
Bacterial contamination and sepsis associated with transfusion: current status in Latin America
Introduction
Blood transfusion is one of the therapeutic strategies employed in clinical practice (1). However, its use carries not only benefits but also potential risks. Transfusion-transmitted infections (TTIs) comprise several biological agents (viruses, parasites, bacteria, fungi, and prions) transmitted during the administration of whole blood or blood components (2). To date, there are more than 68 potentially causative agents of TTI (3,4). However, currently, the spread of viruses transmitted by transfusion has been substantially reduced, so that bacterial contamination (BC) of blood components is today the most frequent cause of TTI (5). Sepsis associated with red blood cell transfusion has decreased rapidly in the last twenty years, possibly due to the more widespread application of leukoreduction filters (6). Therefore, most cases of post-transfusion sepsis today involve platelet components stored at room temperature. With the introduction of better skin disinfection, first aliquot diversion techniques, and bacterial detection in platelets, the rate of clinically significant septic reactions has decreased but not eliminated. Today it is known that the severity of a septic reaction will depend on various factors related to bacteria (gram-positive or gram-negative, the type of strain) and receptors (comorbidities, immune status, use of antibiotics), as well as the bacterial concentration at the time of transfusion (7,8).
Multiple aerobic culture surveillance studies have shown BC frequencies of 1: 1,000 to 1: 3,000 in platelet units (PT) (6). Despite contamination estimates, actual rates of septic adverse transfusion reactions (ADRs) are lower, approximately 1 in 25,000 units of PT (range 1:13,000 to 1:100,000) (6). Underreport of sepsis caused by the transfusion is frequent. Jacobs et al. found that during periods of active culture screening, contaminated platelet components and sepsis were 32.0 and 10.6 times more likely to be documented than during a period based solely on clinical recognition reports (9). Likewise, other authors have shown that the active search for transfusion-associated sepsis could be 35.9 times greater than passive surveillance (10). Hong et al. performed active and passive surveillance for bacterially contaminated platelets between 2007-2013 at the University Hospitals Case Medical Center. The authors showed no reports of the five cases of transfusion-associated sepsis during a seven-year study period. Documentation of these cases was the result of an active surveillance program (8).
Between January 2010 and December 2016, the United States of America’s haemovigilance system reported 111 cases of TTI in 7.9 million blood components transfused. Babesia spp was identified in 16 cases in red blood cell units (14.4%), while Staphylococcus aureus was the most frequent microorganism isolated from PT units (10.8%). A TTI rate = 1.95 per 100,000 transfused PT units and a rate of 0.53 TTI per 100,000 units of red blood cells transfused were estimated (5). In 2017, the Food and Drug Administration (FDA) reported five mortality cases related to transfusion, with definite or probable imputability, associated with BC (Staphylococcus epidermidis, Klebsiella pneumoniae, and Clostridium perfringens) (11). To date, the primary sources of transmitted-transfusion bacterial infection (TTBI) are flora on the skin (74.7%), oropharyngeal flora (16.4%), and intestinal flora (8.9%) (12).
In 2017, Canadian Blood Services reported the results of routine screening and quality control sterility tests in PT units obtained between 2010 to 2016 and reports of septic ATR (13). Quality control tests allowed estimating similar false negative rates between the PT pools produced with the buffy coat method (8 per 10,000) and PT apheresis units (9 per 10,000) (P=0.79). There were five septic ATR with definitive imputability. The agents identified were coagulase-negative Staphylococcus, S. epidermidis, and S. aureus. A non-fatal septic reaction rate of approximately 1 per 100,000 PT units and a fatal septic reaction rate of 1 per 500,000 PT units were estimated (13). Recently, Canada presented the results of incorporating a new BC detection algorithm in PT concentrates. They demonstrate a reduction of cases to 1: 350,000 and zero cases of mortality (7). Spain in 2017 reported 20 suspected cases of BC (two of them with imputability and severity >2) (14), while in 2018, the United Kingdom investigated 98 suspected cases of BC (none confirmed) (15). In 2017, New Zealand reported twelve confirmed positive BC cases in PT (seven cases associated with Cutibacterium acnes). Four patients received these PT units because the culture was positive after transfusion. Only one of the patients developed symptoms (16). They also reported that the rate of true positives was similar to that reported by the Australian Red Cross Blood services (0.12%) (17). In 2017 the French haemovigilance system reported two BC cases (18). The Netherlands’ haemovigilance system investigated in 2017, 72 cases of TTBI, four confirmed (three cases with Cutibacterium acnes and one case due to Staphylococcus saccharolyticus) (19). The 2018 New Zealand haemovigilance report informed that of 15,108 PT screened after 36 hours of collection, there was a confirmed bacterial infection in nineteen (0.13%) (20). None of the five patients who received contaminated PT had symptoms, although four received antibiotics at the transfusion time. From 2016 to 2018, there were fifteen PT transfused with confirmed positive cultures. In fourteen cases, Cutibacterium acnes was the organism found (20). The other case was Staphylococcus saccharolyticus. Likewise, the 2019 Austrian hemovigilance report notified five cases of platelets transfused with confirmed positive bacterial cultures. None of the five recipients developed symptoms, probably because they were receiving antibiotics due to their underlying condition (21).
Hume et al. reported that seven studies were published in Africa evaluating BC frequency up to 2016 (22). TTBI was 8.8% in whole blood and erythrocytes, ranging between 3.1% in Zimbabwe and 17.5% in Ghana. This value contrasts with those rates reported by high-income countries (0.01% to 0.07%) (22). In the specific case of PT concentrates, Hume et al. reported a frequency of BC between 0.3% and 2.1% in Uganda (22). In Latin America, there is an absence of reports of TTBI, except for Brazil, which informed four cases in 2016 and two in 2017 (23). In 2017, Ramírez-Arcos et al. (24) published a survey carried out in four Latin American countries. The authors evaluate the status of the BC of PT concentrates in blood banks. The detection practices and mitigation strategies varied considerably between sites, highlighting the need to standardize Latin American procedures. However, the inadequate response of the actors (less than 22%) limited the conclusions. This review aims to establish the strategies implemented to reduce TTBI in Latin American countries and know the number of cases recorded. Likewise, we determine the limitations that prevent TTBI notification. Finally, we estimate the number of events that should be presented based on more experienced haemovigilance programs.
Information collection method
We included for this review the haemovigilance reports available for Brazil and Colombia, and the report on the Supply of Blood for transfusions in the Latin American countries (Argentina, Bolivia, Brazil, Chile, Colombia, Costa Rica, Cuba, Ecuador, El Salvador, Guatemala, Honduras, Mexico, Nicaragua, Panama, Paraguay, Peru, Dominican Republic, and Uruguay) between 2016 and 2017, published by the Pan American Health Organization (PAHO) (23). Contributed to this review national leaders at the governmental level of the blood network and the health surveillance (Brazil, Colombia, El Salvador, and Honduras), presidents of scientific societies (Chile, Guatemala, Paraguay, and Peru), or members of them (Mexico), as well as opinion leaders interested in haemovigilance (Ecuador and Honduras). The corresponding author sends an invitation email to participate as co-authors to people in 24 countries. People from Argentina, Brazil, Chile, Colombia, Ecuador, El Salvador, Guatemala, Honduras, Mexico, Paraguay, and Peru accept participating. The review’s design included a virtual personal interview with the co-author, using a standardized survey. We estimate the number of events that should be presented based on more experienced haemovigilance programs when no records of TTBI from a country were available.
Results
What strategies have been implemented in Latin American countries to reduce TTBI?
There are at least six effective strategies to reduce the risk of posttransfusion sepsis (6): (I) transfusion of blood components according to evidence-based guidelines; (II) standardized donor survey, skin preparation, and diversion of the first milliliters of blood; (III) reducing storage duration of blood components; (IV) bacterial detection during storage; (V) pathogen reduction technologies (PRT) and (VI) identification of bacteria in blood components at the same day of transfusion. Table 1 briefly shows the primary safety measures relative to platelet component collection, manufacturing, processing, preservations, issuing, and outdating in Latin America. In no country is there a single method to obtain platelets. However, the whole-blood derived prepared by the buffy coat method or by the platelet-rich-plasma methods predominates in most countries. It was impossible to know the number of units collected by apheresis in the Latin-American countries during 2018–2020 (except Brazil and Colombia). Therefore, we analyzed the data reported by PAHO in 2017. The total number of components obtained by apheresis was 474.756, of which 75.6% corresponded to platelets. This value represented 5.4% (95% CI: 1.1–6.1%) of the total collection of blood components in Latin America for that year.
