
Bacterial culture of platelets with the large volume delayed sampling approach: a narrative review
Introduction
Bacterial contamination of blood components leading to post-transfusion sepsis (PTS) has long been recognized as a major infectious risk related to transfusion (1), particularly of platelet concentrates. This led blood centers to introduce various measures with the aim of reducing that risk: optimised skin disinfection, diversion of the first milliliters of blood collected into a derivation pouch, and bacterial culture of platelet concentrates at 24 hours post-collection (with most sites using a single blood culture bottle). Not all countries introduced bacterial culture as a risk mitigation strategy.
These measures, although useful (2-4), did not eliminate the risk of PTS. The American Red Cross reported a residual risk of PTS of 1 in 100,000 following transfusion of culture-negative apheresis platelets (sampled at 24 hours, single aerobic bottle) (5). At Hema-Quebec, three cases of bacterial sepsis were reported to us by the Quebec Hemovigilance System following the transfusion of close to 300,000 culture-negative platelet concentrates (culture at 18–24 hours, one bottle), one of which was fatal. A similar observation was reported by Ramirez-Arcos and colleagues (6). These residual risk figures were based on passive surveillance. Hong et al. in Cleveland cultured all platelets transfused to their patients at the time of transfusion over a seven-year period; all the platelets transfused to their patients had been culture-negative when sampled at 24 hours (7). They found bacterial contamination in 20 of 51,440 transfused platelet component: 5 of these contaminated platelets caused septic transfusion reactions, one of which was fatal. None of these cases were reported to the blood bank and would have been missed had cultures not been systematically done on all platelets just before transfusion. Therefore, with an active surveillance approach, they found a risk of PTS of 1 in 10,000 despite a negative culture after sampling at 24 hours. Finally, Walker et al. did a meta-analysis of studies that evaluated the detection rate of bacterial contamination of platelets whose 24-hour culture was negative, using either secondary culture or rapid testing: they found that the rate of detection by culture was 0.94 per 1,000 (95% CI, 0.54–1.32) and 0.09 per 1,000 (95% CI, 0.01–0.25) by rapid testing (8).
The FDA in 2019 published a guidance on bacterial risk control strategies for blood collection establishments and transfusion services to enhance the safety and availability of platelets for transfusions (9). In this document, various options were proposed, one of which is a large volume delayed sampling (LVDS) strategy. This paper will describe what LVDS culture is, what is the rationale behind its use, the evidence supporting it as a safety enhancing measure, and certain practical considerations around its implementation. We present the following article in accordance with the narrative review checklist (available at http://dx.doi.org/10.21037/aob-21-4).
What is LVDS culture?
Basically, it is the culture of a platelet component using a volume greater and a delay longer than a culture done at 24 hours using a single aerobic bottle. In its recent guidance (9), FDA defines LVDS culture as a culture done at least 36 hours (for a 5-day expiration) or 48 hours (for a 7-day expiration) after collection with at least 16 mL of product, of which 8 mL are inoculated into both an aerobic and an anaerobic blood culture bottle. If the product is a double or triple apheresis, each daughter bag must undergo the 16 mL sampling. In addition, FDA requires a 12-hour hold before releasing the platelets into inventory. The blood operators that have implemented LVDS diverge somewhat from the FDA requirements. At National Health Service Blood and Transplant (NHSBT), the delay is 36–48 hours and the hold is 6 hours for a 7-day expiration (10). At Canadian Blood Services (CBS), the delay is 36 hours, and for double apheresis one anaerobic bottle and three aerobic bottles are inoculated, all sampled from the mother bag; products are held for 6 hours before release, for a 7-day expiration (11). CBS does not collect triples. At Héma-Québec, the delay is a minimum of 48 hours, and 20 mL of product are collected from the mother bag and inoculated into aerobic and anaerobic bottles, whether the product is a pooled platelet, a single or a double apheresis platelet: we do not collect triples. Products are held for 12 hours before release and have a 7-day expiration.
