
Incidence and management of non-immune platelet transfusion refractoriness: a narrative review
Introduction: Incidence of non-immune platelet transfusion refractoriness
Platelet transfusions are indicated therapeutically for trauma and surgical patients, or prophylactically for patients with thrombocytopenia or platelet dysfunction. Although bleeding is a concern in patients with severe thrombocytopenia, the correlation between platelet count and bleeding risk is unclear. Hence, bleeding risk should be clinically assessed by underlying diseases, prior bleeding episodes, presence of purpura and so on (1). In invasive procedures such as surgery, prophylactic transfusion is used to maintain a platelet count of above 50×109/L until hemostasis is ensured (2,3). For spinal fluid testing, the British Committee for Standards in Haematology recommends a platelet count of above 50×109/L (4) and the American Society of Clinical Oncology recommends a platelet count of above 20×109/L (5). These thresholds should be used as guides, and it is important to prioritize clinical judgment based on individual patient and disease factors.
As platelet concentrate (PC) products are stored at room temperature, they have a shorter shelf-life and a higher risk of bacterial contamination compared to other products such as red blood cells and plasma (6,7). Native plasma, in which supplied PCs are suspended, can increase the risk of non-hemolytic transfusion reactions such as febrile and allergic reactions (8,9). Moreover, 30% to 40% of cases with platelet transfusions can show inadequate platelet count increment due to various causes (10,11).
Platelet transfusion refractoriness (PTR) is defined as a response significantly lower than expected to at least two consecutive platelet transfusions (12). Typically, PTR can develop in patients with hematological cancers, who usually require repeated platelet transfusions (13-17). The exact incidence of PTR is unknown, but it has been reported to occur in 30–70% of multi-transfused patients (13,14,17-20). PTR is often multifactorial and it can be due to non-immune and immune causes. About two-thirds of refractory cases are classified as non-immune PTR, in which platelet survival is shortened by various underlying conditions such as fever/sepsis, splenomegaly, hematopoietic stem cell transplantation (HSCT), disseminated intravascular coagulation (DIC), graft-versus-host disease (GVHD), vaso-occlusive diseases (VOD), drug-induced thrombocytopenia and hemorrhage (10,11,12,17). The remaining cases are classified as immune PTR. Among them, anti-human leukocyte antigen (HLA) antibodies are detected in 80–90% of cases, and antibodies to platelet-specific antigens including human platelet antigens (HPAs) and CD36 isoantigen or isoagglutinin to ABO antigens are the causative in the remaining cases. Alloimmunization to these antigens can be through previous transfusion, pregnancy or transplantation (11,17,21). Patients with immune PTR require transfusions of compatible platelets reflecting their alloimmunization profiles (12). (See the review article on immune PTR in this issue of Annals of Blood.) Appropriate assessment of PTR is critical, since poor response to platelet transfusion is associated with poor clinical outcomes such as inferior survival, longer hospitalization and higher hospitalization costs (22-24). When PTR is suspected, non-immune causes should be carefully assessed prior to investigating immune causes.
In this article, we describe the practical approaches for the diagnosis and the management of non-immune PTR, by referring to existing related literature and our clinical experience. We present this article in accordance with the narrative review reporting checklist (available at http://dx.doi.org/10.21037/aob-20-93).
Literature search strategy
The medical literature of published observational/investigational studies, randomized controlled trials or systematic reviews and meta-analyses regarding non-immune and immune PTR were analyzed. The PubMed/Medline electronic database and Google Scholar were searched in November 2020 using the primary phrases such as “platelet transfusion”, “platelet transfusion refractoriness”, “non-immune platelet transfusion refractoriness”, “immune platelet transfusion refractoriness”, “corrected count increment”, “incidence”, “management”. A hand search of ISBT Science Series and Annals of Blood was also added. Peer-reviewed articles in English or Japanese were considered and no constraints on publication type or date were imposed. Titles/abstracts of retrieved articles were checked for relevance, and other relevant papers were identified by manual searching of reference lists and the authors’ personal literature collections. When similar findings were reported in multiple articles, priority was given to those most recently published.
Diagnosis of platelet transfusion refractoriness
Following a platelet transfusion, platelet count can rise with a peak at 10 minutes to one hour and show a gradual decline over 72 hours. Typical dosing of platelet transfusion for an adult is a pool of 6 whole blood derived platelets or one apheresis platelet, by which the platelet count increment 24-hour post-transfusion in a 70 kg patient is clinically expected to be 30×109/L to 60×109/L. Prior to platelet transfusion, “predicted platelet increase” can be calculated by the following formula:
(Multiplication by 2/3 reflects the accumulation of 1/3 of transfused platelets in the spleen)
In Japan, all PCs transfused are leukoreduced single-donor apheresis-derived, and supplied by the Japanese Red Cross Society (JRCS). In the clinical practice, a 10-unit PC bag (volume of about 200 mL), containing about 2.0×1011 platelets, is commonly used, which gives a predicted platelet count increase of about 30×109/L in a patient with a body weight of 70 kg (25,26).
If suboptimal platelet increases are suspected, corrected count increment (CCI) is helpful in assessing the effectiveness of PC transfusion based on the amount of platelets transfused compared to the body surface area (26). The formula of CCI is as follows:
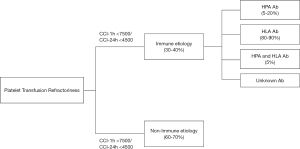
For assessing suspected PTR cases, measurement of CCI 1-hour post-transfusion (CCI-1h) as well as CCI 24-hour post-transfusion (CCI-24h) is encouraged, since CCI-1h can help differentiate immune PTR from non-immune PTR (27). CCI-24h higher than 4,500 indicates normal platelet survival in vivo, while CCI-1h higher than 7,500 indicates normal platelet recovery in vivo (5,11,17,27-29). The previous guidelines from Western countries recommended CCI-1h measurement with an interval of one hour or more after the transfusion, which was a hurdle for clinicians and patients, especially those receiving transfusion at an outpatient setting. In the revised guidelines, including the Japanese one, the interval for the measurement of CCI-1h was changed to “10 minutes to one hour” after transfusion (30,31). Presently, CCI-1h measurement can be performed between 10-min and 1-hour post-transfusion, and CCI-24h between 16 and 24 hours post-transfusion. Figure 1 shows the differentiation between immune and non-immune PTR, according to CCI-24h and CCI-1h (32). Typically, non-immune PTR cases present with CCI-1h above 7,500 and CCI-24h below 4,500, reflecting normal platelet recovery and reduced platelet survival. On the other hand, typical immune PTR cases present with CCI-1h below 7,500 and the CCI-24h below 4,500, reflecting reduced platelet recovery almost immediately after transfusion (within one hour). According to the report by Legler et al., among 145 consecutive patients receiving platelet transfusions, 28% (40/145) developed PTR, with non-immune PTR (predominantly due to fever and sepsis) accounting for the majority (63%), and immune-alone PTR accounted for only 18% (10).