Table 1
| Variable | Argentina | Bolivia | Brazil | Chile | Colombia | Costa Rica | Cuba | Ecuador | El Salvador | Guatemala | Honduras | Mexico | Nicaragua | Panama | Paraguay | Peru | Dominican Republic | Uruguay |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type (s) of platelets produced | ||||||||||||||||||
| Apheresis and Whole-blood derived prepared by the platelet-rich-plasma method | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Unknown | Some centers | Some centers | Some centers | Unknown | Some centers |
| Apheresis and Whole-blood derived prepared by the buffy coat method | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Unknown | Some centers | Some centers | Some centers | Unknown | Some centers |
| Whole-blood derived prepared by the buffy coat method | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Unknown | Some centers | Some centers | Some centers | Unknown | Some centers |
| Whole-blood derived prepared by the platelet-rich-plasma method | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Unknown | Some centers | Some centers | Some centers | Unknown | Some centers |
| Apheresis | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Unknown | Some centers | Some centers | Some centers | Unknown | Some centers |
| Platelet shelf life | ||||||||||||||||||
| 5 days | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |
| 7 days | X | X | ||||||||||||||||
| Unknown | X | X | ||||||||||||||||
| Strategies implemented | ||||||||||||||||||
| Leukocyte reduction | Some centers | Unknown | Some centers | Some centers | Some centers | Some centers | Unknown | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers | Some centers |
| Donor arm skin disinfection | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| First aliquot diversion | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Some centers | No | Some centers | Yes | Unknown | Some centers | Some centers | Some centers | Some centers | Yes |
| Platelet screening for bacterial contamination | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Pathogen reduction technologies | Some centers | Unknown | Some centers | Some centers | Some centers | Some centers | Unknown | No | No | No | Some centers | Some centers | Unknown | Some centers | No | No | Unknown | Some centers |
| PT screening for bacterial contamination | ||||||||||||||||||
| Routine screening (100%) | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| Quality control | 1% | 1% | 1% | 1% | 1% | 1% | 1% | 1% | 2% | 1% | 1% | 5% | 1% | 1% | 1% | 1% | 1% | 1% |
| Quarantine postsampling (hours) | No | No | Some centers | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| Testing method (BACT/ALERT, eBDS) | Some centers | Unknown | Some centers | Some centers | Some centers | Some centers | Unknown | Some centers | No | No | No | Some centers | Unknown | Some centers | No | Some centers | Unknown | Some centers |
| Culture bottle (Aerobic/anaerobic) | Some centers | Unknown | Some centers | Some centers | Some centers | Some centers | Unknown | Some centers | No | No | No | Some centers | Unknown | Some centers | No | Some centers | Unknown | Some centers |
| Blood culture (plate) | Some centers | Unknown | Some centers | Some centers | Some centers | Some centers | Unknown | Some centers | Some centers | Some centers | No | Some centers | Unknown | Some centers | No | Some centers | Unknown | Some centers |
| Swirling | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Visual inspection | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| pH, glucose | Some centers | Unknown | Some centers | Some centers | Some centers | Some centers | Unknown | Some centers | Some centers | Some centers | Some centers | Some centers | Unknown | Some centers | Some centers | Some centers | Some centers | Some centers |
| Rapid Test | No | No | No | No | No | No | Unknown | No | No | No | No | No | No | No | No | No | No | No |
All participating countries reported having a unified national donor survey. Similarly, it is unified skin donor disinfection before the phlebotomy (Table 1). What is not standardized is the procedure or quality controls for these actions. There is evidence that even asepsis with isopropanol, povidone-iodine, or chlorhexidine gluconate, can leave remnants of microorganisms that later cause contamination of blood components (6). There is no national regulation in any Latin American country that establishes as mandatory to screen 100% of PT units from the data collected. The countries’ regulations fluctuate between requesting microbiological control performance at 1–5% of the collected (Table 1) as a quality control measure. However, private institutions in some countries voluntarily perform the microbiological control of 100% of the collected components (for example Garraham Hospital in Argentina, Instituto Distrital de Ciencia Biotecnología e innovación, IDCBIS, among others), but it is not standardized conduct. Not all Latin American countries require detecting BC of the components, but it is mandatory for AABB establishments accredited (25). At the end of 2020, there were eight Latin American Blood Banks, Transfusion Services, or Blood Centers accredited by AABB (Brazil: HemoRio- IEHE and Hospital Israelita Albert Einstein; Colombia: Laboratorio Medico Las Americas and Sub Red Integrada De Servicios De Salud Centro Oriente ESE; Dominican Republic: Referencia Banco De Sangre Luperon; Honduras: Centro Nacional de Sangre, Cruz Roja Hondureña and Centro Regional de Sangre, Cruz Roja Hondureña; Mexico: Centro Estatal De La Transfusion Sanguinea Del Estado de Pachuca) (26). Other certifying entities such as the Transfusion Accreditation Committee, CAT (27), of the Spanish Society of Blood Transfusion, SETS establish microbiological control or PRT within the voluntary standards.
Several public and private institutions in Latin America already have PRT. For example, Honduras has several public and private collection and processing centers, including the Cruz Roja Hondureña (CRH). Currently, CRH has three blood screening centers that process what is collected by ten blood banks. CRH distributes 70% of the platelets throughout the country. Since 2016, PRT is made to 100% of the platelets within the first 8 to 24 hours. In 2019 CRH collected 28,070 platelet concentrates. Three thousand three hundred seventy-eight pools of platelets were prepared and inactivated (Cerus). CRH has transitioned from making pH, followed by BACT/ALERT™, and subsequently implementing PRT. CRH makes quality control to 1% of expired units. Currently, they do not perform plasma inactivation. It is worth highlighting the case of CRH because it shows that it was possible to adopt PRT even though Honduras is one of the poorest countries in Latin America.
In Brazil, blood therapy services are inspected annually by health surveillance (28,29). The risk assessments are subsequently sent to the Agência Nacional de Vigilância Sanitária (Anvisa), to collect the data at the national level (30). In 2019, Anvisa received data from 1,055 (around 49%) of the 2,175 blood therapy services in Brazil. On average, less than 10% of the evaluated services showed some non-compliance with skin disinfection regulation, visual inspection of the bag before transfusion, and quality control. Around 14% of the services evaluated presented some non-compliance with the regulation items in the storage of blood components, mainly regarding equipment qualification. Like Brazil, Colombia and Chile have regulatory authorities responsible for the vigilance of the blood banks [National Institute for Food and Drug Surveillance, INVIMA (31) and Superintendency of Health (32) respectively]. However, these institutions do not publish reports or give feedbacks to national stakeholders.
The 18 countries analyzed in this review reported in 2017 that there were 1,898 collection centers and 1,152 processing centers, 67% located in Brazil (26.5%), Mexico (24.8%), and Argentina (15.3%) (23). Similarly, fifteen countries reported that 7956 hospitals performed transfusions (63.0% in Mexico, 14.3% in Argentina, and 7.4% in Colombia). Brazil, Cuba, and the Dominican Republic did not report data. The 18 countries analyzed had a specific law regarding the national blood system, except for the Dominican Republic, Honduras, and El Salvador. Chile has a blood policy but not associated with a decree or law that guaranteed its national adoption (33). As for a specific blood budget, all except Costa Rica, Cuba, El Salvador, the Dominican Republic, and Uruguay had one. Regarding a national blood policy, only Bolivia and Costa Rica lacked it. Guatemala and Honduras had a partial national policy (23). Regarding norms that standardized the selection of blood donors and the operation of the collection and processing centers, the 18 Latin American countries reported that they had them (23). All countries except for Costa Rica, Honduras, and Peru had guidelines for the clinical use of blood.
Regarding continuing education programs related to blood, of the 18 participating countries, there was no program until 2017 in Colombia, Costa Rica, Honduras, Panama, or the Dominican Republic. There was a partial program in Uruguay and Honduras and no registration in Cuba. Concerning a national quality assurance program, Costa Rica, Guatemala, Honduras, Panama, the Dominican Republic, and Uruguay did not have one. Furthermore, about the existence of a national external evaluation program (serology-TTI), only Guatemala, Honduras, Panama, and Uruguay reported that they did not have it. In all countries, there was an inspection program for 2017 except in Honduras. In Costa Rica and El Salvador, it was partial, and no response in Uruguay (23). Until 2017, 50% of the countries analyzed (Argentina, Bolivia, Brazil (30,34), Chile (35), Colombia (36,37), Mexico, Nicaragua, Peru, and Panama) request certification of personnel to perform specific tasks related to blood banking and transfusion medicine. Argentina, Bolivia, Brazil, Colombia (38), Chile, Costa Rica, Cuba, and Perú required accreditation of blood services (23).
