Physicians 1964;77:65-78. [PubMed]
- Shulman NR, Marder VJ, Weinrach RS. Similarities between known antiplatelet antibodies and the factor responsible for thrombocytopenia in idiopathic purpura. Physiologic, serologic and isotopic studies. Ann N Y Acad Sci 1965;124:499-542. [Crossref] [PubMed]
- Shulman NR, Weinrach RS, Libre EP, et al. The role of the reticuloendothelial system in the pathogenesis of idiopathic thrombocytopenic purpura. Trans Assoc Am Physicians 1965;78:374-90. [PubMed]
- Shulman NR, Aster RH, Leitner A, et al. Immunoreactions Involving Platelets. V. Post-Transfusion Purpura Due to a Complement-Fixing Antibody against a Genetically Controlled Platelet Antigen. A Proposed Mechanism for Thrombocytopenia and Its Relevance in “Autoimmunity”. J Clin Invest 1961;40:1597-620. [Crossref] [PubMed]
- Shulman NR, Marder VJ, Aledort LM, et al. Complement-fixing isoantibodies against antigens common to platelets and leukocytes. Trans Assoc Am Physicians 1962;75:89-98. [PubMed]
- Ballem PJ, Segal GM, Stratton JR, et al. Mechanisms of thrombocytopenia in chronic autoimmune thrombocytopenic purpura. Evidence of both impaired platelet production and increased platelet clearance. J Clin Invest 1987;80:33-40. [Crossref] [PubMed]
- Gernsheimer T, Stratton J, Ballem PJ, et al. Mechanisms of response to treatment in autoimmune thrombocytopenic purpura. N Engl J Med 1989;320:974-80. [Crossref] [PubMed]
- McMillan R, Wang L, Tomer A, et al. Suppression of in vitro megakaryocyte production by antiplatelet autoantibodies from adult patients with chronic ITP. Blood 2004;103:1364-9. [Crossref] [PubMed]
- Semple JW, Rebetz J, Maouia A, et al. An update on the pathophysiology of immune thrombocytopenia. Curr Opin Hematol 2020;27:423-9. [Crossref] [PubMed]
- Houwerzijl EJ, Blom NR, van der Want JJ, et al. Ultrastructural study shows morphologic features of apoptosis and para-apoptosis in megakaryocytes from patients with idiopathic thrombocytopenic purpura. Blood 2004;103:500-6. [Crossref] [PubMed]
- Karpatkin S, Garg SK. The megathrombocyte as an index of platelet production. Br J Haematol 1974;26:307-11. [Crossref] [PubMed]
- Karpatkin S. Autoimmune (idiopathic) thrombocytopenic purpura. Lancet 1997;349:1531-6. [Crossref] [PubMed]
- Stasi R, Sarpatwari A, Segal JB, et al. Effects of eradication of Helicobacter pylori infection in patients with immune thrombocytopenic purpura: a systematic review. Blood 2009;113:1231-40. [Crossref] [PubMed]
- Hollenhorst MA, Al-Samkari H, Kuter DJ. Markers of autoimmunity in immune thrombocytopenia: prevalence and prognostic significance. Blood Adv 2019;3:3515-21. [Crossref] [PubMed]
- Al-Samkari H, Kuter DJ. Lactate dehydrogenase is elevated in immune thrombocytopenia and inversely correlates with platelet count. Br J Haematol 2019;187:e61-4. [Crossref] [PubMed]
- Makar RS, Zhukov OS, Sahud MA, et al. Thrombopoietin levels in patients with disorders of platelet production: diagnostic potential and utility in predicting response to TPO receptor agonists. Am J Hematol 2013;88:1041-4. [Crossref] [PubMed]
- Al-Samkari H, Kuter DJ. Thrombopoietin level predicts response to treatment with eltrombopag and romiplostim in immune thrombocytopenia. Am J Hematol 2018;93:1501-8. [Crossref] [PubMed]
