
An analysis of the factors associated with successful re-entry of blood donors
Introduction
According to the Chinese blood donation law and in order to reduce the risk of transfusion-transmitted infections, the Chongqing blood center screens for major blood-borne pathogens, including hepatitis B virus (HBV), hepatitis C virus (HCV), human immunodeficiency virus (HIV) 1 and 2 (HIV-1/2) and Treponema pallidum (TP). If a test for any of the four blood-borne pathogens is reactive, the suspicious donations will be discarded, leading to deferral of the donors of those samples.
In order to ensure the highest possible safety of clinical transfusion, the blood center uses a highly sensitive test method which is performed several times and includes several makers of infections (1). Therefore, the occurrence of false positive (FP) results is a possibility (2).
A study in the USA found that, between 1995 and 2002, about 90,000 first-time blood donors were deferred for unconfirmed reactive results from HBV, HCV and HIV (3). A national study in China estimated that more than 800,000 donations between 2009 and 2011 were discarded based on results of reactive serological tests, and at the same time those donors were permanently deferred (4).
Therefore, the Chinese society of blood transfusion has developed guidelines for re-entry of reactive blood donors in blood screening tests (5). These guidelines set out the conditions under which deferred donors can participate in the re-entry program, but do not take into account the different characteristics of blood donors. For example, blood donors with HIV or TP reactivity should wait 3 months before participating in the re-entry program, while those with HBV or HCV reactivity should wait 6 months. Several studies in China investigated the eligibility for re-entry and the re-entry rates of deferred donors (6). So far, most studies on re-entry strategies in provinces and cities of China have focused on evaluating the overall participation of blood donors in the re-entry program and the rates of re-entering the blood donor team (7,8). A follow-up study of 888 re-entry blood donors in China found that 61.9% of them were FPs (9). In addition, blood centers in Huizhou and Jiaxing, China, studied the feasibility of the guidelines for re-entry of reactive blood donors in blood screening tests in their local area (10,11).
So far, there have been few studies investigating the factors underlying the success of re-entry of blood donors in China. Two large studies in the United States (one of them based on more than two million blood donors) have shown that certain donor demographic factors are associated to FP results, although these results are likely show differences over time, and to vary among different regions and different methods used (12,13). Specific donor demographic factors, including first-time donors, women, and African Americans or Hispanic ethnicities in serological analyses of several viral markers were associated with a FP result. However, it is possible that other factors, such as gender, age, and time interval, may also affect the success of re-entry of blood donors.
Therefore, we set out to investigate the factors that influence the success of re-entry of blood donors, and the success of blood donation upon re-entry. Our findings further our understanding in the process of re-entry of blood donors, and may provide a scientific basis for high-level decisions of the blood service, increasing trust between donors and the blood service and helping guide staff in the selection of target groups, particularly in setting of limited resources. Based on our results, the outcome and benefits of blood donor programs can be further optimized to increase retention of safe and committed donors. We present the following article in accordance with the MDAR reporting checklist (available at https://aob.amegroups.com/article/view/10.21037/aob-20-95/rc).
Methods
Setting
We collected data from the Chongqing Blood Center, which receives about 240,000 blood units per year across the city during community sessions. The population served by Chongqing Blood Center is about 10,612,600. Chongqing is a city of 32 million people located in the southwest of China.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Chongqing Blood Center in April 2016 and informed consent was obtained from all donors.
Screening of blood donations
According to national government regulations, all blood donations must be simultaneously tested with two enzyme immunoassays (EIAs) to detect HBsAg (WANTAI Bio and DiaSorin); HCV antibodies (HCVAb: WANTAI Bio and Ortho); HIV antibodies alone or in combination with HIV antigen (HIVAg/Ab) (WANTAI Bio and Bio RAD); and antibodies to Treponema pallidum (TPAb: WANTAI Bio and KRHUA Bio). At the same time, donations must be tested by viral nucleic acid testing (NAT) for HBV DNA, HCV RNA, and HIV RNA.
In the event of reactivity for one of these test items, the donation will be discarded and the donor will be permanently deferred.
Participants and sample collection
Donors with reactive tests are notified via text message by the blood center. Donors can call or visit the blood donation site for consultation, at which point the staff will provide information regarding their general health, donor status, as well as professional support and medical advice to the donor, and whether and how they can participate in the re-entry programme.