Concerning national haemovigilance programs, they only exist in five countries: Argentina (39), Brazil (30,40), Colombia (41), Cuba, and Ecuador (42). Chile (32,35), Mexico, Nicaragua, and Paraguay (43) have local programs. Of these, only Brazil (44-46) and Colombia (47-49) have unified case definitions at the national level (adoption of international definitions suggested by ISBT-IHN-AABB) (50,51) and issue annual reports of adverse reactions to Donation and Transfusion. Of the 18 countries, only two have a national transfusion committee (Colombia and Cuba), although Costa Rica, El Salvador, and Paraguay have a partial national committee. Regarding the intrahospital transfusion committees, Argentina, Brazil, Chile, Colombia, Cuba, Ecuador, and El Salvador reported their existence. In other countries, its presence is partial. For example, in Mexico, of the 5010 hospitals estimated to perform transfusions, 320 had hospital transfusion committees and ATR notification mechanisms. Mexican legislation only requires an intrahospital transfusion committee in those that regularly transfuse more than 50 units of blood per month (23).
Finally, we investigated the presence of informed consent for transfusion. In Brazil, there is no mandatory informed consent form for blood transfusion. It does exist in Colombia, Chile, Guatemala, Ecuador, Paraguay, Peru, Mexico, Honduras, and El Salvador. Ecuador, Paraguay, Peru, and El Salvador, have a unified national format. The informed consents in Latin-American countries warn about acquiring TTI, but sepsis’s possible development is not specified, except for Chile.
Are there reports of cases of BC and sepsis associated with blood transfusion in Latin American countries?
According to the blood supply report for transfusions in Latin America and the Caribbean countries, only Brazil reported six BC cases associated with blood components’ transfusion (23). Four events in 2016 after the transfusion of 3,701,988 units (58.9% red blood cell units and 23.7% PT components) and two reactions in 2017 after transfusing 2,187,430 blood components (60.8% red blood cells units and 22.5% PT units). However, Anvisa in Brazil has 296 BC cases associated with transfusion investigated between 2007 and 2020 (Table 2). Actors in Brazil notify ATR through the health surveillance notification system (Notivisa), following national guidelines (30,34,40,45). In 2016, there were 42 cases to investigate, and sixteen were confirmed, probable, or possible. In 2017, of the 27 cases investigated, eight were confirmed, probable, or possible. Between 2018 and until September 2020, Anvisa investigated 88 possible ATR in components contaminated with bacteria. Seven confirmed, ten were probable, and twelve possible. Some adverse events such as BC are subject to further analyses, investigation, and retro vigilance. Therefore, the Notivisa system allows rectification and complementary information to be added to the original report; thus, the numbers are dynamic even for past events. In Colombia, blood banks and transfusion services report adverse reactions to the National Haemovigilance System (SIHEVI-INS) (48,52,53) administered by the National Institute of Health (INS). In 2018 and 2019, there were two BC case reports in PT units, but none confirmed. Until October 2020, SIHEVI-INS received notification of six BC cases (all of them in PT units). Three had definitive imputability and grade 3 severity, and one case had probable imputability and grade 1 severity. There were no records of BC cases, TTBI, or deaths, in other countries consulted by direct interview (Argentina, Chile, Paraguay, Ecuador, Guatemala, Honduras, El Salvador, and Mexico).
Table 2
| Year of presentation | Excluded | Probable | Inconclusive | Confirmed | Possible | Unlikely | Not concluded | Total |
|---|---|---|---|---|---|---|---|---|
| 2006 | 2 | 2 | ||||||
| 2007 | 2 | 2 | 2 | 1 | 7 | |||
| 2008 | 4 | 7 | 1 | 12 | ||||
| 2009 | 3 | 1 | 2 | 6 | ||||
| 2010 | 1 | 3 | 2 | 4 | 10 | |||
| 2011 | 2 | 1 | 2 | 4 | 1 | 10 | ||
| 2012 | 3 | 8 | 5 | 1 | 17 | |||
| 2013 | 4 | 2 | 4 | 3 | 3 | 4 | 20 | |
| 2014 | 6 | 9 | 7 | 3 | 2 | 1 | 28 | |
| 2015 | 5 | 3 | 11 | 3 | 2 | 3 | 27 | |
| 2016 | 9 | 5 | 14 | 6 | 5 | 3 | 42 | |
| 2017 | 3 | 4 | 11 | 1 | 3 | 5 | 27 | |
| 2018 | 6 | 11 | 3 | 4 | 6 | 30 | ||
| 2019 | 12 | 6 | 6 | 1 | 6 | 6 | 1 | 38 |
| 2020 | 4 | 4 | 3 | 2 | 1 | 6 | 20 | |
| Total | 64 | 38 | 80 | 45 | 32 | 30 | 7 | 296 |
†, The data were exported from the Brazilian database of “Sistema de Notificación de Vigilancia Sanitaria” Notivisa system for the period from 2007 until 09.30.2020, per year. The notifications include information about the diagnosis of the transfusion reaction. The evolution of the clinical picture is not included on the notification. The notification of reaction due to bacterial contamination follows the “Marco Conceitual e Operacional de Hemovigilância: Guia para a Hemovigilância no Brasil” (45) (page 28).
What are the limitations in Latin American countries that prevent the notification of TTBI?
Whitaker et al. (54) recently highlighted the characteristics of the world’s most successful haemovigilance programs. For example, transparent system; bidirectional; efficient reporting of donor and recipient adverse reactions; adoption and implementation of standard definitions; robust participation with high quality in the reports and translation of the events reported into recommended actions to be applied by the participants of the transfusion chain, through regular publication reports. In 2015, Muñiz et al. published the Ibero-American Manual of Hemovigilance, which aimed to unify the definitions and procedures related to haemovigilance (55). Wood et al. recently highlighted this initiative as one of the key strategies to strengthen national haemovigilance programs and exchange experiences internationally (56). Unfortunately, not all Latin American countries have adopted such definitions to date, nor have they unified their national programs. The identification of TTBI first requires the presence of a national haemovigilance system. The fact that Brazil and Colombia are the countries that have reported cases of TTBI indicates that they are the ones with the longest track record in their programs. However, we ask the main limitations to consolidate the haemovigilance programs and notify cases of TTBI in Latin America. The lack of knowledge prevents the authorities from showing the relevance and magnitude of the problem, impacting the resources allocated to implement haemovigilance systems. We postulated TTBI is underreporting due to each country’s different limitations.
To establish the magnitude of the underreporting of ATR, we compare the rates of accumulative ADRs/10,000 transfused blood components and Febrile non-hemolytic ART (Table 3). If the reported frequency of fever or chills were significantly lower than in the published series, we assumed that TTBI would have at least an equivalent underreporting. Considering that bacteria may have contaminated the product, these could not be viable at the time of transfusion. However, its metabolic products (particularly lipopolysaccharides) would be present and could give rise to a Febrile non-hemolytic transfusion reaction. The proportion of accumulative ATR reported by Latin American countries to PAHO compared to the Netherlands and New Zealand suggests an underreporting 24 and 20 times, respectively (Table 3). However, the analysis carried out based on the latest available report from Brazil (2014) would indicate that the underreporting is 32.5% (44), and in the case of Colombia (2018), it would be 77.1% (59). Until the authors are aware, the other Latin American countries do not have annual reports available for consultation. In non-hemolytic febrile reactions, no events were recorded for PAHO, while Brazil reported 4,468 (44) and Colombia 264 (59). The comparison of proportions between the Netherlands and Brazil indicated that the rate of febrile reactions is 2.0 times higher in the South American country. On the contrary, for Colombia, there is an underreporting of 68.2%. Therefore, the data suggest that the unexplained underreporting in BC cases associated with transfusion is not limited but extends to other ATR. The preceding emphasizes the relevance of making a diagnosis of each Latin American country’s current state and determining the behaviors to reduce the gaps for other countries with more experience in haemovigilance.