The rationale behind the LVDS approach
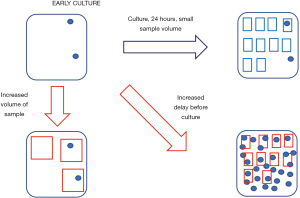
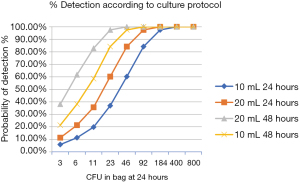
The problem with bacterial culture of platelets is that the number of bacteria in the platelet pouch, even at 24 hours after collection, is quite low (12). This is illustrated in Figure 1. American Red Cross investigators have found that the mean concentration of bacteria at 24 hours post-collections is 0.154 CFU/mL (5). This corresponds to 77 CFUs in a 500 mL platelet component. They also found that increasing the volume of the sample from 4 to 8 mL increased the detection rate and decreased the number of septic reactions reported to them, although that decrease was not statistically significant. As further support for increasing the sample volume in order to increase bacterial detection, a recent meta-analysis of studies reporting on bacterial contamination rates of cultured platelets found that there was a positive relationship between the sample volume and the rate of positivity (13). The improvement predicted with increased volume and delay has been explored using a statistical model (14,15). We used the model developed by Steven Wagner of the American Red Cross (12,14,15) to predict the effect of doubling the culture volume and of increasing the delay between collection and sampling, separately and in combination. The results appear in Figure 2. The conditions of the model were the following: a 500 mL product, a total number of bacteria in the bag that was varied from 3 to 800 CFUs, a comparison of 10 vs. 20 mL samples at 24 vs. 48 hours. A four-fold increase in the number of CFUs between 24 and 48 hours after collection was conservatively modeled. As we can see, below 200 CFUs, doubling the sample volume and increasing the delay both increase the probability of detection. When combined, these two approaches appear to act synergistically. However, it should be noted that increasing the delay alone leads to a better improvement in detection capacity than increasing the volume alone with the parameters used in our model. CBS has also done similar modelling using data generated from spiking experiments and arrived at similar conclusions (11).
The evidence that LVDS results in enhanced product safety
The evidence for this is two-fold. The first line of evidence concerns culture results. The operators who changed from a 24-hour single aerobic bottle culture to LVDS evaluated if its implementation led to an increased bacterial detection rate. At Héma-Québec, after the implementation of LVDS in late October 2015, the detection rate of true positives (defined as a positive culture confirmed by detection of the same organism upon reculture of the component or culture of a co-component) went from 0.013% (59/465,375) to 0.019% (26/136,613), excluding cultures positive for Cutibacterium acnes. C. acnes was frequently isolated after the change and represented almost 50% (26/52) of all our true positives. CBS did not observe an increase in the detection rate of facultative anaerobes with the switch to LVDS. However, C. acnes was frequently isolated, and represented close to 75% (149/217) of their total yield (11). The status of C. acnes as a transfusion-related pathogen is unclear at the present time. Because of a mean three-day delay in detection of this organism, products were often transfused before the culture result was available; no PTS was observed after transfusion of these products either at Héma-Québec or at CBS. Whether the transfusion of C. acnes-contaminated units could lead to infection of implants remains a question that requires further investigation. Concerning cultures that were isolated from one bottle only (excluding obligate anaerobes and obligate aerobes that can only grow in either the anaerobic or aerobic bottle respectively), two true positives were grown from only one bottle at Héma-Québec, and nine at CBS. At NHSBT, out of 403 true positives, 267 grew only from the anaerobic bottle, 31 only from the aerobic bottle and 105 from both (10). If the 240 strains who were strictly anaerobic and could only grow in the anaerobic bottle are excluded, it leaves 68 facultative aerobes that grew only from one of the two bottles. This suggests that increasing the volume does result in improved yield.
Regarding cultures at outdate, only 1 out of 4,536 was found positive at Héma-Québec after implementation of LVDS, compared to 5 out of 9,165 done during the period when we cultured at 24 hours using only one bottle. Although the difference is not statistically significant, it is consistent with increased detection capacity with LVDS leading to lower rates of false-negative components detected at outdate. CBS reported 5 positive cultures out of close to 5,400 (11). At NHSBT, of 4515 cultures done at outdate, none became positive (10).