Major causes of non-immune PTR
Non-immune PTR accounts for about two-thirds of all refractory cases, and the reported causes consist of the patient's underlying disorders (33), as well as factors related to PC products such as platelet counts in the transfused products, the period of storage, and others (34). Major causes of non-immune PTR are described below.
Fever/infection/sepsis
Fever is the most frequently reported cause of non-immune PTR (12,35). Since fever can be secondary to various conditions, such as infection/sepsis, DIC, drug allergy, and HSCT, it is still unclear whether fever is an independent cause of poor response to platelet transfusion (12,36). Freireich et al. showed that the percentage platelet recovery (PPR) after transfusion is worse in patients with higher body temperature, and the worst in the presence of sepsis (37). In an observational study of HLA-alloimmunized patients who received transfusions of random donor PC, most febrile patients had significantly reduced PPRs, while sufficient PPRs were achieved in those receiving HLA-matched platelets (38). Among the various infections implicated in thrombocytopenia and PTR (39,40), mycoplasma pneumoniae infection has been reported to be associated with idiopathic thrombocytopenic purpura (ITP) (41) and thrombotic microangiopathy (TMA) (42). Sepsis, which can cause secondary thrombocytopenia through the production of platelet-associated IgG and the immune destruction of platelets (43,44), has been reported to negatively affect the post-transfusion platelet count increment, and to be related with an unfavorable prognosis in severely ill patients (45,46). Kelton et al. identified increased platelet-associated IgG in septicemic patients with thrombocytopenia, providing some insights for the immune destruction of platelets. In septicemic patients, it has been hypothesized that bacteria in the bloodstream can trigger platelet destruction and shortened lifespan through induction of vascular damage, which leads to platelet consumption (47-49).
In patients with hematological malignancies, the combination of fever, infection and antibiotic therapy has been reported to be the most common cause of PTR (11), but the precise mechanism of this interaction is still unclear (33). In our experience of 224 platelet transfusions in 13 hematological patients with non-immune PTR, the CCI-1h of those who developed post-transfusion fever was comparable to that of patients without fever pre- and post-transfusion, which also suggests uncertain relevance of transfusion-related fever to platelet recovery (31).
DIC
DIC is characterized by systemic activation of blood coagulation and increased platelet consumption, which results in life-threatening hemorrhage (50). Despite lack of evidence, platelet transfusion is indicated in DIC patients with serious bleeding or those with a platelet count below 50×109/L who need urgent/emergent surgery (51). These patients can be refractory to platelet transfusion mainly due to the increased platelet consumption (11,29,33,52-55). The bleeding tendency is more severe or apparent in patients with leukemia, solid cancer, obstetric disease or severe infection (56). Of note, aggressive DIC can result in low CCI-1h, resembling immune PTR. Since treatment of the underlying cause is the major principle in DIC management, it is anticipated that proper DIC management can alleviate the related platelet consumption and PTR.
Splenomegaly
The spleen is the most important organ affecting the platelet count increment after transfusion (17,57,58), with about one-third of transfused platelets being retained in the spleen. In patients with splenomegaly, higher amounts of platelets accumulate in the enlarged spleen, reducing the platelet count in the peripheral blood. Studies using radiolabeled platelets have indicated that when platelets are transfused into patients with splenomegaly, large amounts of platelets are pooled into the spleen, thus not exerting their hemostatic function in the periphery (59). Therefore, the evaluation on the required amount of PC to be transfused as well as the indication for transfusion should be carefully considered. In fact, in patients with splenomegaly, up to 85% of transfused platelets, compared to 61% in normal patients, are destroyed; the recovery of peripheral platelet count immediately after transfusion was 26% in patients with splenomegaly, compared to 59% in normal patients, and 97–98% in asplenic patients; transfused platelets were found in the spleen in 80% of splenomegaly patients 30 minutes after transfusion, but only 40% of normal patients (60). A case report showed that splenectomy reduced the frequency of platelet transfusions and improved CCI in a patient with myelodysplastic syndrome (MDS) developing PTR due to splenomegaly (61). It should be noted that hypersplenism can also result in low CCI-1h, resembling immune PTR.
Bleeding
Clinical bleeding is often listed as a non-immune cause of PTR (62,63), but it is thought that bleeding itself is a consequence rather than a cause of reduced survival of platelets (22). Actually, in patients who were refractory to pooled random-donor platelet transfusions, fever and splenomegaly, but not bleeding, were found to correlate with the reduced CCI-1h after HLA-matched platelet transfusion (29). Severely active bleeding can also result in low CCI-1h, resembling immune PTR. Therefore, it is important for clinicians to recognize PTR as a sensitive clinical marker for the occurrence of bleeding and impaired patient survival (22).
Drugs
Drug-induced thrombocytopenia is a clinical condition that affects a small percentage of patients taking certain drugs. Causative agents are listed in Table 1. Patients with a typical history of drug-induced thrombocytopenia may have negative antibody tests (70,71) because metabolites produced in vivo can be sensitizing agents (72,73). Drug-induced thrombocytopenia is usually considered to be immune-mediated (74), and clinically, drug-induced platelet-specific autoimmunity resembles acute ITP (75). Thus, drug-induced thrombocytopenia should be suspected in patients with PTR.