Table 3
| Country | Year | Total AcTR | Rate/10.000 transfused blood components | Febrile non-hemolytic transfusion reaction | Rate /10.000 transfused blood components | Transfused blood components |
|---|---|---|---|---|---|---|
| Netherlands (57) | 2018 | 2055 | 41.5 | 326 | 6.6 | 494,720 |
| New Zealand (20) | 2018 | 448 | 34.4 | 278 | 21.3 | 130,361 |
| Brazil (44) | 2014 | 9346 | 28.0 | 4468 | 13.4 | 3,337,857 |
| France (58) | 2019 | 7168 | 25.1 | 1617 | 5.7 | 2,852,426 |
| Colombia (59) | 2018 | 1176 | 9.5 | 264 | 2.1 | 1,239,059 |
| Spain (60) | 2018 | 1178 | 6.3 | 867 | 4.7 | 1,863,645 |
| Latin America (23) | 2017 | 1544 | 1.7 | NR | NR | 8,913,882 |
We identified other reasons for low ATR notifications, including TTBI. For example, there are not national entities responsible for compiling, analyzing, and publishing the haemovigilance information collected at local institutions. There is a lack of annual haemovigilance reports and continuity in government actors to consolidate blood policies. Finally, we did not find an external audit to guarantee that all countries’ laws, decrees, and blood policies on paper are adopted.
How many cases of BC and sepsis-related to transfusion should Latin America have?
In 2016, Latin American countries informed PAHO 10,175,660 units of whole blood and 210,267 units of apheresis collected. In 2017, there were 10,439,325 units of whole blood and 114,216 units of apheresis collected (23). Of the 20,939,468 blood donations reported for the Latin American and Caribbean region, 60% came from Brazil and Mexico (37.4% and 22.7%, respectively). The remaining 14.6% came from the Andean subregion (Colombia, Ecuador, Peru, and Bolivia) and 13.9% from the southern cone (Argentina, Uruguay, Chile, and Paraguay). In other words, the collection of nine out of every ten blood components in Latin America came from these countries (23). Therefore, the largest number of cases of BC and sepsis associated with transfusion should be there.
In Brazil, according to data from “Sistema Nacional de Informação da Produção Hemoterápica,” HEMOPROD, in 2019 there were collected 3,435,000 whole blood units (97.76%), and 78,831 (2.24%) apheresis. In Peru, at the end of 2018, the collection of 382,586 bags of whole blood was reported. In Colombia, there was 844,846 whole blood units and 51,957 apheresis procedures during 2019 (67). Because it was impossible to obtain a unified national number of total blood collection and apheresis in the other Latin American countries, the information notified to PAHO until 2017 was used to estimate potential BC cases (23).
Lafeuillade et al. reported that for the period 2000 to 2008, PT transfusion was implicated in 87% of BC cases (68). Similarly, the SHOT 2019 report compiled all the historical cases (44) from 1996 to 2019 of TTBI, establishing PT components in 84% of the cases (62). It is worth noting that between 2010 and 2019, the United Kingdom had no notifications of TTBI (62). Before implementing skin disinfection, diversion of the initial volume of collected blood, and detection of BC, the incidence of BC was approximately 1 in 1,000 platelet units and reports of sepsis were 1 in 15–100,000 transfusions (6). The American Red Cross experience showed that the implementation of skin disinfection, diversion of the initial volume of collected blood, and detection of BC reduced the rate of sepsis and deaths associated with BC in platelets by 60% (69,70). However, BC persists even automated bacterial culture in large part (residual risk) by false-negative cultures due to the low concentration of bacteria in the unit at the time of sampling. The use of PRT can reduce the risk of BC further. However, its ability to reduce contamination will depend on the type of technology, the initial bacterial load, the type of bacteria, and the time elapsed from collection of the unit to inactivation (6).
Based on this information, and considering Latin American countries have different signs of progress in the six major strategies described to reduce BC, we estimated the range of cases of BC, sepsis, and deaths that Latin American countries should inform PAHO if (I) there are no strategies implemented to reduce BC; (II) countries implement skin disinfection, deviation of the first milliliters of blood, and 100% microbiological culture; or if (III) countries incorporate other measures available to date (delayed-sampling bacterial screening algorithm, PRT, and bacterial detection just before transfusion).
Therefore, we estimate the frequency of BC (Table 4) and TTBI in PT units (Table 5), and TTBI in red blood cells (Table 6). According to the PAHO report, in 2017, the Latin American countries collected 4,613,316 units of PT and transfused 2,109,337 units (23). Similarly, these countries reported the transfusion of 6,223,024 units of red blood cells. During that same year, there were 2 cases of TTBI (both in Brazil), indicating a frequency of 1 case per 1,054,669 units of transfused platelets. This result contrasts with the information published by the haemovigilance reports from France (58), the United States of America (5), Canada (13), Spain (60), the United Kingdom (62), Austria (64), Germany (63), the Netherlands (57), European Commission (66), Australia (61), New Zealand (73) and Uganda (22) (Tables 4-6). Figure 1 shows the median, minimum, and maximum values of CB, TTBI, and deaths associated with platelet and red blood cell components in each country. The reported collection calculated that 4494 cases of BC, should have been reported in all Latin America (1,503 cases with the six strategies implemented; or 10,029 events without any strategy implemented). Of them, the majority had to appear in Brazil (median = 1,833; 613 with the six strategies implemented; 4,091 without any strategy implemented), Mexico (median = 835; 279 with the six strategies implemented; 1,863 without any strategy implemented) and Argentina (median = 693; 232 with the six strategies implemented; 1,550 without any strategy implemented) (Figure 1A and Table 4). About the TTBI or sepsis, it was determined that the median of reports should be 21 (minimum 2; maximum 145), which should have been preferentially reported by Brazil (median = 5; minimum = 0; maximum = 34), Argentina (median = 5; minimum = 0; maximum = 34) and Mexico (median = 4; minimum = 0; maximum = 26), Figure 1B and Table 4. The two cases reported in 2017 suggests that there is an underreporting of 91%. These findings coincide with that reported by other authors and emphasize the need to increase active hemovigilance programs (8-10).
Table 4
| Country | Obtained platelets units notified to PAHO in 2017 (23) | Estimated number of cases that should have been reported, based on bacterial detection results performed at the end of storage or on the expiration date. False negatives. Extracted from Table 53.3 Benjamin 2016 (6) | Number of cases that should have occurred from the false negatives in cultures reported by Jacobs et al. (71) | Number of cases that should have occurred from the false negatives in cultures reported by Ramirez-Arcos et al. (13) | Number of cases that should have occurred from the false negatives in cultures reported by Ramirez-Arcos S et al. (7) | Number of cases that should have occurred from the false negatives in cultures reported by McDonald, et al. (12) | Average (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Passport Study v(1: 1,509) | Irish Blood Service Day 8 (1: 460) | Irish Blood Service Day 4 (1: 828) | Welsh Blood Service (1: 1,073) | False negative (1: 3,069) | False negative (1: 1,176) |
False negative (1: 984) |
Indeterminate negative (1: 911) |
False negative | |||||||
| Argentina | 712,777 | 472 | 1550 | 861 | 664 | 232 | 606 | 724 | 782 | 737 (471–1002) | |||||