The other line of evidence concerns the residual risk of septic reactions with LVDS. Following implementation of LVDS, only one case of PTS after transfusion of a culture -negative platelet was reported to CBS, for a residual risk of approximately 1 in 350,000 units transfused (11). At NHSBT, out of 1,239,029 platelet products screened using LVDS, one case of PTS was reported, and 3 near misses who were intercepted by visual inspection prior to transfusion (10). This compares favorably with the 10 PTS cases and five near-misses reported in the five years preceding introduction of bacterial culture of platelets in England. No cases of PTS have to date been reported to Héma-Québec after implementation of LVDS, with close to 200,000 transfused platelet components. Although it is true that the data concerning breakthrough infections is based on passive surveillance, it is important to note that the hemovigilance systems in the two countries from where the studies emanate are quite robust. As an example, in our jurisdiction (Québec), more than half of all febrile transfusion reactions undergo full microbiological investigation, based on guidelines published by a working group of the Public Health Agency of Canada (16). Therefore, we believe that most PTS cases are detected and reported, and certainly those of moderate or greater severity. This is also true for the rest of Canada and England. In conclusion, it appears that LVDS leads to a three-fold reduction in the residual risk of PTS, when compared to 24-hour one-bottle culture.
Practical considerations around use of LVDS
False positive rates
All three operators observed an increased rate of false-positive results with the anaerobic bottle. At Héma-Québec the false positive rate for the aerobic bottle was 0.0315% versus 0.162% (0.068% of platelet pools, 0.180% of apheresis platelets) following the introduction of the anaerobic bottle. Following the advice of our supplier of bacterial culture material, we implemented a technical improvement (installation of a software application called a compensation filter that leads to reduction of the number of false positives due to the drop of temperature when loading new bottles) that dropped the false-positive rate to 0.075% in apheresis platelets. Since around 90% of the platelet components manufactured and distributed by Héma-Québec are apheresis platelets, this was an important improvement.
Outdates
The two operators who moved from a 24-hour one-bottle culture of platelets with a five-day expiration to LVDS with a seven-day expiration evaluated the impact this change had on rates of outdate. Both CBS and Héma-Québec saw a significant drop in outdating of platelets. At CBS, outdates dropped from 18.9% to 13.1% (11). At Héma-Québec the outdates dropped from 23.3% to 12.2%. However, the drop in outdates at Héma-Québec cannot be fully ascribed to the change in culture method, because at the same time LVDS was implemented, so was a new policy on indications for CMV- negative blood components which also led to an improvement in platelet inventory management and reduced outdating.
Age of platelets at transfusion
Inevitably, LVDS with a seven-day expiration will increase the age of platelets transfused. This was an issue of great concern in England at the time they were considering implementation of LVDS. This led NHSBT to fund a study to compare the efficacy of 6 to 7 day-old compared to 2 to 5-day old platelets. They found that 6 to 7-day old platelets were noninferior to 2 to 5-day old platelets as regards corrected count increments, bleeding and interval to next platelet transfusion (17). At Héma-Québec, before the change, 22% of platelet were transfused at 3 days of age, 39% at 4 days and 32% at 5 days. Following the implementation of LVDS, the percentages were the following: 4 days, 6.5%; 5 days, 24.4%; 6 days, 38.7%; 7 days, 29.5%. Rate of platelet consumption per 1,000 persons was monitored: the rate went from 4.6 per 1,000 in 2012 to 4.1 per 1,000 in 2017, suggesting that implementation of LVDS did not lead to increased need for platelet transfusions. At CBS, the impact of the change on post-transfusion increments, time between transfusions, transfusions per patient and patient outcomes is under investigation, as stated in their paper (11).
Costs
Change from a 24-hour one-bottle culture to LVDS results in increased costs that are more than offset for by decreased outdates. CBS estimated a net inferred cost benefit of approximately $1,900,000 over a 27-month period (11). At Hema-Québec, a similar cost benefit ratio was measured. However, at Héma-Québec benefits of reduced outdating were partly due to the simultaneous implementation of the modified policy concerning distribution of CMV-negative blood components.