Table 1
| Drug Category | Drugs |
|---|---|
| Antithrombotics | Heparin, Clopidogrel/ticlopidine, GPIIb/IIIa antagonists |
| Infectious disease agents | Ampicillin, Amoxicillin, Cephalosporins, Ciprofloxacin/levofloxacin, Linezolid, Metronidazole, Nafcillin, Penicillin, Piperacillin/tazobactam, Rifampin, Sulfonamides, Vancomycin, Amphotericin |
| Histamine-receptor antagonists | Cimetidine, Famotidine, Ranitidine |
| Analgesic agents | Acetaminophen, Diclofenac, Fentanyl, Ibuprofen, Naproxen, Salicylates |
| Chemotherapeutic and immunosuppressant agents | Bleomycin, Cyclosporine, Oxaliplatin, Fludarabine, Rituximab |
| Cinchona alkaloids | Quinine, Quinidine |
| Platelet inhibitors | Abciximab, Eptifibatide, Tirofiban |
| Antirheumatic agents | Gold salts, D-penicillamine |
| Sedatives and anticonvulsant agents | Carbamazepine, Phenytoin, Valproic acid, Diazepam |
| Diuretic agents | Chlorothiazide, Hydrochlorothiazide |
Drug-induced thrombocytopenia does not require specific treatment other than the discontinuation of the sensitizing agent. Patients with drug-induced thrombocytopenia are often treated with corticosteroids, intravenous immunoglobulin (64) and plasma exchange (76), however, the benefit of such treatments is unknown.
HSCT
HSCT has been shown to be associated with an impaired response to platelet transfusion (19,52,77-79). Complications such as fever, infection, acute GVHD, and sepsis have been shown as factors affecting platelet transfusion in patients undergoing HSCT (80,81). GVHD-associated TMA (82) may lead to increased production of anti-platelet autoantibodies and accelerated platelet destruction (83). In one series by Rio et al., 100% (13/13) of patients with sinusoidal obstruction syndrome (SOS) developed PTR as early as 6±2 days after transplantation (84).
Factors related to PC products
The period of PC storage, the ABO compatibility of the transfused PC and the irradiation of PC may also affect the CCI. Recently, pathogen-reduction/inactivation (PI) treatment of PC has also been shown to affect CCI (85).
In hematological patients, longer storage period (up to 5 to 7 days) of PC is associated with a lower CCI compared to shorter storage period (less than 3 days) (86,87). Platelet count recovery of patients receiving ABO-incompatible platelets is reported to be as low as one-third of those receiving ABO-identical platelets (88,89). In the previous studies investigating the effects of ABO incompatibility in thrombocytopenic patients who were refractory to platelet transfusion due to HLA antibodies, ABO-incompatible HLA-matched platelet transfusions were inferior to ABO-identical HLA-matched platelet transfusions in terms of platelet recovery and CCI (88,90,91). Our experience with multiple transfusions of HLA-matched PC to a single MDS patient with multiple specificity HLA antibodies also confirmed the lower effectiveness of ABO-incompatible PCs compared to the compatible ones (92).
Previously, Button et al. showed that the gamma-irradiation of PC at a dose of 5,000 cGy prior to transfusion was required to negatively affect platelet recovery (93), while Read et al. showed that up to 3,000 cGy had no effect on in vivo recovery or survival of irradiated platelets (94). Slichter et al. has shown that lower doses of 2,500 to 3,000 cGy can reduce CCI-1h, but not CCI-24h (17). Thus, the impact of gamma irradiation of platelets is still controversial (95).
PI treatment of donor platelets can negatively affect platelet recovery of recipients. Patients transfused with PI-platelets can have lower CCI-1h and CCI-24h than those with standard platelets, with a relative risk of platelet refractoriness of about 2.7 (96). Therefore, patients receiving PI-platelets require more frequent platelet transfusions than those with standard platelets. Despite the potential benefit of PI treatment in preventing post-transfusion GVHD, it has been recently reported that PI-platelets have no impact on preventing HLA alloimmunization (97).
Others
Repeated platelet transfusions in hematological patients lead to PTR (17), and this can be observed even in cases in which alloantibodies are not produced. It is possibly triggered by the vascular endothelial damage caused by the chemotherapy agents. It has been reported that hemophagocytic syndrome (HPS) is associated with PTR. A case report showed that treatment of relapsed acute leukemia with etoposide effectively relieved the HPS as well as the PTR (98).
Studies have shown that pooled whole blood-derived PC (WB-PC) and single-donor apheresis PC have similar rates of alloimmunization (20), but that single-donor apheresis PC shows significantly higher CCI-1h to CCI-24h compared to WB-PC (99).
Volume-reduced washed platelets may be beneficial for PTR cases because equal volumes can increase platelet count more efficiently than standard platelets (100). Despite the other advantages of shortened transfusion times and reduced volume loads (101), volume reduction can lead to spontaneous activation and aggregability of platelets (100,102).
Management of non-immune PTR
When PTR develops in patients receiving PC transfusion, the implementation of the appropriate management is essential. We propose below a multi-step approach for the management of PTR, especially focusing on non-immune-mediated PTR, based on the reported literature and our experience. By following these steps, clinicians can avoid unnecessary transfusions that may cause adverse transfusion reactions as well as immune-mediated PTR, consequently contributing for the improvement of healthcare economics.
Consider appropriate indication of platelet transfusion to avoid adverse events and alloimmunization
Suspected PTR patients should be carefully evaluated for various factors such as the current platelet count, the changes of platelet count over time, the presence/absence of bleeding symptoms, the primary disease and underlying conditions including the degree of organ damage, and the history of transplantation. Although a platelet count of 7×109/L is required to maintain the strength of the vessel wall (103), the majority of patients in a stable condition do not experience bleeding even when the count is below 5×109/L (104).