| Bolivia | 59,649 | 40 | 130 | 72 | 56 | 19 | 51 | 61 | 65 | 62 (39–84) | |||||
| Brazil | 1,881,885 | 1247 | 4091 | 2.273 | 1.754 | 613 | 1.600 | 1.912 | 2066 | 1945 (1244–2645) | |||||
| Chile | 182,953 | 121 | 398 | 221 | 171 | 60 | 156 | 186 | 201 | 189 (121–257) | |||||
| Colombia | 229,781 | 152 | 500 | 278 | 214 | 75 | 195 | 234 | 252 | 237 (152–323) | |||||
| Costa Rica | 55,295 | 37 | 120 | 67 | 52 | 18 | 47 | 56 | 61 | 57 (37–78) | |||||
| Cuba | 66,452 | 44 | 144 | 80 | 62 | 22 | 57 | 68 | 73 | 69 (44–93) | |||||
| Ecuador | 119,613 | 79 | 260 | 144 | 111 | 39 | 102 | 122 | 131 | 124 (79–168) | |||||
| Guatemala | 49,618 | 33 | 108 | 60 | 46 | 16 | 42 | 50 | 54 | 51 (33–70) | |||||
| Honduras | 33,284 | 22 | 72 | 40 | 31 | 11 | 28 | 34 | 37 | 34 (22–47) | |||||
| Mexico | 857,150 | 568 | 1.863 | 1.035 | 799 | 279 | 729 | 871 | 941 | 886 (567–1205) | |||||
| Nicaragua | 48,117 | 32 | 105 | 58 | 45 | 16 | 41 | 49 | 53 | 50 (32–68) | |||||
| Panama | 35,844 | 24 | 78 | 43 | 33 | 12 | 30 | 36 | 39 | 37 (24–50) | |||||
| Paraguay | 53,691 | 36 | 117 | 65 | 50 | 17 | 46 | 55 | 59 | 55 (36–75) | |||||
| Peru | 173,639 | 115 | 377 | 210 | 162 | 57 | 148 | 176 | 191 | 179 (115–244) | |||||
| Dominican Republic | 3713 | 2 | 8 | 4 | 3 | 1 | 3 | 4 | 4 | 4 (2–5) | |||||
| Uruguay | 49,855 | 33 | 108 | 60 | 46 | 16 | 42 | 51 | 55 | 52 (33–70) | |||||
| Latin America | 4,613,316 | 3057 | 10,029 | 5572 | 4299 | 1503 | 3923 | 4688 | 5064 | 4767 (3051–6483) | |||||
Table 5
| Country | Transfused platelets notified to PAHO in 2017 (23) | TTBI/SEPSIS notified to PAHO in 2017 (23) | France 1996–1998 (72) | France 2000–2008 (68) | USA 1995–2004 (6) | USA 2005–2013 (6) | American Red Cross 2006–2011 (6) | USA 2010–2016 (5) | Spain 2018 (60) | Germany 2018 (64) | Germany 2000–2018 (63) | Australia 2017–2018 (17) | Canada 2010–2016 (13) |
Canada 2017–2019 (7) | New Zealand 2018 (20) | UK 2011–2015 | European Commission 2018 (66) | Average (95% CI) | Average (95% CI) | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TTBI/SEPSIS (1: 29,375) | DEATHS (1: 235,000) | TTBI/SEPSIS (1: 40,417) | DEATHS (1: 216,000) | DEATHS (1: 250,000) | DEATHS (1: 642,857) | TTBI/SEPSIS (1: 106,921) | DEATHS (1: 1,015,750) | TTBI/SEPSIS (1: 51,282) | TTBI/SEPSIS (1: 45,275) | DEATHS (1: 226,376) | TTBI/SEPSIS (1: 168,350) | DEATHS (1: 505,312) | TTBI/SEPSIS (1: 121,065) | TTBI/SEPSIS (1: 75,000) | TTBI/SEPSIS (1: 100,000) | DEATHS (1: 500,000) | TTBI/SEPSIS (1: 350,000) | TTBI/SEPSIS (1: 14,515) | TTBI/SEPSIS (1: 1,239,029) (12) | TTBI/SEPSIS (1: 384,903) | DEATHS (1: 1,347,162) | TTBI/SEPSIS | DEATHS | |||||||||||||||||||
| Argentina | 491,700 | 0 | 17 | 2 | 12 | 2 | 2 | 1 | 5 | 0 | 10 | 11 | 2 | 3 | 1 | 4 | 7 | 5 | 1 | 1 | 34 | 0 | 1 | 0 | 8.4 (3.5–13.3) | 1.3 (0.8–1.8) | ||||||||||||||||
| Bolivia | 28,409 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0.5 (0.2–0.8) | 0.1 (0.0–0.1) | ||||||||||||||||
| Brazil | 491,640 | 2 | 17 | 2 | 12 | 2 | 2 | 1 | 5 | 0 | 10 | 11 | 2 | 3 | 1 | 4 | 7 | 5 | 1 | 1 | 34 | 0 | 1 | 0 | 8.4 (3.5–13.3) | 1.3 (0.8–1.8) | ||||||||||||||||
| Chile | 96,674 | 0 | 3 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 2 | 2 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 7 | 0 | 0 | 0 | 1.7 (0.7–2.6) | 0.3 (0.2–0.4) | ||||||||||||||||
| Colombia | 216,918 | 0 | 7 | 1 | 5 | 1 | 1 | 0 | 2 | 0 | 4 | 5 | 1 | 1 | 0 | 2 | 3 | 2 | 0 | 1 | 15 | 0 | 1 | 0 | 3.7 (1.5–5.9) | 0.6 (0.4–0.8) | ||||||||||||||||
| Costa Rica | 32,084 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0.5 (0.2–0.9) | 0.1 (0.1–0.1) | ||||||||||||||||
| Cuba | 40,847 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0.7 (0.3–1.1) | 0.1 (0.1–0.2) | ||||||||||||||||
| Ecuador | 39,437 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0.7 (0.3–1.1) | 0.1 (0.1–0.1) | ||||||||||||||||
| Guatemala | 28,202 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0.5 (0.2–0.8) | 0.1 (0.0–0.1) | ||||||||||||||||
| Honduras | 22,238 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0.4 (0.2–0.6) | 0.1 (0.0–0.1) | ||||||||||||||||
| Mexico | 370,934 | 0 | 13 | 2 | 9 | 2 | 1 | 1 | 3 | 0 | 7 | 8 | 2 | 2 | 1 | 3 | 5 | 4 | 1 | 1 | 26 | 0 | 1 | 0 | 6.3 (2.6–10.1) | 1.0 (0.6–1.4) | ||||||||||||||||
| Nicaragua | 36,052 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0.6 (0.3–1.0) | 0.1 (0.1–0.1) | ||||||||||||||||
| Panama | 19,532 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0.3 (0.1–0.5) | 0.1 (0.0–0.1) | ||||||||||||||||
| Paraguay | 29,581 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0.5 (0.2–0.8) | 0.1 (0.1–0.1) | ||||||||||||||||
| Peru | 129,089 | 0 | 4 | 1 | 3 | 1 | 1 | 0 | 1 | 0 | 3 | 3 | 1 | 1 | 0 | 1 | 2 | 1 | 0 | 0 | 9 | 0 | 0 | 0 | 2.2 (0.9–3.5) | 0.4 (0.2–0.5) | ||||||||||||||||
| Dominican Republic | 851 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | ||||||||||||||||
| Uruguay | 35,149 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0.6 (0.2–1.0) | 0.1 (0.1–0.1) | ||||||||||||||||
| Latin America | 2,109,337 | 2 | 72 | 9 | 52 | 10 | 8 | 3 | 20 | 2 | 41 | 47 | 9 | 13 | 4 | 17 | 28 | 21 | 4 | 6 | 145 | 2 | 5 | 2 | 36.1 (15.0–57.2) | 5.8 (3.6–7.9) | ||||||||||||||||
Table 6
| Country | Transfused red blood cells notified to PAHO in 2017 (23) | France 1996–1998 (72) | France 2000–2008 (68) | France 2019 (58) | Netherlands 2018 (57) | USA 1995–2004 (6) | USA 2005–2013 (6) | USA 2010–2016 (5) | Germany 2018 (63) | Germany 2000–2018 (63) | Australia 2017–2018 (61) | New Zealand 2018 (73) | Austria 2019 (64) | Average (95% CI) | Average (95% CI) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TTBI/SEPSIS (1: 141,379) | DEATHS (1: 1,025,000) | TTBI/SEPSIS (1: 2,571,429) | DEATHS (1: 9,000,000) | TTBI/SEPSIS (1: 1,426,213) | TTBI/SEPSIS (1: 494,720) | DEATHS (1: 4,800,000) | DEATHS (1: 32,000,000) | TTBI/SEPSIS (1: 729,390) | TTBI/SEPSIS (1: 3,448,275) | TTBI/SEPSIS (1: 2,040,816) | TTBI/SEPSIS (1: 500,000) | TTBI/SEPSIS (1: 98,850) | TTBI/SEPSIS (1: 317,312) | TTBI/SEPSIS | DEATHS | |||||||||||||||
| ARGENTINA | 1,016,808 | 7 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 1 | 0 | 0 | 2 | 10 | 3 | 2.8 (0.7–4.9) | 0.3 (0.1–0.6) | |||||||||||||
| BOLIVIA | 78,100 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0.2 (0.1–0.4) | 0.0 (0.0–0.0) | |||||||||||||
| BRAZIL | 1,329,853 | 9 | 1 | 1 | 0 | 1 | 3 | 0 | 0 | 2 | 0 | 1 | 3 | 14 | 4 | 3.7 (0.9–6.4) | 0.4 (0.1–0.8) | |||||||||||||
| CHILE | 231,708 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0.6 (0.2–1.1) | 0.1 (0.0–0.1) | |||||||||||||
| COLOMBIA | 835,367 | 6 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 1 | 0 | 0 | 2 | 9 | 3 | 2.3 (0.6–4.1) | 0.3 (0.1–0.5) | |||||||||||||
| COSTA RICA | 64,044 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0.2 (0.0–0.3) | 0.0 (0.0–0.0) | |||||||||||||
| CUBA | 219,808 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0.6 (0.2–1.1) | 0.1 (0.0–0.1) | |||||||||||||
| ECUADOR | 107,445 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0.3 (0.1–0.5) | 0.0 (0.0–0.1) | |||||||||||||
| EL SALVADOR | 78,198 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0.2 (0.1–0.4) | 0.0 (0.0–0.0) | |||||||||||||
| GUATEMALA | 131,507 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0.4 (0.1–0.6) | 0.0 (0.0–0.1) | |||||||||||||
| HONDURAS | 71,056 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0.2 (0.1–0.3) | 0.0 (0.0–0.0) | |||||||||||||
| MEXICO | 1,500,941 | 11 | 1 | 1 | 0 | 1 | 3 | 0 | 0 | 2 | 0 | 1 | 3 | 15 | 5 | 4.2 (1.1–7.3) | 0.5 (0.1–0.9) | |||||||||||||
| NICARAGUA | 70,969 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0.2 (0.1–0.3) | 0.0 (0.0–0.0) | |||||||||||||
| PANAMA | 48,613 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0.1 (0.0–0.2) | 0.0 (0.0–0.0) | |||||||||||||