Between January 2017, and December 2019, the number of deferred blood donors who met the guidelines for re-entry of reactive blood donors in blood screening test were 4,643. Only 18.2% (844 of 4,643) of the donors that were informed by blood center by text message of their test reactivity volunteered to participate in the re-entry programme. For a successful re-entry into the programme, [T/CSBT 002—2019: the guideline for re-entry of reactive blood donors in blood screening test], reactive blood donors or their tests must meet the following criteria:
- Blood donors who previously participated in voluntary blood donation, for whom reactivity for serological markers of the pathogens of blood-transmitted diseases (HBV, HCV, HIV, TP) in a single reagent were detected, and without reactivity of HBV, HCV and HIV markers by NAT.
- Blood donors whose HIV markers were tested by serological single reagent for reactivity with a negative confirmation test and without NAT reactivity who were deferred for more than 3 months.
- Blood donors whose HBV or HCV markers were detected by a single serological reagent and NAT was not reactive, and who were deferred for more than 6 months.
- Blood donors whose test reactivity was detected by a single serological reagent with TP markers who were deferred for more than 3 months.
- Blood donors whose NAT multiplex was reactive, but non-reactive on all discriminatory assays, and who were deferred for more than 6 months (these blood donors will no longer be included in the program according to guidelines for re-entry of reactive blood donors in blood screening test released in April 2019).
Blood donors who did not meet the criteria for participating in the re-entry programme have not been included in this study.
Blood samples were collected from donors who met the above conditions and volunteered for the project. In brief, 10 mL of whole blood was collected into two tubes containing EDTA-K2 anticoagulant for testing. Informed consent for blood collection from blood donors participating in the re-entry programme has been obtained.
Re-entry programme
The procedure and determination rules for enzyme-linked immunosorbent assay reactive blood donors are shown in Figures 1-4. Blood donors whose NAT multiplex was reactive, but non-reactive on all discriminatory assays, were followed up after 6 months of positive testing. Follow-up was done before April 2019. All blood donations underwent serological testing for HBV, HCV, HIV and TP with two EIAs. At the same time, the same blood samples were also tested for nucleic acids. In the event that all results are non-reactive, the donors will be eligible for re-entry in the blood donation programme. If any of the above-mentioned experimental tests are reactive, the donor will continue to be deferred.
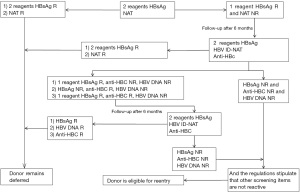
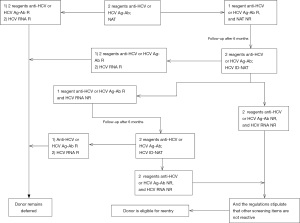
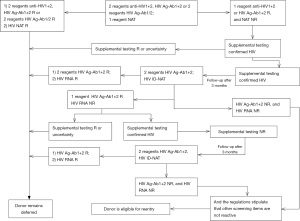
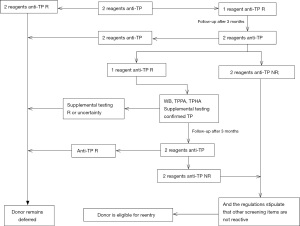
Staff at the blood center will be responsible for informing the donors of their re-entry results as soon as possible. Donors eligible for re-entry can participate in blood donation 3 months after deferring. Donors who remain deferred will be given medical information and guidance.
The number of people willing to re-enroll in the re-entry programme and donate blood after three months will be monitored in the information system.
Data collection
Eight hundred and forty-four blood donors volunteered to participate in the re-entry programme, 409 (48%) of whom were men and 435 (52%) were women. The average age of all study participants is 36.02 years. Five hundred and sixty blood donors had test results that qualified for re-entry and returned successfully to the programme. Demographic and related experimental data were collected and included: age; occupation; education; donor status [first or repeat; sex; reactive items (HBV, HCV, HIV, TP or NAT); blood type (A, B, O or AB)]; and time interval (the time between the first test failure and participation in the re-entry programme). All of the above-mentioned data were retrieved from the blood donor registration in information system of the blood center. Outcomes for re-entry include 3 groups: donors who are eligible for re-entry; donors who were followed-up after 3 months for re-testing; and donors who remained permanently deferred. Donors who are eligible for re-entry were defined as the successful group and donors who were followed-up but remained permanently deferred were defined as the failure group.