In 1962, Gaydos et al. performed a retrospective analysis of acute leukemia and suggested that a platelet count of 20×109/L should be considered as a transfusion trigger because severe bleeding was rare in patients with platelet counts above 20×109/L (105). The result might have been affected by aspirin, an inhibitor of the platelet function, since it was frequently used as an antipyretic agent in those days. Thereafter, lowering this platelet transfusion trigger to 10×109/L for patients without comorbidities has been shown to have no impact on the frequency of bleeding and result in a 20–30% reduction in platelet use (106-109). Other reports suggest that a trigger value of 5×109/L is acceptable for uncomplicated patients (110), whereas a target of 20×109/L is recommended for patients with acute promyelocytic leukemia due to the strong bleeding tendency associated with DIC (4). Ethical issues make it difficult to design a comparative study with trigger values below 10×109/L, and it is still unclear how far the threshold can be safely lowered. The clinical trigger of prophylactic platelet transfusion is currently thought to be 10×109/L to 20×109/L, and some reports suggest that 10×109/L may be acceptable in patients after HSCT (5,81,109,111). Chronic thrombocytopenia due to aplastic anemia and MDS can be managed according to the above criteria of platelet transfusion, however, for cases requiring iterative transfusions, a platelet count of 5×109/L should be the trigger value (112).
Patients with consumptive thrombocytopenia such as ITP, thrombotic thrombocytopenic purpura (TTP) or heparin-induced thrombocytopenia (HIT), are not usually eligible for prophylactic platelet transfusion. But platelet transfusion should be considered when bleeding is severe, or an invasive treatment is needed, or it remains difficult to stop bleeding even with other treatments. A large-scale retrospective study by Goel et al. showed that platelet transfusions are associated with higher odds of arterial thrombosis and mortality among patients with TTP and HIT (113). Therefore, clinicians should keep in mind that platelet transfusion has been identified as a potential exacerbator of TTP and HIT.
Investigate the causes of PTR
The clinical evaluation of the possible non-immune causes is essential (114). Combined measurements of CCI-1h and CCI-24h values can help differentiation between immune and non-immune PTR (See Diagnosis section and Figure 1). A CCI-1h in the normal range accompanied with a low CCI-24h indicates a shortened platelet survival, which is typically seen in non-immune PTR.
Select the best treatment plan according to the etiology of PTR
For non-immune PTR cases, the best way to improve the transfusion response is appropriate treatment of the underlying diseases (29).
When PTR persists after the appropriate treatment of the non-immune causes, immune PTR, alone or in combination, should be suspected. Since about 80–90% of immune PTR cases are due to anti-HLA antibodies (11), screening for anti-HLA antibodies is indicated, concomitantly with testing for anti-HPA antibodies. Anti-CD36 isoantibodies, which are found in a higher frequency among Asian and African populations (115), may also cause PTR, so it should be considered when other antibodies are not identified. For immune PTR due to anti-HLA antibodies, transfusion of HLA-matched platelets is strongly recommended. Since HLA matching of platelets requires recruitment of compatible donors, testing of HLA/HPA antigens/antibodies and crossmatching, HLA-matched platelets are usually expensive and not always available in many countries. Thus, antibody specificity prediction method is applied in some countries as a reasonable way to find “HLA-compatible” platelets (38,65) (See the review article on immune PTR in this issue of Annals of Blood).
Tranexamic acid, a fibrinolytic inhibitor, has been shown to prevent bleeding and reduce the need of blood transfusion, including PC, in surgical patients, but its role as an alternative to platelet transfusion in PTR cases remains to be investigated (116). The TREATT trial is underway to assess the safety and efficacy of using prophylactic tranexamic acid during a period of intensive chemotherapy and associated thrombocytopenia in hematological patients (66).
Consider the coexistence of non-immune and immune causes
When managing PTR cases, it is important for clinicians to consider the coexistence of non-immune and immune causes. Actually, the combination of immune and non-immune factors is identified in a substantial proportion of PTR cases. In a prospective study of 266 PC transfusions in 26 patients with hematological malignancies, PTR developed in 44% (116/266). Among them, 67% (78/116) were due to non-immune, and 4% (5/116) due to immune factors alone, but the remaining 21% (24/116) was due to both non-immune and immune causes (11).
Limitations and future perspective
This article has several limitations. First, this article could not cover all literature regarding non-immune and immune PTR due to the nature of narrative review. Second, although we aimed to review the non-immune PTR, it is not feasible to describe non-immune PTR without mentioning to immune PTR, because of their close association. Third, dependent on the various etiologies, there are no consistent approaches for the diagnosis and management of the condition, mostly relying on the management of the underlying condition. There is a need to accumulate experience and data related to non-immune PTR and develop concise and consistent algorithms for the appropriate diagnosis and management of this condition.