| PARAGUAY | 72,404 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0.2 (0.1–0.4) | 0.0 (0.0–0.0) | |||||||||||||
| PERU | 288,592 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 1 | 0.8 (0.2–1.4) | 0.1 (0.0–0.2) | |||||||||||||
| DOMINICAN REPUBLIC | 3,889 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | |||||||||||||
| URUGUAY | 73,722 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0.2 (0.1–0.4) | 0.0 (0.0–0.0) | |||||||||||||
| LATIN AMERICA | 6,223,024 | 44 | 6 | 2 | 1 | 4 | 13 | 1 | 0 | 9 | 2 | 3 | 12 | 64 | 20 | 17.3 (4.4–30.2) | 2.1 (0.4–3.7) | |||||||||||||
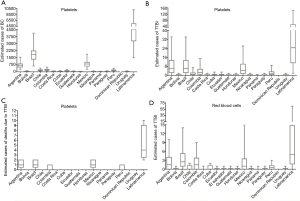
Similarly, we estimated the number of deaths expected due to platelets’ transfusion contaminated with bacteria in Latin America (Figure 1C and Table 5). The PAHO report did not inform any death associated with the transfusion of blood components contaminated with bacteria (23), but we estimated that only for this component a median of 4 deaths should have occurred (minimum 2, maximum 10). Once again, these findings suggest an underreporting of events more significant than 99% in these countries and that it is necessary to consolidate strategies between governments and scientific societies to increase notifications.
Kuehnert estimated the risk of death due to BC in red blood cells transfused in the US between 1998–2000 in 0.13 per million (74). In the period 1995–2004, FDA estimated the risk of death at 0.21 per million. Between 2005 to 2013, the FDA determined the risk of death at 0.031 per million (6). Likewise, France estimated the risk of death from BC of transfused red blood cells at 0.98 per million between 1996–1998 (72). That same risk of death went to 0.11 between 2000–2008 (68). Lafeuillade et al. suggested a massive introduction of pre-storage leukoreduction as the cause of this fall in deaths (68). Therefore, prestorage leukoreduction reduced mortality risk due to BC in erythrocytes by approximately 76–89%. Because not all blood banks in Latin America perform leukoreduction, we estimate the number of deaths that Latin American countries should inform to PAHO based on the US and France’s risks before and after implementing leukoreduction (Figure 1D and Table 6). We estimated an average underreporting of at least 89%.
Our review has several limitations. To date, there are 43 countries in Latin America and the Caribbean (23). However, the information collected in this article was obtained directly from eight (that is, 18.6%) and indirectly from eighteen (41.8%). It was impossible to precisely know the total number of units collected during 2018 or 2019 in most countries. Nor was it possible to precisely define the number of institutions that have adopted leukoreduction or PRT strategies. It was essential to have the PAHO report to make the case estimates. However, the information provided first-hand by Brazil and Colombia suggests some inaccurate data reported in the final PAHO document. For example, according to the INS, Colombia collected in the 2016 and 2017 44,037 (75) and 66,715 (76) apheresis, while the PAHO report mentioned 217,427 and 167,412, respectively. Besides, Anvisa recorded 69 cases of TTBI in 2016–2017 period, but the PAHO report mentioned six. Likewise, in Table 5, despite Brazil’s population is 4.6 that of Argentina, both countries have a similar value of transfused platelets reported to PAHO. These discrepancies are because notification systems like Novitisa and SIHEV-INS are dynamic. Therefore, a later notification is allowed, and this data changes with time. However, we consider that this first estimate of BC in Latin America will represent a starting point for further investigations.
Conclusions
Latin American countries have progressed in the implementation of strategies aimed at reducing BC in blood components. Although most of the contamination will probably occur in the PT component, there is a remained risk in red blood cells because leukoreduction practices are not widely used. There was an underreporting of 20-24 times in accumulative ATRs and Febrile non-hemolytic ART. Likewise we calculated in this review a potential risk of TTBI between 7 to 29 times higher than reported to PAHO. Based upon this survey, we recommend that the priorities to improve the safety of blood components for BC should be: (I) guarantee a national entity that collects, analyzes, and provides feedback to all stakeholders regarding the annual findings of hemovigilance; (II) Implement external audits to corroborate the reliability of the reports; (III) successful exchange experiences from Brazil and Colombia with the other Latin American countries to strengthen their haemovigilance systems; (IV) widespread adoption of PRT. Although some people may consider the implementation of PRT inviable due to costs, the experience of various public and private institutions in Brazil, Colombia, Chile, Argentina, and Honduras, suggests that PRT adoption is possible.
Acknowledgments
The authors want to thank Fernando Palomino Quintana (Fundación para alternativas a la transfusión sanguínea, FUATS) for his invaluable comments, suggestions, and critical review of this work.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Sandra Ramirez-Arcos) for the series “Bacterial Contamination of Platelet Components” published in Annals of Blood. The article has undergone external peer review.
Peer Review File: Available at https://dx.doi.org/10.21037/aob-20-92
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob-20-92). The series “Bacterial Contamination of Platelet Components” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- OMS. El uso clínico de la sangre en medicina, obstetricia, pediatría y neonatología,cirugía y anestesia, trauma y quemaduras. Organización Mundial de la Salud; 2001. Available online: https://www.who.int/bloodsafety/clinical_use/en/Manual_S.pdf?ua=1
- Vaillant A, Sticco K. Transfusion transmitted disease [Updated 2020 Jul 2]. StatPearls Publishing LLC; 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538427/
- Stramer S, Galel S. Infectious Disease Screening. In: Fung M, Eder A, Spitalnik S, editors. Technical Manual. Nineteenth Ed. Bethesda, Maryland. USA: AABB; 2017:179.
- Stramer SL. Current perspectives in transfusion-transmitted infectious diseases: emerging and re-emerging infections. ISBT Sci Ser 2014;9:30-6. [Crossref] [PubMed]
- Haass KA, Sapiano MRP, Savinkina A, et al. Transfusion-Transmitted Infections Reported to the National Healthcare Safety Network Hemovigilance Module. Transfus Med Rev 2019;33:84-91. [Crossref] [PubMed]
- Benjamin R. Bacterial contamination of blood components. In: Simon T, McCullough J, Snyder E, Solheim B, RG. S, editors. Rossi’s Principles of Transfusion Medicine. 1. Fifth Edition. ed: John Wiley & Sons, Ltd.; 2016:11.
- Ramirez-Arcos S, Evans S, McIntyre T, et al. Extension of platelet shelf life with an improved bacterial testing algorithm. Transfusion 2020;60:2918-28. [Crossref] [PubMed]
- Hong H, Xiao W, Lazarus HM, et al. Detection of septic transfusion reactions to platelet transfusions by active and passive surveillance. Blood 2016;127:496-502. [Crossref] [PubMed]
- Jacobs MR, Good CE, Lazarus HM, et al. Relationship between bacterial load, species virulence, and transfusion reaction with transfusion of bacterially contaminated platelets. Clin Infect Dis 2008;46:1214-20. [Crossref] [PubMed]
- Raval JS, Mazepa MA, Russell SL, et al. Passive reporting greatly underestimates the rate of transfusion-associated circulatory overload after platelet transfusion. Vox Sang 2015;108:387-92. [Crossref] [PubMed]
- FDA. Fatalities Reported to FDA Following Blood Collection and Transfusion. Annual Summary for Fiscal Year 2017. Food and drugs administration; 2019.