In the successful group, some of the blood donors decided to participate in the donation process again while others did not. Data collection in the successful group is consistent with the blood donors participating in the re-entry programme. Donors who participated in blood donation again were defined as the “re-participation group” and donors who did not participate in blood donation again were defined as the “re-participate not yet group”.
Statistics
Statistical analysis was performed by using SPSS (Statistical Package for Social Sciences) for windows, version 23.0 (SPSS Inc., Chicago, IL, USA).
Analysis was performed as follows: we first applied a t-test for independent samples to investigate the influence of measurement data. Then, we applied a chi-square test to investigate the influence of count data. Multivariable logistic regression analysis was used to explore the factors influencing success of re-entry. Logistic regression analysis was performed for parameters that were statistically significant for the outcome (P<0.1). After preliminary data fitting using a multivariable model, variables with a test p value of less than 0.05 were retained in the final model. All P values reported are two-tailed.
Results
The clinical characteristics of donors in this study
Between January 2017, and December 2019, 441,377 donations were collected in Chongqing blood center, China, of which 2.8% [12,193] were discarded due to test results that did not meet the qualifying criteria for blood donation. Of 12,193 tests, 2,657 (0.60%), 1,287 (0.29%) and 716 (0.16%) donations showed reactivity for markers of HBV, HCV, and HIV infections, respectively. Anti-TP was detected in 1,343 (0.30%) donations. As of December 2019, 844 of these blood donors had volunteered to participate in the re-entry programme. As shown in Table 1, of the 844 blood donors, 265 (31.40%) were initially deferred for HBsAg (10.90% of HBV deferrals), 248 (29.38%) were initially deferred for HCV (19.41% of HCV deferrals), 102 (12.09%) were initially deferred for HIV (15.34% of HIV deferrals), 111 (13.15%) were initially deferred for TP (8.79% of TP deferrals) and 118 (13.98%) were initially deferred for NAT (3.80% of NAT deferrals).
Table 1
| Test items | Deferred (n=441,377) | Eligible for re-entry | Re-entry | |||||
|---|---|---|---|---|---|---|---|---|
| Number | Percent (%) | Number | Percent (%) | Number | Percent (%) | |||
| HBV | 2,657 | 0.60 | 1,131 | 42.57 | 265 | 23.43 | ||
| HCV | 1,287 | 0.29 | 1,227 | 95.34 | 248 | 20.21 | ||
| HIV | 716 | 0.16 | 550 | 76.82 | 102 | 18.55 | ||
| TP | 1,343 | 0.30 | 506 | 37.68 | 111 | 21.94 | ||
| NAT (before April 2019) | 2,450 | 0.56 | 1,229 | 53.16 | 118 | 9.60 | ||
HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; TP, Treponema pallidum; NAT, nucleic acid testing.
As shown in Table 2, among the 844 donors who were tested for possible re-entry and volunteered to participate in the project, 66.35% [560] of the donors presented results where all test items met the qualifying criteria for re-entry in the programme, and were defined as the re-entry group, and 33.65% [284] of the donors presented tests that failed to meet the qualifying criteria. Twenty-six-point-one-eight percent [221] of the donors who obtained once again a single reagent positive result and could return to the team for a follow-up were defined as the follow-up group. Seven-point-four-six percent [63] of the donors who were not eligible to rejoin the team and were permanently deferred were defined as the quarantine group. The number of donors in the three groups with HBV, HCV, HIV, TP and NAT results are shown in Table 2.
Table 2
| Test items | Successful group, n (%) | Failure group, n (%) | Total | ||
|---|---|---|---|---|---|
| Re-entry | Follow-up | Defer | |||
| HBV | 202 (76.23) | 50 (18.87) | 13 (4.91) | 265 | |
| HCV | 123 (49.60) | 118 (47.58) | 7 (2.82) | 248 | |
| HIV | 77 (75.49) | 22 (21.57) | 3 (2.94) | 102 | |
| TP | 63 (56.76) | 25 (22.52) | 23 (20.72) | 111 | |
| NAT | 95 (80.51) | 6 (5.08) | 17 (14.41) | 118 | |
| Total | 560 (66.35) | 221 (26.18) | 63 (7.46) | 844 | |
HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; TP, Treponema pallidum; NAT, nucleic acid testing.