Summary
When PTR is suspected, common and possible non-immune causes such as fever/sepsis, DIC and splenomegaly should be investigated in the early stage, because non-immune PTR is about twice as frequent as immune PTR. This step can prevent unnecessary platelet transfusions, preventing immune PTR. The combined evaluation of CCI-1h and CCI-24h may help differentiate PTR of immune and non-immune etiologies. Typically, in non-immune PTR, CCI-1h is within the normal range, but CCI-24h is usually low, reflecting shortened platelet survival. However, conditions such as aggressive DIC, hypersplenism, and severely active bleeding are known to be associated with low CCI-1h, so caution is needed. When the appropriate management of the non-immune causes does not improve the condition, the presence of immune PTR, alone or in combination, should be suspected. In case immune PTR is suspected, screening for anti-HLA antibodies is indicated, and if confirmed, transfusion of HLA-matched PC is indicated. Due to the multifactorial nature of the non-immune PTR, clinicians are recommended to follow the four steps described in this review for the appropriate diagnosis and management of the condition. Reducing unnecessary transfusions is pivotal in preventing not only the adverse events of transfusion but also alloimmunization and the subsequent immune PTR, consequently contributing for the improvement of the healthcare economics.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Pilar Solves) for the series “Platelet Transfusion” published in Annals of Blood. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/aob-20-93
Peer Review File: Available at http://dx.doi.org/10.21037/aob-20-93
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob-20-93). The series “Platelet Transfusion” was commissioned by the editorial office without any funding or sponsorship. Dr. NHT serves as an unpaid editorial board member of Annals of Blood. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- van Bladel ER, Laarhoven AG, van der Heijden LB, et al. Functional platelet defects in children with severe chronic ITP as tested with 2 novel assays applicable for low platelet counts. Blood 2014;123:1556-63. [Crossref] [PubMed]
- Bishop JF, Schiffer CA, Aisner J, et al. Surgery in acute leukemia: a review of 167 operations in thrombocytopenic patients. Am J Hematol 1987;26:147-55. [Crossref] [PubMed]
- Palo R, Capraro L, Hanhela R, et al. Platelet transfusions in adult patients with particular reference to patients undergoing surgery. Transfus Med 2010;20:30-7. [Crossref] [PubMed]
- British Committee for Standards in Haematology, Blood Transfusion Task Force. Guidelines for the use of platelet transfusions. Br J Haematol 2003;122:10-23. [Crossref] [PubMed]
- Schiffer CA, Anderson KC, Bennett CL, et al. American Society of Clinical Oncology. Platelet transfusion for patients with cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol 2001;19:1519-38. [Crossref] [PubMed]
- Kuehnert MJ, Roth VR, Haley NR, et al. Transfusiontransmitted bacterial infection in the United States, 1998 through 2000. Transfusion 2001;41:1493-9. [Crossref] [PubMed]
- Perez P, Salmi LR, Follea G, et al. Determinants of transfusion-associated bacterial contamination: results of the French BACTHEM Case-Control Study. Transfusion 2001;41:862-72. [Crossref] [PubMed]
- Muylle L, Wouters E, De Bock R, et al. Reactions to platelet transfusion: the effect of the storage time of the concentrate. Transfus Med 1992;2:289-93. [Crossref] [PubMed]
- Muylle L, Joos M, Wouters E, et al. Increased tumor necrosis factor alpha (TNF alpha), interleukin 1, and interleukin 6 (IL-6) levels in the plasma of stored platelet concentrates: relationship between TNF alpha and IL-6 levels and febrile transfusion reactions. Transfusion 1993;33:195-9. [Crossref] [PubMed]
- Legler TJ, Fischer I, Dittmann J, et al. Frequency and causes of refractoriness in multiply transfused patients. Ann Hematol 1997;74:185-9. [Crossref] [PubMed]
- Doughty HA, Murphy MF, Metcalfe P, et al. Relative importance of immune and non-immune causes of platelet refractoriness. Vox Sang 1994;66:200-5. [Crossref] [PubMed]
- Murphy MF. Managing the platelet refractory patient ISBT Sci Ser 2014;9:234-8. [Crossref]
- Slichter SJ. Prevention of platelet refractoriness. In: Murawski K, Peetoom F (eds) Transfusion Medicine: Recent Technological Advances. Alan Liss Inc., New York 1986; 83-116.
- Slichter SJ. Transfusion and bone marrow transplantation. Transfus Med Rev 1988;2:1-17. [Crossref] [PubMed]
- Murphy MF, Waters AH. Platelet transfusions: the problem of refractoriness. Blood Rev 1990;4:16-24. [Crossref] [PubMed]
- Hod E, Schwartz J. Platelet transfusion refractoriness. Br J Haematol 2008;142:348-60. [Crossref] [PubMed]
- Slichter SJ, Davis K, Enright H, et al. Factors affecting posttransfusion platelet increments, platelet refractoriness, and platelet transfusion intervals in thrombocytopenic patients. Blood 2005;105:4106-14. [Crossref] [PubMed]
- Ferreira AA, Zulli R, Soares S, et al. Identification of platelet refractoriness in oncohematologic patients. Clinics (Sao Paulo) 2011;66:35-40. [Crossref] [PubMed]
- Li G, Liu F, Mao X, et al. The investigation of platelet transfusion refractory in 69 malignant patients undergoing hematopoietic stem cell transplantation. Transfus Apher Sci 2011;45:21-4. [Crossref] [PubMed]
- Trial to Reduce Alloimmunization to Platelets Study Group. Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. N Engl J Med 1997;337:1861-9. [Crossref] [PubMed]
- Howard JE, Perkins HA. The natural history of alloimmunization to platelets. Transfusion 1978;18:496-503. [Crossref] [PubMed]
- Kerkhoffs JLH, Eikenboom JC, van de Watering LM, et al. The clinical impact of platelet refractoriness: correlation with bleeding and survival. Transfusion 2008;48:1959-65. [Crossref] [PubMed]
- Meehan KR, Matias CO, Rathore SS, et al. Platelet transfusions: utilization and associated costs in a tertiary care hospital. Am J Hematol. 2000;64:251-6. [Crossref] [PubMed]
- Hunt BJ, Allard S, Keeling D, et al. British Committee for Standards in Haematology. A practical guideline for the haematological management of major haemorrhage. Br J Haematol 2015;170:788-803. [Crossref] [PubMed]
- The Japan Red Cross Society. Transfusion Information 1804-159 [Internet]. 2018 [cited 2020 Dec 13]. Available online: http://www.jrc.or.jp/mr/english/pdf/yuketsu johou_1804_159.pdf