- Mithoowani S, Gregory-Miller K, Goy J, et al. High-dose dexamethasone compared with prednisone for previously untreated primary immune thrombocytopenia: a systematic review and meta-analysis. Lancet Haematol 2016;3:e489-96. [Crossref] [PubMed]
- Kitchens CS. Amelioration of endothelial abnormalities by prednisone in experimental thrombocytopenia in the rabbit. J Clin Invest 1977;60:1129-34. [Crossref] [PubMed]
- Kitchens CS, Weiss L. Ultrastructural changes of endothelium associated with thrombocytopenia. Blood 1975;46:567-78. [Crossref] [PubMed]
- Kuter DJ, Newland A, Chong BH, et al. Romiplostim in adult patients with newly diagnosed or persistent immune thrombocytopenia (ITP) for up to 1 year and in those with chronic ITP for more than 1 year: a subgroup analysis of integrated data from completed romiplostim studies. Br J Haematol 2019;185:503-13. [Crossref] [PubMed]
- Moulis G, Rueter M, Lafaurie M, et al. Eltrombopag for Immune Thrombocytopenia in Adult Patients in the Real-World in France. Final Results of the Elextra Study. Blood 2020;136:11-2. [Crossref]
- Kuter DJ, Bussel JB, Newland A, et al. Long-term treatment with romiplostim in patients with chronic immune thrombocytopenia: safety and efficacy. Br J Haematol 2013;161:411-23. [Crossref] [PubMed]
- Saleh MN, Bussel JB, Cheng G, et al. Safety and efficacy of eltrombopag for treatment of chronic immune thrombocytopenia: results of the long-term, open-label EXTEND study. Blood 2013;121:537-45. [Crossref] [PubMed]
- Kuter DJ, Bussel JB, Lyons RM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet 2008;371:395-403. [Crossref] [PubMed]
- Kuter DJ, Rummel M, Boccia R, et al. Romiplostim or standard of care in patients with immune thrombocytopenia. N Engl J Med 2010;363:1889-99. [Crossref] [PubMed]
- Bussel JB, Cheng G, Saleh MN, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med 2007;357:2237-47. [Crossref] [PubMed]
- Bussel JB, Kuter DJ, Aledort LM, et al. A randomized trial of avatrombopag, an investigational thrombopoietin-receptor agonist, in persistent and chronic immune thrombocytopenia. Blood 2014;123:3887-94. [Crossref] [PubMed]
- Bussel JB, Kuter DJ, George JN, et al. AMG 531, a thrombopoiesis-stimulating protein, for chronic ITP. N Engl J Med 2006;355:1672-81. [Crossref] [PubMed]
- Bussel JB, Provan D, Shamsi T, et al. Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: a randomised, double-blind, placebo-controlled trial. Lancet 2009;373:641-8. [Crossref] [PubMed]
- Kuter DJ. Thrombopoietin Receptor Agonists. In: Michelson A, Cattaneo M, Frelinger A, et al. editors. Platelets. 4 ed. Cambridge, Massachusetts: Academic Press, 2019:1085-110.
- Lozano ML, Godeau B, Grainger J, et al. Romiplostim in adults with newly diagnosed or persistent immune thrombocytopenia. Expert Rev Hematol 2020;13:1319-32. [Crossref] [PubMed]
- Kuter DJ. General aspects of thrombocytopenia, platelet transfusions, and thrombopoietic growth factors. In: Kitchens CS, Kessler CM, Konkle B, et al. editor. Consultative Hemostasis and Thrombosis, 4th edition. Philadelphia: Elsevier Saunders, 2018:108-26.