Analysis of factors affecting the success of re-entry
The characteristics of the donors in this study, including clinical and blood donation parameters and the outcome of re-entry, are shown in Table 3. Seven hundred and twenty-six (86.02%) donors were deemed to have FP serology results and 118 (13.98%) were deemed to have FP NAT results. The results in Table 3 indicate that sex and age do not affect re-entry outcomes. Re-entry outcomes of deferred donors were influenced by blood type, donor status, occupation, education, reactive items and time interval (P<0.1). We have therefore incorporated these six factors into the multivariable logistic regression model. The results presented on Table 4 suggest that donor status, occupation, reactive items and time interval were the factors most likely to be associated with re-entry outcomes. Logistic regression model, tested by likelihood ratio (χ2=216.824, P<0.001) was significant and was the model that provided the best degree of fit. Our logistic regression model shows that the risk of a reactive test result at qualification decreased with the donors’ occupation. Specifically, medical personnel, civil servant/public institution/army, students, workers or others (compared to not report) had a decreased likelihood of providing a reactive test result while an increased likelihood was associated with shorter time interval, the HIV or NAT group and first-time donor groups. However, the risk of a reactive test at qualification was not associated with sex, blood type and education.
Table 3
| Factors | Successful group (n=560) | Failure group (n=284) | χ2/t | P |
|---|---|---|---|---|
| Gender | 0.003 | 0.956 | ||
| Female | 289 | 146 | ||
| Male | 271 | 138 | ||
| Blood type | 8.099 | 0.044 | ||
| A | 179 | 103 | ||
| B | 110 | 72 | ||
| AB | 46 | 18 | ||
| O | 225 | 91 | ||
| Donor status | 51.853 | <0.001 | ||
| Repeat | 359 | 108 | ||
| First | 201 | 176 | ||
| Occupation | 13.900 | 0.031 | ||
| Medical personnel | 40 | 12 | ||
| Civil servant/public institution/army | 44 | 27 | ||
| Student | 76 | 62 | ||
| Workers | 82 | 38 | ||
| Farmer | 44 | 26 | ||
| Other | 242 | 103 | ||
| Not reported | 32 | 16 | ||
| Education | 63.980 | <0.001 | ||
| Middle school/less | 153 | 62 | ||
| Trade school | 25 | 13 | ||
| High school | 109 | 51 | ||
| Junior college | 117 | 71 | ||
| Undergraduate | 96 | 44 | ||
| Master’s degree | 9 | 35 | ||
| Other | 15 | 8 | ||
| Not reported | 36 | 0 | ||
| Reactive items | 61.741 | <0.001 | ||
| HBV | 202 | 63 | ||
| HCV | 123 | 125 | ||
| HIV | 77 | 25 | ||
| TP | 63 | 48 | ||
| NAT | 95 | 23 | ||
| Age (years), mean ± SD | 36.46±10.33 | 35.14±11.72 | 1.680 | 0.093 |
| Time interval (months), mean ± SD | 37.75±43.40 | 27.60±34.60 | 3.427 | 0.001 |
HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; TP, Treponema pallidum; NAT, nucleic acid testing; SD, standard deviation.
Table 4
| Factors | B | OR | 95% CI lower | 95% CI upper | P |
|---|---|---|---|---|---|
| Donor status (relative to first) | 1.018 | 0.361 | 0.252 | 0.519 | <0.001 |
| Occupation (relative to not reported) | |||||
| Medical personnel | −2.609 | 0.074 | 0.020 | 0.266 | <0.001 |
| Civil servant/public institution/army | −1.623 | 0.197 | 0.060 | 0.654 | 0.008 |
| Student | −1.894 | 0.15 | 0.046 | 0.459 | 0.002 |
| Workers | −1.669 | 0.188 | 0.059 | 0.800 | 0.005 |
| Farmer | −1.166 | 0.312 | 0.090 | 1.083 | 0.066 |
| Other | −1.764 | 0.171 | 0.057 | 0.518 | 0.002 |
| Reactive items (relative to HBV) | |||||
| HCV | 0.191 | 1.21 | 0.67 | 2.170 | 0.523 |
| HIV | 1.551 | 4.716 | 2.608 | 8.526 | <0.001 |
| TP | 0.629 | 1.875 | 0.912 | 3.857 | 0.088 |
| NAT | 1.008 | 2.739 | 1.391 | 5.394 | 0.004 |
| Time interval | −0.022 | 0.978 | 0.971 | 0.985 | <0.001 |
HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; TP, Treponema pallidum; NAT, nucleic acid testing; CI, confidence interval; OR, odds ratio.