- Sato T, Tsuno NH, Goto N, et al. Incidence and severity of adverse effects related to platelet transfusion: a narrative review of the literature and the recent hemovigilance data of Japan. Ann Blood 2021; in press. [Crossref]
- McFarland JG, Anderson AJ, Slichter SJ. Factors influencing the transfusion response to HLA-selected apheresis donor platelets in patients refractory to random platelet concentrates. Br J Haematol 1989;73:380-6. [Crossref] [PubMed]
- Daly PA, Schiffer CA, Aisner J, et al. Platelet transfusion therapy. One-hour posttransfusion increments are valuable in predicting the need for HLA-matched preparations. JAMA 1980;243:435-8. [Crossref] [PubMed]
- Bishop JF, Matthews JP, Yuen K, et al. The definition of refractoriness to platelet transfusions. Transfus Med 1992;2:35-41. [Crossref] [PubMed]
- O'Connell B, Lee EJ, Schiffer CA. The value of 10-minute posttransfusion platelet counts. Transfusion 1988;28:66-7. [Crossref] [PubMed]
- Matsui R, Hagino T, Tsuno NH, et al. Does time of CCI measurement affect the evaluation of platelet transfusion effectiveness? Transfus Apher Sci 2021; in press. [Crossref] [PubMed]
- Pavenski K, Freedman J, Semple JW. HLA alloimmunization against platelet transfusions: pathophysiology, significance, prevention and management. Tissue Antigens 2012;79:237-45. [Crossref] [PubMed]
- Bishop JF, McGrath K, Wolf MM, et al. Clinical factors influencing the efficacy of pooled platelet transfusions. Blood 1988;71:383-7. [Crossref] [PubMed]
- Novotny VM. Prevention and management of platelet transfusion refractoriness. Vox Sang 1999;76:1-13. [Crossref] [PubMed]
- Delaflor-Weiss E, Mintz PD. The evaluation and management of platelet refractoriness and alloimmunization. Transfus Med Rev 2000;14:180-96. [Crossref] [PubMed]
- Böck M, Muggenthaler KH, Schmidt U, et al. Influence of antibiotics on posttransfusion platelet increment. Transfusion 1996;36:952-4. [Crossref] [PubMed]
- Freireich EJ, Kliman A, Gaydos LA, et al. Response to repeated platelet transfusion from the same doner. Ann Intern Med 1963;59:277-87. [Crossref] [PubMed]
- Petz LD, Garratty G, Calhoun L, et al. Selecting donors of platelets for refractory patients on the basis of HLA antibody specificity. Transfusion 2000;40:1446-56. [Crossref] [PubMed]
- Yam P, Petz LD, Scott EP, et al. Platelet crossmatch tests using radiolabelled staphylococcal protein A or peroxidase anti-peroxidase in alloimmunized patients. Br J Haematol 1984;57:337-47. [Crossref] [PubMed]
- Cohen P, Gardner FH. Thrombocytopenia as a laboratory sign and complication of gram-negative bacteremic infection. Arch Intern Med 1966;117:113-24. [Crossref] [PubMed]
- Okoli K, Gupta A, Irani F, et al. Immune thrombocytopenia associated with Mycoplasma pneumoniae infection: a case report and review of literature. Blood Coagul Fibrinolysis 2009;20:595-8. [Crossref] [PubMed]
- Alves FC, Aguiar R, Pessegueiro P, et al. Thrombotic microangiopathy associated with Mycoplasma pneumoniae infection. BMJ Case Rep 2018;17;2018:bcr2017222582.
- Davis RB, Meeker WR, McQuarrie DG. Immediate effects of intravenous endotoxin on serotonin concentrations and blood platelets. Circ Res 1960;8:234-9. [Crossref] [PubMed]
- Kelton JG, Neame PB, Gauldie J, et al. Elevated platelet-associated IgG in the thrombocytopenia of septicemia. N Engl J Med 1979;300:760-4. [Crossref] [PubMed]
- Nijsten MW, ten Duis HJ, Zijlstra JG, et al. Blunted rise in platelet count in critically ill patients is associated with worse outcome. Crit Care Med 2000;28:3843-6. [Crossref] [PubMed]
- Vanderschueren S, De Weerdt A, Malbrain M, et al. Thrombocytopenia and prognosis in intensive care. Crit Care Med 2000;28:1871-6. [Crossref] [PubMed]
- McGrath JM, Stewart GJ. The effects of endotoxin on vascular endothelium. J Exp Med 1969;129:833-48. [Crossref] [PubMed]
- Gaynor E, Bonnier CA, Spaet TH. Circulating endothelial cells in endotoxin-treated rabbits. Clin Res 1968;16:535.
- Thorne KJ, Oliver RC, MacIntyre DE, et al. Endotoxin-induced platelet aggregation and secretion. II. Changes in plasma membrane proteins. J Cell Sci 1977;28:225-36. [Crossref] [PubMed]
- Harker LA, Slichter SJ. Platelet and fibrinogen consumption in man. N Engl J Med 1972;287:999-1005. [Crossref] [PubMed]
- Squizzato A, Hunt BJ, Kinasewitz GT, et al. Supportive management strategies for disseminated intravascular coagulation. An international consensus. Thromb Haemost 2016;115:896-904. [Crossref] [PubMed]
- Klumpp TR, Herman JH, Innis S, et al. Factors associated with response to platelet transfusion following hematopoietic stem cell transplantation. Bone Marrow Transplant 1996;17:1035-41. [PubMed]
- Norol F, Kuentz M, Cordonnier C, et al. Influence of clinical status on the efficacy of stored platelet transfusions. Br J Haematol 1994;86:125-9. [Crossref] [PubMed]
- Friedberg RC, Donnally SF, Boyd JC, et al. Clinical and blood bank factors in the management of platelet refractoriness and alloimmunization. Blood 1993;81:3428-34. [Crossref] [PubMed]
- Alcorta I, Pereira A, Ordinas A. Clinical and laboratory factors associated with platelet transfusion refractoriness: a case-control study. Br J Haematol 1996;93:220-4. [Crossref] [PubMed]
- Asakura H. Classifying types of disseminated intravascular coagulation: clinical and animal models. J Intensive Care 2014;2:20. [Crossref] [PubMed]
- Harker LA. The role of the spleen in thrombokinetics. J Lab Clin Med 1971;77:247-53. [PubMed]
- Banaji M, Bearman SI, Buckner CD, et al. The effects of splenectomy on engraftment and platelet transfusion requirements in patients with chronic myelogenous leukemia undergoing marrow transplantation. Am J Hematol 1986;22:275-283. [Crossref] [PubMed]
- Aster RH, Jandl JH. Platelet sequestration in man. II. Immunological and clinical studies. J Clin Invest 1964;43:856-69. [Crossref] [PubMed]
- Hill-Zobel RL, McCandless B, Kang SA, et al. Organ distribution and fate of human platelets: studies of asplenic and splenomegalic patients. Am J Hematol 1986;23:231-8. [Crossref] [PubMed]
- Kawai Y, Fukushima T, Ookura K, et al. Improvement of bleeding tendency and normalized platelet count increment following splenectomy in a patient with refractory anemia. Rinsho Ketsueki. 1997;38:532-8. [PubMed]
- Aster RH. Platelet transfusion therapy: in Brinkhaus KM, Shermer RW, Mostofi FK (eds): The Platelet. Baltimore, Williams and Williams, 1971, pp 153-171.