- McDonald C, Allen J, Brailsford S, et al. Bacterial screening of platelet components by National Health Service Blood and Transplant, an effective risk reduction measure. Transfusion 2017;57:1122-31. [Crossref] [PubMed]
- Ramirez-Arcos S, DiFranco C, McIntyre T. Transfusion 2017;57:2174-81. [Crossref] [PubMed]
- Hemovigilancia Ud, y AdHSGdPdlS, Epidemiología Dirección General de Salud Pública Calidad e Innovación E. Hemovigilancia año 2017. Available online: https://www.mscbs.gob.es/profesionales/saludPublica/medicinaTransfusional/hemovigilancia/docs/Informe2017.pdf
- Narayan S, Poles D, et a. The 2018 Annual SHOT Report (2019). Available online: https://www.shotuk.org/wp-content/uploads/myimages/SHOT-Report-2018_Web_Version-1.pdf
- Ratonga T, Aotearoa T. New Zeland National Haemovigilance Programme. Annual Report 2017. Available online: https://www.nzblood.co.nz/assets/Haemovigilance/Haemovigilance-Annual-Report-2017.pdf
- Australian R. Transfusion-transmissible infections in Australia 2017 Surveillance Report. Available online: https://kirby.unsw.edu.au/sites/default/files/kirby/report/SERP_Transfusion-transmissible-infections-in-Australia-Surveillance-Report-2017.pdf
- ANSM F. Rapport d’activité hémovigilance 2018. 15e rapport national d’hémovigilance ed. Available online: https://www.ansm.sante.fr/var/ansm_site/storage/original/application/02e815958aa822f7f5e2274b47837d00.pdf
- Schipperus M, Soons J, de-Vooght K. TRIP REPORT 2017. Hemovigilance. Extended version. Available online: https://www.notifylibrary.org/sites/default/files/TRIP%20hemovigilance%202017.pdf
- Badami K, Dagger J, Sadani D. National Haemovigilance Programme. Annual Report 2018. Available online: https://www.nzblood.co.nz/assets/Uploads/Haemovigilance-Annual-Report-2018.pdf
- BASC. Hämovigilanz-Bericht 2019. Available online: https://www.basg.gv.at/fileadmin/redakteure/Blut_Gewebe/Berichte/Haemovigilanz_Bericht_2019.pdf
- Hume HA, Ddungu H, Angom R, et al. Platelet transfusion therapy in sub-Saharan Africa: bacterial contamination, recipient characteristics, and acute transfusion reactions. Transfusion 2016;56:1951-9. [Crossref] [PubMed]
- OPS. Suministro de Sangre para transfusiones en los países de américa latina y el caribe 2016-2017. Available online: https://www.paho.org/es/documentos/suministro-sangre-para-transfusiones-paises-america-latina-caribe-2016-2017
- Ramirez‐Arcos S, McDonald CP, Benjamin RJ, et al. Survey for bacterial testing of platelet components in Latin America. ISBT Science Series 2017;12:336-9. [Crossref]
- AABB. Accreditation Program. Available online: http://www.aabb.org/sa/overview/Pages/program.aspx
- AABB. AABB Accredited Blood Banks, Transfusion Services, and Blood Centers. Available online: https://www.aabb.org/standards-accreditation/accreditation/accredited-facilities/aabb-accredited-blood-banks-transfusion-services-and-blood-centers
- CAT. Procedimiento de Certificación de Centros y Servicios de Transfusión. I Available online: https://www.catransfusion.es/media/upload/arxius/documentos/procedimientos/PR-CAT-01%20Procedimiento%20de%20certificacion%20centros%20y%20servicios%20de%20transfusion%20Ed%2015%20Rev%20%202020.pdf
- Presidência-da R. LEI No 10.205, DE 21 DE MARÇO DE 2001. Available online: https://www.planalto.gov.br/ccivil_03/leis/leis_2001/l10205.htm
- República P-d. DECRETO Nº 3.990, DE 30 DE OUTUBRO DE 2001. Available online: https://www.planalto.gov.br/ccivil_03/decreto/2001/d3990.htm
- MS A. RDC 34/2014. RESOLUÇÃO DA DIRETORIA COLEGIADA –RDC N° 34, DE 11 DE JUNHO DE 2014. Available online: http://antigo.anvisa.gov.br/documents/10181/2867975/%282%29RDC_34_2014_COMP.pdf
- INVIMA. Banco de Sangre y Hemoderivados. Available online: Available online: https://www.invima.gov.co/sangre-y-hemoderivados
- Supersalud. APTr 1.2. En: Pauta de Cotejo, Manual de Atención cerrada. Available online: https://www.supersalud.gob.cl/observatorio/671/articles-4530_pauta_AC_pdf
- Minsal. Norma general Técnica N° 0146. Norma que regula el procedimiento de atención de donantes de sangre (En sitio fijo o móvil). Available online: https://www.hematologia.org/bases/arch1087.pdf
- Saúde M-d. PRT 05/17 Anexo IV. Portaria de ConsolidaÇÃo nº 5, de 28 de Setembro de 2017. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/gm/2017/prc0005_03_10_2017.html#ANEXOIV: Ministério-da Saúde
- Minsal. Guía Técnica: Orientaciones sobre las unidades de medicina transfusional. Unidades de Medicina Transfusional. 1026. Available online: https://www.minsal.cl/wp-content/uploads/2017/03/2013-Res-Ex-1026-Guia-Tecnica-_-Orientaciones-sobre-las-UMT.pdf
- Ministerio S. Decreto 1571 de 1993. Available online: Available online: https://www.minsalud.gov.co/Normatividad_Nuevo/DECRETO%20%201571%20DE%201993.pdf
- MinSalud PS, Salud. IdETe. Guía de Práctica ClínicaTransfusión de sangre y de sus componentes. Available online: http://www.iets.org.co/gpc_adopcion_2016/Documents/GPC_USO_DE_LASANGRE_version_preliminar.pdf
- Ministerio d-S. Resolución 3100 de 2019. Available online: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/DE/DIJ/resolucion-3100-de-2019.pdf
- AAHI. Manual de Hemovigilancia. Available online: http://www.aahi.org.ar/wp-content/uploads/2014/10/MANUAL-HV-AAHI.pdf
- MS A. INSTRUÇÃO NORMATIVA Nº 1, DE 17 DE MARÇO DE 2015. Available online: http://antigo.anvisa.gov.br/documents/10181/2867325/IN_01_2015_COMP2.pdf
- Peñuela O, Beltran M, Rebollo S, et al. Manual de hemovigilancia. In: INS, editor. Bogotá: Instituto Nacional de Salud; 2010:16-7.
- Lama T, Hurado H, Rubio J, et al. Manual Técnico de Hemovigilancia en Bancos de Sangre y Servicios de Medicina Transfusional. Available online: https://aplicaciones.msp.gob.ec/salud/archivosdigitales/documentosDirecciones/dnn/archivos/MANUAL%20T%C3%89CNICO%20DE%20HEMOVIGILANCIA%20EN%20BANCOS%20DE%20SANGRE%20Y%20SERVICIOS%20DE%20MEDICINA%20TRANSFUSIONAL.pdf
- Poder L. Ley 3441. Available online: https://www.bacn.gov.py/archivos/999/20131112092748.pdf
- ANVISA. Boletim de Hemovigilância nº 7. Available online: https://www.gov.br/anvisa/pt-br/centraisdeconteudo/publicacoes/fiscalizacao-e-monitoramento/publicacoes-de-fiscalizacao-e-monitoramento/boletim-de-hemovigilancia-no-7.pdf/@@download/file/Boletim de Hemovigil%C3%A2ncia n%C2%BA 7.pdf: Agência Nacional de Vigilância Sanitária; 2015:14.