Analysis of factors influencing re-participation in blood donation
Five hundred and sixty (66.35%) donors whose all test items qualified for re-entering the blood donation programme are able to donate blood again after 3 months. Three hundred and twenty-one blood donors decided to donate again after being told they were eligible for the test 3 months later. Table 5 presents data on those who participated in blood donation again and those who did not. The decision whether to donate again was influenced by the donor status (first donor or repeat donor), type of reactive test and time interval (P<0.05).
Table 5
| Factors | Re-participate not yet | Re-participate | χ2 | P |
|---|---|---|---|---|
| Gender | 2.55 | 0.110 | ||
| Female | 114 | 175 | ||
| Male | 125 | 146 | ||
| Blood type | 3.69 | 0.290 | ||
| A | 71 | 108 | ||
| B | 55 | 55 | ||
| AB | 17 | 29 | ||
| O | 96 | 129 | ||
| Donor status | 23.49 | <0.001 | ||
| Repeat | 126 | 233 | ||
| New | 113 | 88 | ||
| Occupation | 8.572 | 0.190 | ||
| Medical personnel | 10 | 30 | ||
| Civil servant/public institution/army | 18 | 26 | ||
| Student | 36 | 40 | ||
| Workers | 30 | 52 | ||
| Farmer | 20 | 24 | ||
| Other | 112 | 130 | ||
| Not reported | 13 | 19 | ||
| Education | 8.28 | 0.310 | ||
| Middle school/less | 71 | 82 | ||
| Trade school | 5 | 20 | ||
| High school | 44 | 65 | ||
| Junior college | 47 | 70 | ||
| Undergraduate | 47 | 49 | ||
| Master’s degree | 4 | 5 | ||
| Others | 6 | 9 | ||
| Not reported | 15 | 21 | ||
| Reactive items | 24.97 | <0.001 | ||
| HBV | 99 | 103 | ||
| HCV | 51 | 72 | ||
| HIV | 23 | 54 | ||
| TP | 14 | 49 | ||
| NAT | 52 | 43 | ||
| Age (years), mean ± SD | 35.87±10.39 | 36.91±10.28 | 1.18 | 0.239 |
| Time interval (months), mean ± SD | 32.03±41.10 | 42.00±44.62 | 2.71 | 0.007 |
HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; TP, Treponema pallidum; NAT, nucleic acid testing; SD, standard deviation.
Discussion
In Chongqing, China, 2.76% [12,193/441,377] donations were reactive to at least one marker of HBV, HCV, HIV, TP infections, NAT or ALT test, resulting in 1.58% [6,981/441,377] donors being permanently deferred. Of the donors that were permanently deferred, 48.90% donors [3,414/6,981] were deferred because of NRR serology and could potentially participate in the re-entry programme. Along the same lines, a Chinese national survey reported a single EIA reactivity rate in 48.5% of the deferred donors (4). In our study, we included donors deferred because of NRR serology or deferred because of NAT results before April 2019. Eight hundred and forty-four donors participated in our study. Based on our results, 66.35% of blood donors [560/844] returned to the blood donor team successfully. Similar results (63.9%) were reported by Yu et al. (14). Our studies show that among the blood donors deferred for HBV, HCV, HIV and TP, the success rate of returning was of 76.23% [202/265] for HBV, 49.60% [123/248] for HCV, 64.17% [77/120] for HIV and 56.76% [63/111] for TP.
There are recommended guidelines regarding the procedures for re-enrolling in a blood donation programme in China. However, the time of re-entry of blood donors mentioned in most guidelines is basically regulated. In this work, we only formulated the re-entry programme of blood donors according to the guidelines, so that more blood donors might not be able to re-entered successfully. The aim of this study was to analyze the factors impacting re-entry in order to retain blood donors and provide guidance to carry out blood donation work more efficiently.
Our results suggest that the successful rate of re-entry depends on the donor status, occupation, type of reactivity, and time interval. Repeat donors had a lower risk of failure of returning to the blood donation team than first-time donors (odds ratio =0.361, 95% CI: 0.252–0.519, P<0.001). These results confirm prior studies (15). One explanation for our results is that repeat donors may be living healthier lives and are more likely to have FP. In addition, after repeated blood screening tests, the probability of blood donors having a true positive test was lower than that of first-time blood donors. Certain occupations such as medical personnel, civil servant/public institution/army, students, workers had a lower risk of failure of returning to the blood team than those not reported occupation. One possible explanation for this statistical difference is that these donors paid were more diligent about their health and were more willing to participate in the re-entry programme.