- McFarland JG, Slichter SJ, Appelbaum F. Alloimmune versus consumptive platelet refractoriness in AML. Blood 1982;60:18a.
- Ray JB, Brereton WF, Nullet FR. Intravenous immune globulin for the treatment of presumed quinidine-induced thrombocytopenia. DICP 1990;24:693-5. [Crossref] [PubMed]
- Cohn CS. Platelet transfusion refractoriness: how do I diagnose and manage? Hematology Am Soc Hematol Educ Program 2020;2020:527-532. [Crossref] [PubMed]
- Estcourt LJ, McQuilten Z, Powter G, et al. The TREATT Trial (TRial to EvaluAte Tranexamic acid therapy in Thrombocytopenia): safety and efficacy of tranexamic acid in patients with haematological malignancies with severe thrombocytopenia: study protocol for a double-blind randomised controlled trial. Trials 2019;20:592. [Crossref] [PubMed]
- Warkentin TE, Kelton JG. Thrombocytopenia due to platelet destruction and hypersplenism. In: Hoffman R, Benz EJ Jr, Shattil SJ, et al. eds. Hematology: basic principles and practice. 4th ed. Philadelphia: Elsevier, 2005:2305-25.
- Tinmouth AT, Semple E, Shehata N, et al. Platelet immunopathology and therapy: a Canadian Blood Services Research and Development Symposium. Transfus Med Rev 2006;20:294-314. [Crossref] [PubMed]
- George JN, Raskob GE, Shah SR, et al. Drug-induced thrombocytopenia: a systematic review of published case reports. Ann Intern Med 1998;129:886-90. [Crossref] [PubMed]
- Aster RH. Drug-induced thrombocytopenia. In: Michelson AD, ed. Platelets. New York: Academic Press, 2007:887-902.
- Aster RH. Drug-induced immune cytopenias. Toxicology 2005;209:149-53. [Crossref] [PubMed]
- Mueller-Eckhardt C, Salama A. Drug-induced immune cytopenias: a unifying pathogenetic concept with special emphasis on the role of drug metabolites. Transfus Med Rev 1990;4:69-77. [Crossref] [PubMed]
- Bougie D, Aster R. Immune thrombocytopenia resulting from sensitivity to metabolites of naproxen and acetaminophen. Blood 2001;97:3846-50. [Crossref] [PubMed]
- Aster RH, Bougie DW. Drug-induced immune thrombocytopenia. N Engl J Med 2007;357:580-7. [Crossref] [PubMed]
- Aster RH. Can drugs cause autoimmune thrombocytopenic purpura? Semin Hematol 2000;37:229-238. [Crossref] [PubMed]
- Pourrat O. Treatment of drug-related diseases by plasma exchanges. Ann Med Interne (Paris) 1994;145:357-60. [PubMed]
- Balduini CL, Salvaneschi L, Klersy C, et al. Factors influencing post-transfusional platelet increment in pediatric patients given hematopoietic stem cell transplantation. Leukemia 2001;15:1885-91. [Crossref] [PubMed]
- Bishop JF, Matthews JP, McGrath K, et al. Factors influencing 20-hour increments after platelet transfusion. Transfusion 1991;31:392-6. [Crossref] [PubMed]
- Zhang X, Xiao Y, Ran Q, et al. Clinical observation of factors in the efficacy of blood component transfusion in patients following hematopoietic stem cell transplantation. PLoS One 2012;7:e36912 [Crossref] [PubMed]
- Diedrich B, Remberger M, Shanwell A, et al. prospective randomized trial of a prophylactic platelet transfusion trigger of 10×10(9) per L versus 30×10(9) per L in allogeneic hematopoietic progenitor cell transplant recipients. Transfusion 2005;45:1064-72. [Crossref] [PubMed]
- Oka S. Evaluation of Platelet transfusion triggers in hematopoietic stem cell transplant patients. Japanese Journal of Transfusion and Cell Therapy 2017;63:599-606. [Crossref]
- Daly AS, Hasegawa WS, Lipton JH, et al. Transplantation-associated thrombotic microangiopathy is associated with transplantation from unrelated donors, acute graft-versus-host disease and venoocclusive disease of the liver. Transfus Apher Sci 2002;27:3-12. [Crossref] [PubMed]
- Anasetti C, Rybka W, Sullivan KM, et al. Graft‐v‐host disease is associated with autoimmune‐like thrombocytopenia. Blood 1989;73:1054-8. [Crossref] [PubMed]
- Rio B, Andreu G, Nicod A, et al. Thrombocytopenia in venocclusive disease after bone marrow transplantation or chemotherapy. Blood 1986;67:1773-6. [Crossref] [PubMed]
- Kerkhoffs JLH, Putten WLJ, Novotny VMJ, et al. Clinical effectiveness of leucoreduced, pooled donor platelet concentrates, stored in plasma or additive solution with and without pathogen reduction. Br J Haematol 2010;150:209-17. [Crossref] [PubMed]
- Aubron C, Flint AWJ, Ozier Y, et al. Platelet storage duration and its clinical and transfusion outcomes: a systematic review. Crit Care 2018;22:185. [Crossref] [PubMed]
- Tynngård N, Boknäs N, Trinks M, et al. Storage-induced change in platelet transfusion response evaluated by serial transfusions from one donor to one patient. Transfusion 2019;59:723-8. [Crossref] [PubMed]
- Aster RH. Effect of anticoagulant and ABO incompatibility on recovery of transfused human platelets. Blood 1965;26:732-743. [Crossref] [PubMed]
- Kelton JG, Hamid C, Aker S, et al. The amount of blood group A substance on platelets is proportional to the amount in the plasma. Blood 1982;59:980-5. [Crossref] [PubMed]
- Duguesnoy RJ, Anderson AJ, Tomasulo PA, et al. ABO compatibility and platelet transfusions of alloimmunized thrombocytopenic patients. Blood 1979;54:595-9. [Crossref] [PubMed]