- Ghanima W, Cooper N, Rodeghiero F, et al. Thrombopoietin receptor agonists: ten years later. Haematologica 2019;104:1112-23. [Crossref] [PubMed]
- Virk ZM, Kuter DJ, Al-Samkari H. An evaluation of avatrombopag for the treatment of thrombocytopenia. Expert Opin Pharmacother 2021;22:273-80. [Crossref] [PubMed]
- Kuter DJ, Arnold DM, Rodeghiero F, et al. Safety and efficacy of self-administered romiplostim in patients with immune thrombocytopenia: Results of an integrated database of five clinical trials. Am J Hematol 2020;95:643-51. [Crossref] [PubMed]
- Kuter DJ, Allen LF. Avatrombopag, an oral thrombopoietin receptor agonist: results of two double-blind, dose-rising, placebo-controlled Phase 1 studies. Br J Haematol 2018;183:466-78. [Crossref] [PubMed]
- Al-Samkari H, Kuter DJ. Relative potency of the thrombopoietin receptor agonists eltrombopag, avatrombopag and romiplostim in a patient with chronic immune thrombocytopenia. Br J Haematol 2018;183:168. [Crossref] [PubMed]
- Kuter DJ, Bain B, Mufti G, et al. Bone marrow fibrosis: pathophysiology and clinical significance of increased bone marrow stromal fibres. Br J Haematol 2007;139:351-62. [Crossref] [PubMed]
- Kuter DJ, Mufti GJ, Bain BJ, et al. Evaluation of bone marrow reticulin formation in chronic immune thrombocytopenia patients treated with romiplostim. Blood 2009;114:3748-56. [Crossref] [PubMed]
- Janssens A, Rodeghiero F, Anderson D, et al. Changes in bone marrow morphology in adults receiving romiplostim for the treatment of thrombocytopenia associated with primary immune thrombocytopenia. Ann Hematol 2016;95:1077-87. [Crossref] [PubMed]
- Li J, Yang C, Xia Y, et al. Thrombocytopenia caused by the development of antibodies to thrombopoietin. Blood 2001;98:3241-8. [Crossref] [PubMed]
- Mytych DT, Park JK, Kim J, et al. Assessment of romiplostim immunogenicity in adult patients in clinical trials and in a global postmarketing registry. Br J Haematol 2020;190:923-32. [Crossref] [PubMed]
- Cines DB, Gernsheimer T, Wasser J, et al. Integrated analysis of long-term safety in patients with chronic immune thrombocytopaenia (ITP) treated with the thrombopoietin (TPO) receptor agonist romiplostim. Int J Hematol 2015;102:259-70. [Crossref] [PubMed]
- Amgen Inc. Nplate® (romiplostim) prescribing information. Available online: http://pi.amgen.com/united_states/nplate/nplate_pi_hcp_english.pdf. Accessed Jsanuary 20 2021.
- Cines DB, Wasser J, Rodeghiero F, et al. Safety and efficacy of romiplostim in splenectomized and nonsplenectomized patients with primary immune thrombocytopenia. Haematologica 2017;102:1342-51. [Crossref] [PubMed]
- Wang B, Nichol JL, Sullivan JT. Pharmacodynamics and pharmacokinetics of AMG 531, a novel thrombopoietin receptor ligand. Clin Pharmacol Ther 2004;76:628-38. [Crossref] [PubMed]
- Novartis Inc. Promacta® (eltrombopag) prescribing information. Available online: https://www.novartis.us/sites/www.novartis.us/files/promacta.pdf. Accessed January 20, 2021.