The risk of having a second reactive screening test is also highly dependent on the transmissible disease marker. In particular, the secondary rate of HBsAg reactivity was only 23.77%, while the rates of TP reactivity (43.24%) and of HCV reactivity (50.40%) were higher. The rate of HCV reactivity is similar to the rate of 51% reported previously Ariaansz-Van zanten et al. (16). It has been previously reported by Kiely et al. that the rate of HBsAg reactivity (14.3%) was lower than that of HCV reactivity (77.4%) following a first reactive test (17). On the other hand, our results based on logistic regression analysis showed that HIV and NAT deferred blood donors were more likely to fail re-entry in the blood donation programme than HBV deferred blood donors (HIV: odds ratio =4.716, 95% CI: 2.608–8.526, P<0.001; NAT: odds ratio =2.739, 95% CI: 1.391–5.394, P=0.004). Similar results were obtained by Grégoire et al. (15). In addition, HCV and TP deferred blood donors were almost as likely to fail re-entry in the blood donation team as HBV deferred donors; this difference was not statistically significant.
Another factor associated with an increased risk of having a repeat reactive outcome is the time elapsed from the initial result. Grégoire et al. have reported that for each year interval between the initial FP test and a qualifying test, the risk appeared to decrease by 7% (15). Similarly, our study suggested that a longer interval between an unqualified test and returning to participation in blood donation team was associated with a greater success of returning (odds ratio =0.978, 95% CI: 0.971–0.985, P<0.001). These findings indicate that a longer time gap should be considered when implementing a donor re-entry program. It might be more productive for the blood donors to wait longer before re-enrolling in the programme, in order to increase their chances of successful re-entry.
Among all blood donors who re-enrolled in blood donation programmes, 57.32% [321/560] participated in the donation, a rate that is much higher than that of the general population (20.69%). The rate of donation among repeat donors (72.59%, 233/321) was higher than that of first-time donors (41.71%, 88/211) after returning to the group, a difference that was statistically significant (χ2=23.4, P<0.001). The rate of repeated donation was the highest among those deferred for TP (77.78%, 49/63), and the lowest among those deferred for NAT (45.26%, 43/95). A possible reason for NAT deferred blood donors to participate in the re-entry programme may be their wish to confirm their health status than to participate in blood donation again. Blood donors who participated in blood donation again experienced a longer time before re-enrollment than those who did not participate in blood donation again. One reason for these results might be that some blood donors did not take the notice of unqualified blood seriously, and when they received a new recruitment notice for blood donation, they realized that they could not participate in the blood donation process before enrolling in the re-entry programme. In general, such blood donors have the intention to donate blood before they participate in the re-entry programme, therefore the probability of them donating blood is higher after a successful re-entry into the programme. However, upon successful re-entry into the programme, donors who had previously had a reactive result were still at a higher risk of having another reactive result (13.04%, 73/560) than the general population (1.98%, 8,738/441,377). Similar observations have been previously reported in the study of Grégoire et al. (15).
In conclusion, although repeated FP results lead to a predictable loss of blood donors, deferred donors have a higher rate of blood donation than the general population, indicating their motivation to donate, and they can make a valuable contribution to the blood supply. Therefore, it is important to understand the many factors that affect re-entry in the blood donation programme in order to increase its success. These factors should be taken into consideration when providing advice and guidance to blood donors who wish to re-enter the donation programme. For instance, it is advisable that blood donors who are first-time donor, whose occupation is farmer or have not reported it or who were deferred to HIV or NAT reactivity wait longer before participating in the re-entry programme. Such longer time gap is likely to increase the chance of successful re-entry. On the other hand, repeat donors who wait a longer time interval are more likely to be able to participate again. Blood centers should recruit the blood donors who have the highest chance of successful re-entry, and those with a higher chance of donating again, helping them to continue to pursue their valuable contributions to blood supply.
Acknowledgments
Staff at the Department of Quality Management of Chongqing Blood Center, and blood donors are thanked for participating in this study. The authors would like to thank all the reviewers who participated in the review and MJEditor (www.mjeditor.com) for its linguistic assistance during the preparation of this manuscript.