- Heal JM, Blumberg N, Masel D. An evaluation of crossmatching, HLA, and ABO matching for platelet transfusions to refractory patients. Blood 1987;70:23-30. [Crossref] [PubMed]
- Hagino T, Tsuno NH, Azuma F, et al. Multiple HLA-matched platelet transfusions for a single patient with broad anti-HLA antibodies: a case report. Platelets 2019;30:799-801. [Crossref] [PubMed]
- Button LN, DeWolf WC, Newburger PE, et al. The effects of irradiation on blood components. Transfusion 1981;21:419-26. [Crossref] [PubMed]
- Read EJ, Kodis C, Carter CS, et al. Viability of platelets following storage in the irradiated state. A pair-controlled study. Transfusion 1988;28:446-50. [Crossref] [PubMed]
- Stanworth SJ, Navarrete C, Estcourt L, et al. Platelet refractoriness--practical approaches and ongoing dilemmas in patient management. Br J Haematol 2015;171:297-305. [Crossref] [PubMed]
- Butler C, Doree C, Estcourt LJ, et al. Pathogen reduced platelets for the prevention of bleeding. Cochrane Database Syst Rev 2013;CD009072 [PubMed]
- Saris A, Kerkhoffs JL, Norris PJ, et al. The role of pathogen-reduced platelet transfusions on HLA alloimmunization in hemato-oncological patients. Transfusion 2019;59:470-81. [Crossref] [PubMed]
- Ishida Y, Yokota Y, Tauchi H, et al. Effectiveness of etoposide on reactive histiocytosis and refractory state to platelet transfusion during therapy of leukemia: case report. Rinsho Ketsueki 1991;32:970-5. [PubMed]
- Heddle NM, Arnold DM, Boye D, et al. Comparing the efficacy and safety of apheresis and whole blood-derived platelet transfusions: a systematic review. Transfusion 2008;48:1447-58. [Crossref] [PubMed]
- Schoenfeld H, Muhm M, Doepfmer UR, Kox WJ, Spies C, Radtke H. The functional integrity of platelets in volume-reduced platelet concentrates. Anesth Analg 2005;100:78-81. [Crossref] [PubMed]
- Tanaka M, Yanagisawa R, Yamanaka M, et al. Transfusion outcome for volume- and plasma-reduced platelet concentrates for pediatric patients. Transfus Apher Sci 2020;59:102776 [Crossref] [PubMed]
- Smogorzewska A, Dzik W. Transfusion medicine illustrated. Volume-reduced apheresis platelets. Transfusion 2005;45:651. [Crossref] [PubMed]
- Hanson SR, Slichter SJ. Platelet kinetics in patients with bone marrow hypoplasia: evidence for a fixed platelet requirement. Blood. 1985;66:1105-9. [Crossref] [PubMed]
- Slichter SJ. Relationship between platelet count and bleeding risk in thrombocytopenic patients. Transfus Med Rev 2004;18:153-67. [Crossref] [PubMed]
- Gaydos LA, Frereich EJ, Mantel N. The quantitative relation between platelet count and hemorrhage in patients with acute leukemia. N Engl J Med 1962;266:905-9. [Crossref] [PubMed]
- Heckman KD, Weiner GJ, Davis CS, et al. Randomized study of prophylactic platelet transfusion threshold during induction therapy for adult acute leukemia: 10,000/microL versus 20,000/microL. J Clin Oncol 1997;15:1143-9. [Crossref] [PubMed]
- Rebulla P, Finazzi G, Marangoni F, et al. The threshold for prophylactic platelet transfusions in adults with acute myeloid leukemia. Gruppo Italiano Malattie Ematologiche Maligne dell'Adulto. N Engl J Med 1997;337:1870-5. [Crossref] [PubMed]
- Wandt H, Frank M, Ehninger G, et al. Safety and cost effectiveness of a 10 x 10(9)/L trigger for prophylactic platelet transfusions compared with the traditional 20 x 10(9)/L trigger: a prospective comparative trial in 105 patients with acute myeloid leukemia. Blood 1998;91:3601-6. [Crossref] [PubMed]
- Kumar A, Mhaskar R, Grossman BJ, et al. Platelet transfusion: a systematic review of the clinical evidence. Transfusion 2015;55:1116-27; quiz 1115. [Crossref] [PubMed]
- Gmür J, Burger J, Schanz U, et al. Safety of stringent prophylactic platelet transfusion policy for patients with acute leukaemia. Lancet 1991;338:1223-6. [Crossref] [PubMed]
- Estcourt L, Stanworth S, Doree C, et al. Prophylactic platelet transfusion for prevention of bleeding in patients with haematological disorders after chemotherapy and stem cell transplantation. Cochrane Database Syst Rev 2012;CD004269 [Crossref] [PubMed]
- Sagmeister M, Oec L, Gmür J. A restrictive platelet transfusion policy allowing long-term support of outpatients with severe aplastic anemia. Blood 1999;93:3124-6. [Crossref] [PubMed]
- Goel R, Ness PM, Takemoto CM, et al. Platelet transfusions in platelet consumptive disorders are associated with arterial thrombosis and in-hospital mortality. Blood 2015;125:1470-6. [Crossref] [PubMed]
- Dzik S. How I do it: platelet support for refractory patients. Transfusion 2007;47:374-8. [Crossref] [PubMed]
- Xia W, Xu X, Fu Y, et al. CD36 deficiency among South‐East Asian populations. ISBT Sci Ser 2016;52:33-6. [Crossref]
- Cornelissen LL, Caram-Deelder C, Meier RT, et al. Platelet transfusion and tranexamic acid to prevent bleeding in outpatients with a hematological disease: A Dutch nationwide survey. Eur J Haematol 2021;106:362-70. [Crossref] [PubMed]
Cite this article as: Hagino T, Sato T, Tsuno NH, Tasaki T. Incidence and management of non-immune platelet transfusion refractoriness: a narrative review. Ann Blood 2021;6:28.