- Al-Samkari H, Kuter DJ. An alternative intermittent eltrombopag dosing protocol for the treatment of chronic immune thrombocytopenia. Br J Clin Pharmacol 2018;84:2673-7. [Crossref] [PubMed]
- Farrell C, Hayes SC, Wire M, et al. Population pharmacokinetic/pharmacodynamic modelling of eltrombopag in healthy volunteers and subjects with chronic liver disease. Br J Clin Pharmacol 2014;77:532-44. [Crossref] [PubMed]
- Gibiansky E, Zhang J, Williams D, et al. Population pharmacokinetics of eltrombopag in healthy subjects and patients with chronic idiopathic thrombocytopenic purpura. J Clin Pharmacol 2011;51:842-56. [Crossref] [PubMed]
- Hayes S, Ouellet D, Zhang J, et al. Population PK/PD modeling of eltrombopag in healthy volunteers and patients with immune thrombocytopenic purpura and optimization of response-guided dosing. J Clin Pharmacol 2011;51:1403-17. [Crossref] [PubMed]
- Afdhal NH, Dusheiko GM, Giannini EG, et al. Eltrombopag increases platelet numbers in thrombocytopenic patients with HCV infection and cirrhosis, allowing for effective antiviral therapy. Gastroenterology 2014;146:442-52.e1. [Crossref] [PubMed]
- Punzo F, Tortora C, Argenziano M, et al. Iron chelating properties of Eltrombopag: Investigating its role in thalassemia-induced osteoporosis. PLoS One 2018;13:e0208102 [Crossref] [PubMed]
- Dova, Inc.® Doptelet (avatrombopag) prescribing information. Available online: https://dova.com/wp-content/uploads/2019/06/doptelet-prescribing-information.pdf
- Nomoto M, Ferry J, Hussein Z. Population Pharmacokinetic/Pharmacodynamic Analyses of Avatrombopag in Patients With Chronic Liver Disease and Optimal Dose Adjustment Guide With Concomitantly Administered CYP3A and CYP2C9 Inhibitors. J Clin Pharmacol 2018;58:1629-38. [Crossref] [PubMed]
- Nomoto M, Pastino G, Rege B, et al. Pharmacokinetics, Pharmacodynamics, Pharmacogenomics, Safety, and Tolerability of Avatrombopag in Healthy Japanese and White Subjects. Clin Pharmacol Drug Dev 2018;7:188-95. [Crossref] [PubMed]
- Nomoto M, Zamora CA, Schuck E, et al. Pharmacokinetic/pharmacodynamic drug-drug interactions of avatrombopag when coadministered with dual or selective CYP2C9 and CYP3A interacting drugs. Br J Clin Pharmacol 2018;84:952-60. [Crossref] [PubMed]
- Jurczak W, Chojnowski K, Mayer J, et al. Phase 3 randomised study of avatrombopag, a novel thrombopoietin receptor agonist for the treatment of chronic immune thrombocytopenia. Br J Haematol 2018;183:479-90. [Crossref] [PubMed]
- Kuter DJ, Macahilig C, Grotzinger KM, et al. Treatment patterns and clinical outcomes in patients with chronic immune thrombocytopenia (ITP) switched to eltrombopag or romiplostim. Int J Hematol 2015;101:255-63. [Crossref] [PubMed]
- Khellaf M, Viallard JF, Hamidou M, et al. A retrospective pilot evaluation of switching thrombopoietic receptor-agonists in immune thrombocytopenia. Haematologica 2013;98:881-7. [Crossref] [PubMed]
- Patel VL, Mahevas M, Lee SY, et al. Outcomes 5 years after response to rituximab therapy in children and adults with immune thrombocytopenia. Blood 2012;119:5989-95. [Crossref] [PubMed]
- Marangon M, Vianelli N, Palandri F, et al. Rituximab in immune thrombocytopenia: gender, age, and response as predictors of long-term response. Eur J Haematol 2017;98:371-7. [Crossref] [PubMed]