Conclusions
LVDS culture of platelets is one of a number of measures that can enhance safety of transfused platelets when compared to a single-bottle culture at 24 hours. It has the advantage of relative simplicity when compared to multiple-step approaches developed to meet the same goal. If LVDS allows one to move from a 5-day to a 7-day expiration, it can lead to reduced outdates and cost savings. The price to pay is the increased age of platelets at transfusion. If a blood center is considering implementation of LVDS, this is an issue that needs to be addressed with hospital customers in order to get their buy-in.
Is the enhanced safety sufficient? In a commentary published in the January 2013 edition of Transfusion, Brecher et al. acknowledged that the 24-hour culture had greatly improved the safety of platelet transfusion in regard to bacterial contamination, at the same time recognising the need for further measures (18). LVDS is regarded as an important step in that direction. The FDA acknowledged this since it included LVDS as one of the options available to make platelets safer in their recent guidance (9). The approach is not 100% effective at eliminating bacterially contaminated platelets, as illustrated by the fact that there are breakthrough infections, albeit at a lesser rate than with the 24-hour aerobic bottle culture and a certain number of positive cultures at outdate, again at a lesser rate than previously in our experience. There is always room for improvement. That being said, LVDS, in conjunction with other measures, has decreased the residual risk of PTS following platelet transfusion to a level that now approaches what is reported with transfusion transmissible viral infections for which screening programs are in place (19).
Are other culture approaches superior in terms of safety? In a recent paper, Walker et al., with the use of modelling to simulate various hypothetical contamination scenarios, compared the performance of the nine risk control strategies contained in the recent FDA guidance (20). They concluded that two-step policies involving secondary culture were generally safer. The 11 by 10 matrices of scenarios in the paper compared various combinations of lag times (from 0 to 120 hours) and doubling times (from 1 to 10 hours). Most of the scenarios in these risk matrices had a lag time of 48 hours or more (63%), and all scenario in the matrices were considered to have an equal probability of occurrence. This methodological approach obviously disadvantaged LVDS, since in any scenario with a 48-hour lag time LVDS will perform poorly. The equal weighing of scenarios in their modeling exercise is recognised as a limitation by the authors. In a paper on the growth characteristics of 20 strains of Staphylococcus epidermidis originally isolated from contaminated platelets, only 5 of the strains showed slow growth when inoculated into platelet products, and even in these five, there was evidence of growth, albeit reduced when compared to the other 15 strains, at 48 hours (21). Furthermore, they used a single starting inoculum of 10 CFU, which is lower than the estimated mean level of 77 CFU in contaminated platelets at 24 hours in a ARC paper previously cited in this article (5). We therefore feel that the data in the Walker et al. paper, although interesting, do not allow a head-to-head comparison of the various culture strategies found in the FDA guidance. Only time, and ongoing evaluation of the various strategies, once implemented, will allow to eventually determine if one does have a more favorable performance than the others.
Acknowledgments
We wish to acknowledge the major contribution of Julie Beaudoin and Jocelyne Dion to the successful implementation of large volume delayed sampling bacterial culture of platelets.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Sandra Ramirez-Arcos) for the series “Bacterial Contamination of Platelet Components” published in Annals of Blood. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/aob-21-4
Peer Review File: Available at http://dx.doi.org/10.21037/aob-21-4
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob-21-4). The series “Bacterial Contamination of Platelet Components” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brecher ME, Hay SN. Bacterial contamination of blood components. Clin Microbiol Rev 2005;18:195-204. [Crossref] [PubMed]