Funding: This work was supported by Chongqing Municipal Health Commission (No. 2016ZDXM035).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://aob.amegroups.com/article/view/10.21037/aob-20-95/rc
Data Sharing Statement: Available at https://aob.amegroups.com/article/view/10.21037/aob-20-95/dss
Peer Review File: Available at https://aob.amegroups.com/article/view/10.21037/aob-20-95/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aob.amegroups.com/article/view/10.21037/aob-20-95/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Chongqing Blood Center in April 2016 and informed consent was obtained from all donors.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dean CL, Wade J, Roback JD. Transfusion-Transmitted Infections: an Update on Product Screening, Diagnostic Techniques, and the Path Ahead. J Clin Microbiol 2018;56:e00352-18. [Crossref] [PubMed]
- Kiely P, Hoad VC, Wood EM. False positive viral marker results in blood donors and their unintended consequences. Vox Sang 2018;113:530-9. [Crossref] [PubMed]
- Cable R, Musavi F, Notari E, et al. Limited effectiveness of donor deferral registries for transfusion-transmitted disease markers. Transfusion 2008;48:34-42. [PubMed]
- Deng X, Zhang L, Gao Y, et al. Blood screening and blood donor deferral in 357 Chinese blood banks. Chinese Journal Blood Transfusion 2012;25:1241-3.
- T/CSBT 002—201. Guideline for reentry of reactive blood donors in blood screening test. Available online: https://www.csbt.org.cn/plus/view.php?aid=10195
- Chen J, Xie G, Liang H, et al. Evaluating the process of conducting Guideline for Deferral and Reentry of Blood Donors of Reactive Test Results (Second Edition) in Guangzhou Blood Center. Chinese Journal Blood Transfusion 2019;32:485-8.
- Zhou H, Wang Y, Sang L, et al. Application and establishment of the regionally integrated strategy for the re-entry of blood screening reactive donors. Chinese Journal Blood Transfusion 2019;32:918-21.
- Shi J, Jia L, Xu A, et al. Analysis of return of reactive blood donors in Nanjing. Journal of Clinical Hematology 2019;32:620-1.
- Deng X, Zang L, Wang X, et al. Follow-up program for blood donors with unconfirmed screening results reveals a high false-positive rate in Dalian, China. Transfusion 2020;60:334-42. [Crossref] [PubMed]
- Xu L, Li J, Wang L, et al. The feasibility study of reentry for blood Syphilis reaction donors in Jiaxing. Chin J Blood Transfusion 2017;30:528-530.
- Li X, Zhong Z. Investigation and analysis on the feasibility of regrouping blood donors with anti-HIV antibody single test agent. Experimental and Laboratory Medicine 2015;33:246-7.
- Ownby HE, Korelitz JJ, Busch MP, et al. Loss of volunteer blood donors because of unconfirmed enzyme immunoassay screening results. Retrovirus Epidemiology Donor Study. Transfusion 1997;37:199-205. [Crossref] [PubMed]
- Vo MT, Bruhn R, Kaidarova Z, et al. A retrospective analysis of false-positive infectious screening results in blood donors. Transfusion 2016;56:457-65. [Crossref] [PubMed]
- Yu Y, Liu Z, Li J, et al. Study on rejoin detection mode for unpaid blood donors of positive reactivity in Shunde. International Journal of Laboratory Medicine 2019;40:149-51.
- Grégoire Y, Germain M, Delage G. Factors associated with a second deferral among donors eligible for re-entry after a false-positive screening test for syphilis, HCV, HBV and HIV. Vox Sang 2018;113:339-44. [Crossref] [PubMed]
- Ariaansz-Van zanten M, Vrielink H, van den Burg PJM, et al. Re-entry of blood donors with a false positive anti-HTLV ELISA result. Transfusion 2000;40:10S.
- Kiely P, Stewart Y, Castro L. Analysis of voluntary blood donors with biologic false reactivity on chemiluminescent immunoassays and implications for donor management. Transfusion 2003;43:584-90. [Crossref] [PubMed]
Cite this article as: Yang D, Li X, Qin W, Dai H, Yang J, Luo Z, Wang F, Hu W, Yang P, Duan H, Huang X. An analysis of the factors associated with successful re-entry of blood donors. Ann Blood 2022;7:27.