- Deshayes S, Khellaf M, Zarour A, et al. Long-term safety and efficacy of rituximab in 248 adults with immune thrombocytopenia: Results at 5 years from the French prospective registry ITP-ritux. Am J Hematol 2019;94:1314-24. [Crossref] [PubMed]
- Khellaf M, Charles-Nelson A, Fain O, et al. Safety and efficacy of rituximab in adult immune thrombocytopenia: results from a prospective registry including 248 patients. Blood 2014;124:3228-36. [Crossref] [PubMed]
- Provan D, Butler T, Evangelista ML, et al. Activity and safety profile of low-dose rituximab for the treatment of autoimmune cytopenias in adults. Haematologica 2007;92:1695-8. [Crossref] [PubMed]
- Cattaneo C, Spedini P, Casari S, et al. Delayed-onset peripheral blood cytopenia after rituximab: frequency and risk factor assessment in a consecutive series of 77 treatments. Leuk Lymphoma 2006;47:1013-7. [Crossref] [PubMed]
- Venna N, Gonzalez RG, Camelo-Piragua SI. Case records of the Massachusetts General Hospital. Case 11-2010. A 69-year-old woman with lethargy, confusion, and abnormalities on brain imaging. N Engl J Med 2010;362:1431-7. [Crossref] [PubMed]
- van Assen S, Holvast A, Benne CA, et al. Humoral responses after influenza vaccination are severely reduced in patients with rheumatoid arthritis treated with rituximab. Arthritis Rheum 2010;62:75-81. [Crossref] [PubMed]
- Yri OE, Torfoss D, Hungnes O, et al. Rituximab blocks protective serologic response to influenza A (H1N1) 2009 vaccination in lymphoma patients during or within 6 months after treatment. Blood 2011;118:6769-71. [Crossref] [PubMed]
- Nazi I, Kelton JG, Larche M, et al. The effect of rituximab on vaccine responses in patients with immune thrombocytopenia. Blood 2013;122:1946-53. [Crossref] [PubMed]
- Bussel J, Arnold DM, Grossbard E, et al. Fostamatinib for the treatment of adult persistent and chronic immune thrombocytopenia: Results of two phase 3, randomized, placebo-controlled trials. Am J Hematol 2018;93:921-30. [Crossref] [PubMed]
- Boccia R, Cooper N, Ghanima W, et al. Fostamatinib is an effective second-line therapy in patients with immune thrombocytopenia. Br J Haematol 2020;190:933-8. [Crossref] [PubMed]
- Hou M, Peng J, Shi Y, et al. Mycophenolate mofetil (MMF) for the treatment of steroid-resistant idiopathic thrombocytopenic purpura. Eur J Haematol 2003;70:353-7. [Crossref] [PubMed]
- Zhang WG, Ji L, Cao XM, et al. Mycophenolate mofetil as a treatment for refractory idiopathic thrombocytopenic purpura. Acta Pharmacol Sin 2005;26:598-602. [Crossref] [PubMed]
- Provan D, Moss AJ, Newland AC, et al. Efficacy of mycophenolate mofetil as single-agent therapy for refractory immune thrombocytopenic purpura. Am J Hematol 2006;81:19-25. [Crossref] [PubMed]
- Taylor A, Neave L, Solanki S, et al. Mycophenolate mofetil therapy for severe immune thrombocytopenia. Br J Haematol 2015;171:625-30. [Crossref] [PubMed]
- Godeau B, Durand JM, Roudot-Thoraval F, et al. Dapsone for chronic autoimmune thrombocytopenic purpura: a report of 66 cases. Br J Haematol 1997;97:336-9. [Crossref] [PubMed]
- Damodar S, Viswabandya A, George B, et al. Dapsone for chronic idiopathic thrombocytopenic purpura in children and adults--a report on 90 patients. Eur J Haematol 2005;75:328-31. [Crossref] [PubMed]
- Patel AP, Patil AS. Dapsone for immune thrombocytopenic purpura in children and adults. Platelets 2015;26:164-7. [Crossref] [PubMed]