- Robillard P, Delage G, Itaj NK, et al. Use of hemovigilance data to evaluate the effectiveness of diversion and bacterial detection. Transfusion 2011;51:1405-11. [Crossref] [PubMed]
- Goldman M, Roy G, Fréchette N, et al. Evaluation of skin disinfection methods. Transfusion 1997;37:309-12. [Crossref] [PubMed]
- McDonald CP, Roy A, Mahajan P, et al. Relative value of the interventions of diversion and improved donor-arm disinfection to reduce the bacterial risk from blood transfusion. Vox Sang 2004;86:178-82. [Crossref] [PubMed]
- Eder AF, Kennedy JM, Dy BA, et al. Limiting and detecting bacterial contamination of apheresis platelets: inlet-line diversion and increased culture volume improve component safety. Transfusion 2009;49:1554-63. [Crossref] [PubMed]
- Ramirez-Arcos S, DiFranco C, McIntyre T, et al. Residual risk of bacterial contamination of platelets: six years of experience with sterility testing. Transfusion 2017;57:2174-81. [Crossref] [PubMed]
- Hong H, Xiao W, Lazarus HM, et al. Detection of septic transfusion reactions to platelet transfusions by active and passive surveillance. Blood 2016;127:496-502. [Crossref] [PubMed]
- Walker BS, White SK, Schmidt RL, et al. Residual bacterial detection rates after primary culture as determined by secondary culture and rapid testing in platelet components: a systematic review and meta-analysis. Transfusion 2020;60:2029-37. [Crossref] [PubMed]
- Bacterial Risk Control Strategies for Blood Collection Establishments and Transfusion Services to Enhance the safety and Availability of Platelets for Transfusion. Guidance for Industry. Available online: https://www.fda.gov/vaccines-blood-biologics/guidance-compliance-regulatory-information-biologics/biologics-guidances
- McDonald C, Allen J, Brailsford S, et al. Bacterial screening of platelet components by National Health Service Blood and Transplant, an effective risk reduction measure. Transfusion 2017;57:1122-31. [Crossref] [PubMed]
- Ramirez-Arcos S, Evans S, McIntyre T, et al. Extension of platelet shelf life with an improved bacterial testing algorithm. Transfusion 2020;60:2918-28. [Crossref] [PubMed]
- Benjamin RJ, Wagner SJ. The residual risk of sepsis: modeling the effect of concentration on bacterial detection in two-bottle culture systems and an estimation of false-negative culture rates. Transfusion 2007;47:1381-9. [Crossref] [PubMed]
- White SK, Schmidt RL, Walker BS, et al. Bacterial contamination rate of platelet components by primary culture: a systematic review and meta-analysis. Transfusion 2020;60:986-96. [Crossref] [PubMed]
- Wagner SJ, Eder A. A model to predict the improvement of automated blood culture bacterial detection by doubling platelet sample volume. Transfusion 2007;47:430-3. [Crossref] [PubMed]
- Tomasulo PA, Wagner SJ. Predicting improvement in detection of bacteria in apheresis platelets by maintaining constant component sampling proportion. Transfusion 2013;53:835-42. [Crossref] [PubMed]
- Transfusion Transmitted Injuries Section, Blood Safety Surveillance and Health Care Acquired Infections Division, Public Health Agency of Canada. Guideline for investigation of suspected transfusion transmitted bacterial contamination. Can Commun Dis Rep 2008;34:1-8. [PubMed]
- MacLennan S, Harding K, Llewelyn C, et al. A randomized noninferiority crossover trial of corrected count increments and bleeding in thrombocytopenic hematology patients receiving 2 to 5- versus 6-or 7-day old platelets. Transfusion 2015;55:1856-65. [Crossref] [PubMed]
- Brecher ME, Blajchman MA, Yomtomian R, et al. Addressing the risk of bacterial contamination of platelets within the United States: a history to help illuminate the future. Transfusion 2013;53:221-31. [Crossref] [PubMed]
- Dodd RY, Crowder LA, Haynes JM, et al. Screening blood donors for HIV, HCV and HBV at the American Red Cross: 10-year trends in prevalence, incidence, and residual risk, 2007-2016. Transfus Med Rev 2020;34:81-93. [Crossref] [PubMed]
- Walker BS, Schmidt RL, Fisher MA, et al. The comparative safety of bacterial risk control strategies for platelet components: a simulation study. Transfusion 2020;60:1723-31. [Crossref] [PubMed]
- Ali H, Greco-Stewart V, Jacobs MR, et al. Characterisation of the growth dynamics and biofilm formation of Staphylococcus epidermidis strains isolated from contaminated platelet units. J Med Microbiol 2014;63:884-91. [Crossref] [PubMed]
Cite this article as: Delage G, Bernier F. Bacterial culture of platelets with the large volume delayed sampling approach: a narrative review. Ann Blood 2021;6:30.