- ANVISA. Marco Conceitual e Operacional de Hemovigilância:Guia para a Hemovigilância no Brasil. In: ANVISA ANdVS-, editor. Available online: https://www.gov.br/anvisa/pt-br/assuntos/fiscalizacao-e-monitoramento/hemovigilancia/publicacoes/marco-conceitual-e-operacional-de-hemovigilancia-guia-para-a-hemovigilancia-no-brasil.pdf/@@download/file/Marco Conceitual e Operacional de Hemovigil%C3%A2ncia - Guia para a Hemovigil%C3%A2ncia no Brasil.pdf
- ANVISA. Relatórios e boletins de hemovigilância 2007-2015. In: Anvisa ANdVS-, editor. Available online: https://www.gov.br/anvisa/pt-br/assuntos/fiscalizacao-e-monitoramento/hemovigilancia/publicacoes
- Bermúdez-Forero M, García-Otálora M. Áreas estratégicas. Categoría 2: Hemovigilancia. Subcategoría: Informes 2016-2018. Available online: http://www.ins.gov.co/Direcciones/RedesSaludPublica/DonacionSangre/Paginas/bancos-de-sangre.aspx
- Bermúdez-Forero MI, Soto-Viáfara JA, Anzola-Samudio D, et al. Notificación y reporte RAT SIHEVI-INS. In: Salud CRNBdSySdTINd, editor. Available online: https://www.ins.gov.co/Direcciones/RedesSaludPublica/DonacionSangre/Publicaciones/DRSP_%20Notificacion-Reporte-RAT_2017_07_23.pdf
- Bermúdez-Forero M, García-Otálora M. Informe Nacional de Hemovigilancia 2016. Available online: http://www.ins.gov.co/Direcciones/RedesSaludPublica/DonacionSangre/AreasEstrategicas/Informe%20Hemovigilancia%20Colombia%202016.pdf
- ISBT A, IHN. Standard for Surveillance of Complications Related to Blood Donation International Society of Blood Transfusion 2014.
- ISBT I. Proposed standard definitions for surveillance of non infectious adverse transfusion reaction. Available online: https://www.isbtweb.org/fileadmin/user_upload/Proposed_definitions_2011_surveillance_non_infectious_adverse_reactions_haemovigilance_incl_TRALI_correction_2013_TACO_correction_2018.pdf
- Bermúdez-Forero M, Gardeazabal-Acuña P, Soto-Viafara J, et al. Design, development and implementation of a haemovigilance system in Colombia (SIHEVI). Abstract of the 35th international congress of the ISBT. Vox Sang 2018;113:P-785. Available online: https://onlinelibrary.wiley.com/doi/10.1111/vox.12658
- INS C. Lineamiento técnico para la selección de donantes de sangre en Colombia. Available online: https://www.ins.gov.co/Direcciones/RedesSaludPublica/DonacionSangre/Publicaciones/Lineamiento%20tecnico%20Selecci%C3%B3n%20de%20donantes%202018.pdf
- Whitaker BI, Belov A, Anderson SA. Progress in US hemovigilance: can we still learn from others? Transfusion 2019;59:433-6. [Crossref] [PubMed]
- Muñiz-Diaz E, Leon G, Torres O. Manual iberoamericano de hemovigilancia. BST G, OPS, editor. Barcelona, España2015.
- Wood EM, Ang AL, Bisht A, Bolton-Maggs PH, Bokhorst AG, Flesland O, et al. International haemovigilance: what have we learned and what do we need to do next? Transfus Med 2019;29:221-30. [Crossref] [PubMed]
- Bermúdez-Forero MI, García-Otálora MA. Informe de reacciones adversas a la transfusión notificadas a SIHEVI-INS©durante 2018. Available online: http://www.ins.gov.co/Direcciones/RedesSaludPublica/DonacionSangre/AreasEstrategicas/Informe%20de%20Hemovigilancia%20%202018.pdf
- Bermúdez-Forero M-I. Informe Ejecutivo de la Red Nacional Bancos de Sangre Colombia 2019 Available online: https://www.ins.gov.co/BibliotecaDigital/informe-nacional-bancos-de-sangre-2019.pdf
- Lafeuillade B, Eb F, Ounnoughene N, et al. Residual risk and retrospective analysis of transfusion-transmitted bacterial infection reported by the French National Hemovigilance Network from 2000 to 2008. Transfusion 2015;55:636-46. [Crossref] [PubMed]
- Narayan S, Poles D, et al. The 2019 Annual SHOT Report (2020). In: Group SHoTSS, editor. Available online: https://www.shotuk.org/wp-content/uploads/myimages/SHOT-REPORT-2019-Final-Bookmarked-v2.pdf
- Benkebil M, Boudjedir K, Drougard S, et a. 17eme rapport National d'Hemovigilance 2019. Available online: https://ansm.sante.fr/var/ansm_site/storage/original/application/a2de7023c1b7daeda5f675a449aaef2d.pdf
- Ministerio S. Hemovigilancia año 2018. Available online: https://www.mscbs.gob.es/fr/profesionales/saludPublica/medicinaTransfusional/hemovigilancia/docs/Informe2018.pdf
- BASG. Hämovigilanz-Bericht 2019. Available online: https://www.basg.gv.at/fileadmin/redakteure/Blut_Gewebe/Berichte/Haemovigilanz_Bericht_2019.pdf
- Funk M, Heiden M, Müller S, et a. Hämovigilanz-Bericht des Paul-Ehrlich-Instituts 2018. In: AMG. AdMvRuZni, editor. Available online: https://www.pei.de/DE/arzneimittelsicherheit/haemovigilanz/haemovigilanzberichte/haemovigilanzberichte-node.html
- TRIP. Report 2018 Hemovigilance Extended version. Available online: https://www.tripnet.nl/wp-content/uploads/2020/08/Trip.HEMO_uitgebreid_ENGdef2020-4.pdf
- European C. Summary of the 2019 annual reporting of serious adverse reactions and events for blood and blood components. Data collected from 01/01/2018 to 31/12/2018. In: Safety. D-GFHaf, editor. Available online: https://www.basg.gv.at/fileadmin/redakteure/Blut_Gewebe/Berichte/200930_Final_Summary_Report_Blood_2019__data_2018_.pdf
- National-Blood A. Australian Haemovigilance report. Data for 2017-18. In: National Blood Authority A, editor. Available online: https://www.blood.gov.au/system/files/Australian%20Haemovigilance%20Report%202017-18_FINAL%20v2.pdf
- Badami K, Dagger J, Sadani D. Annual Report 2018. National Haemovigilance Programme. New Zealand Blood Service. In: Aotearoa NTRTo, editor. Available online: https://www.nzblood.co.nz/assets/Uploads/Haemovigilance-Annual-Report-2018.pdf
- Fang CT, Chambers LA, Kennedy J, et al. Detection of bacterial contamination in apheresis platelet products: American Red Cross experience, 2004. Transfusion 2005;45:1845-52. [Crossref] [PubMed]
- Benjamin RJ, Dy B, Perez J, et al. Bacterial culture of apheresis platelets: a mathematical model of the residual rate of contamination based on unconfirmed positive results. Vox Sang 2014;106:23-30. [Crossref] [PubMed]
- Kuehnert MJ, Roth VR, Haley NR, et al. Transfusion-transmitted bacterial infection in the United States, 1998 through 2000. Transfusion 2001;41:1493-9. [Crossref] [PubMed]
- Perez P, Salmi LR, Folléa G, et al. Determinants of transfusion-associated bacterial contamination: results of the French BACTHEM Case-Control Study. Transfusion 2001;41:862-72. [Crossref] [PubMed]
- Bermúdez-Forero MI. Informe Anual (2016) Red Nacional de Bancos de Sangre y Servicios de Transfusión. Available online: http://www.ins.gov.co/Direcciones/RedesSaludPublica/DonacionSangre/AreasEstrategicas/Informe%20Anual%20Red%20Sangre%202016%20v2.pdf
- Bermúdez-Forero M. Informe Anual Red Nacional De Bancos De Sangre Y Servicios De Transfusión, Colombia 2017. Available online: https://www.ins.gov.co/BibliotecaDigital/informe-anual-red-sangre-2017-v2.pdf
- Edens C, Haass KA, Cumming M, et al. Evaluation of the National Healthcare Safety Network Hemovigilance Module for transfusion-related adverse reactions in the United States. Transfusion 2019;59:524-33. [Crossref] [PubMed]
- Jacobs MR, Smith D, Heaton WA, et al. Detection of bacterial contamination in prestorage culture-negative apheresis platelets on day of issue with the Pan Genera Detection test. Transfusion 2011;51:2573-82. [Crossref] [PubMed]
Cite this article as: García-Otálora MA, Núñez-Ahumada MA, Kuperman S, Oliveira-Leitão L, Silveira F, Martins R, Pesántez-Pesántez M, Gutiérrez JL, Alcaráz-Paredes RE, Manrique Castagnola ER, Bravo-Lindoro AG, Vinelli E, González ML, Juárez K, Bermúdez-Forero MI. Bacterial contamination and sepsis associated with transfusion: current status in Latin America. Ann Blood 2021;6:26.