- Vancine-Califani SM, De Paula EV, Ozelo MC, et al. Efficacy and safety of dapsone as a second-line treatment in non-splenectomized adults with immune thrombocytopenic purpura. Platelets 2008;19:489-95. [Crossref] [PubMed]
- Zaja F, Marin L, Chiozzotto M, et al. Dapsone salvage therapy for adult patients with immune thrombocytopenia relapsed or refractory to steroid and rituximab. Am J Hematol 2012;87:321-3. [Crossref] [PubMed]
- Marinacci LX, Simeone FJ, Lundquist AL, et al. Case 38-2020: A 52-Year-Old Man with Cancer and Acute Hypoxemia. N Engl J Med 2020;383:2372-83. [Crossref] [PubMed]
- Li HQ, Zhang L, Zhao H, et al. Chronic idiopathic thrombocytopenic purpura in adult Chinese patients: a retrospective single-centered analysis of 1791 cases. Chin Med J (Engl) 2005;118:34-7. [PubMed]
- Ahn YS, Fernandez LF, Kim CI, et al. Danazol therapy renders red cells resistant to osmotic lysis. FASEB J 1989;3:157-62. [Crossref] [PubMed]
- Ambríz R, Pizzuto J, Morales M, et al. Therapeutic effect of danazol on metrorrhagia in patients with idiopathic thrombocytopenic purpura (ITP). Nouv Rev Fr Hematol 1986;28:275-9. [PubMed]
- Fenaux P, Quiquandon I, Huart JJ, et al. The role of danazol in the treatment of refractory idiopathic thrombocytopenic purpura. A report of 22 cases. Nouv Rev Fr Hematol 1990;32:143-6. [PubMed]
- Liu W, Gu X, Fu R, et al. The Effect of Danazol in Primary Immune Thrombocytopenia: An Analysis of a Large Cohort From a Single Center in China. Clin Appl Thromb Hemost 2016;22:727-33. [Crossref] [PubMed]
- Maloisel F, Andres E, Zimmer J, et al. Danazol therapy in patients with chronic idiopathic thrombocytopenic purpura: long-term results. Am J Med 2004;116:590-4. [Crossref] [PubMed]
- Sundar S, Moorleedursingh GS, Kumar K, et al. Danazol therapy in chronic idiopathic thrombocytopenic purpura. J Assoc Physicians India 1992;40:350-1. [PubMed]
- Newland AC, Sanchez-Gonzalez B, Rejto L, et al. Phase 2 study of efgartigimod, a novel FcRn antagonist, in adult patients with primary immune thrombocytopenia. Am J Hematol 2020;95:178-87. [Crossref] [PubMed]
- Schwartz J, Leber MD, Gillis S, et al. Long term follow-up after splenectomy performed for immune thrombocytopenic purpura (ITP). Am J Hematol 2003;72:94-8. [Crossref] [PubMed]
- Kojouri K, Vesely SK, Terrell DR, et al. Splenectomy for adult patients with idiopathic thrombocytopenic purpura: a systematic review to assess long-term platelet count responses, prediction of response, and surgical complications. Blood 2004;104:2623-34. [Crossref] [PubMed]
- Sarpatwari A, Provan D, Erqou S, et al. Autologous 111 In-labelled platelet sequestration studies in patients with primary immune thrombocytopenia (ITP) prior to splenectomy: a report from the United Kingdom ITP Registry. Br J Haematol 2010;151:477-87. [Crossref] [PubMed]
- Sailer T, Lechner K, Panzer S, et al. The course of severe autoimmune thrombocytopenia in patients not undergoing splenectomy. Haematologica 2006;91:1041-5. [PubMed]
- Marshall AL, Scarpone R, De Greef M, et al. Remissions after long-term use of romiplostim for immune thrombocytopenia. Haematologica 2016;101:e476-8. [Crossref] [PubMed]
- Schifferli A, Nimmerjahn F, Kuhne T. Immunomodulation in Primary Immune Thrombocytopenia: A Possible Role of the Fc Fragment of Romiplostim? Front Immunol 2019;10:1196. [Crossref] [PubMed]
Cite this article as: Kuter DJ. The treatment of immune thrombocytopenia (ITP)—focus on thrombopoietin receptor agonists. Ann Blood 2021;6:7.