
Platelet rich plasma: hope or hype?
Introduction
The science of blood transfusion has been documented as early as the 19th century when an obstetrician attempted vein to vein transfusions to treat postpartum hemorrhage (1). From vein to vein transfusions to collection of whole blood stored in glass bottles to the introduction of plastic storage bags, advances in blood transfusion have allowed fractionation of whole blood to component therapy. Transfusion medicine physicians, blood bank staff, and clinical providers are familiar with the collection, indications, and regulations surrounding the use of red blood cells, platelets, plasma, and cryoprecipitate. However, the larger medical community may be unfamiliar with another frequently administered blood product called platelet rich plasma (PRP). This review aims to break down the enigma of PRP and its associated research and selected applications.
Methods
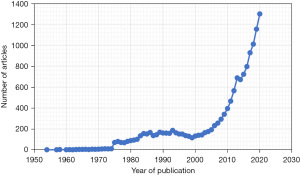
Articles on PRP were first published in the 1950s and a PubMed search reveals that there has been an exponential growth in interest and research in the past seven decades (Figure 1). Publications on PRP are available in almost every subspecialty of medicine and include original articles, systematic reviews, and meta-analyses. Select articles were chosen from the literature search and are reviewed here. Online searches of Google and Google Scholar were also performed to guide selected review of popular PRP treatments, as these are search engines that are readily accessible to consumers of PRP. Only English articles were included. The goal is to provide an overview of PRP composition and preparations, some current applications, gaps in knowledge, and areas for future investigation and development.
Description
PRP consists of concentrated platelets suspended in plasma obtained through the centrifugation of anticoagulated whole blood. All PRP products, by definition, contain a higher platelet concentration than native blood. However, actual concentrations can range from 2 to 12 times baseline concentration with some studies identifying levels as low as 0.52 times baseline despite efforts to standardize preparation methods and protocols (2). There are over 40 commercial systems for PRP preparation aimed at collecting a product that has been hailed as a safe, autologous product with the potential to promote healing (3-5).
Platelets are complex non-nucleated cell fragments containing secretory granules that house multiple chemokines, adhesion molecules, and growth factors that aid in angiogenesis and recruitment of fibroblasts and other cells that are central to collagenesis and healing. These include vascular endothelial growth factor (VEGF), transforming growth factor beta (TGF-β), platelet-derived growth factor (PDGF), epidermal growth factor (EGF), and insulin-like growth factors I and II (IGF-I and IGF-II) (6-8). Upon activation, platelets will release almost all stored growth factors within 1 hour although continued growth factor synthesis will occur until platelet death at around 8 days (9).
PRP encompasses a heterogeneous group of products containing various levels of white blood cells (WBCs) and fibrin and can even be used to describe activation of these products with platelet activators such as thrombin, calcium chloride, or calcium gluconate. PRP can be collected as the serum and buffy coat layers on higher platelet count systems or as the non-cellular component layer using lower platelet count systems (10). This has led to the development of additional classification terminology with a spectrum of pure platelet-rich plasma (P-PRP), leukocyte- and platelet-rich plasma (L-PRP), pure platelet-rich fibrin (P-PRF), etc. (11). WBC content has been observed to vary across PRP preparation methods and the value of including WBCs in PRP formulations has been debated with some support for their role in platelet activation and cell signaling and simultaneous concern for resulting negative inflammatory reactions (12).
In vitro studies have shown activated PRP to release platelet-derived growth factor-AB (PDGF-AB) and transforming growth factor-β1 (TGF-β1) with associated heightened proliferation of stem cells and fibroblasts. Cell proliferation, however, varied with activated PRP dose and optimal dosing varied by cell type (13).
Graziani et al. [2006] identified the optimal concentration for in vitro fibroblast and osteoblast growth to be 2.5 times baseline concentration with adverse effects on proliferation at higher concentrations although this likely differs by product and application (14). Kakudo et al. [2008] observed stimulation of human adipose-derived stem cells and human dermal fibroblast proliferation with 1% and 5% activated PRP with no benefit at the highest studied concentration of 20% (15). It therefore remains unclear whether higher platelet dosages translate into added clinical benefit (9).
Although deemed a safe and generally non-invasive intervention, some suggested contraindications to PRP therapy include platelet dysfunction or critical thrombocytopenia, hypofibroginemia, anticoagulation, hemodynamic instability, sepsis or infection, and chronic liver disease (16).
Regulation
Unlike blood products that are stored within and distributed by the blood bank, PRP is usually not within the operational scope of most transfusion services. In the United States, PRP products are not regulated, rather, the biologics and medical devices (i.e., centrifuges) used to produce PRP are regulated separately by the Food and Drug Administration (FDA). PRP systems currently have 510(k) clearance based on approval of a predicate device for PRP. PRP was initially intended to be combined with bone graft material for improved handling with all other uses considered to be off-label at this time. Of note, clearance is not synonymous with approval and is not indication-specific (17). The current clearance status for PRP systems does not require clinical data as the autologous, homologous, and minimally manipulated nature of these products deem them low risk biologic products that do not require additional regulation by the FDA. In such a way, PRP products are exempt from quality and other control measures that are common for other biologic products, including its components of platelets and plasma (18-20).
PRP applications
There has been increasing interest in PRP and its potential uses in the past several decades (Figure 1). The main investigated applications have been in the fields of dentistry, orthopedics, and aesthetics with numerous trials conducted for soft tissue injuries, arthritis, dermatologic conditions including scars, hair restoration, and breast augmentation. PRP has seen various administration routes ranging from gel forms applied topically to injections to the site of interest (21). Of note, some products designated as PRP may not meet established definitions (9). A review of all published applications is beyond the scope of the current review and several comprehensive reviews have been published elsewhere (5,22). A summary of orthopedic and cosmetic applications will be outlined to demonstrate the scope of PRP therapies and available data supporting these interventions.
Orthopedics and sports medicine
Rachul et al. [2017] investigated media coverage of PRP and demonstrated that 70% were published in newspaper sports sections and over 75% were sports-related stories. Interestingly, of the articles studied, the majority framed PRP as a routine procedure as compared to an experimental therapy (23). Several reviews have been published outlining current data on the effectiveness of PRP for the management of orthopedic conditions including tendinopathy, muscle injury, and arthritis. PRP has been compared to surgical intervention, such as rotator cuff repair, injections (steroid, hyaluronic acid, saline), and physical therapy (17,21,24). These studies tend to use self-report measures such as the visual analogue scale (VAS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), or other subjective scales that assess for pain and function (17,21,24,25). Return to sport has also been used to determine benefit of these treatments (17).
There has been some support for improvement of pain in lateral epicondylitis (also known as “tennis elbow”) but minimal support for benefit in other soft tissue injuries including chronic Achilles tendinopathy, rotator cuff injury, and anterior cruciate ligament injury (21). Furthermore, the mechanism for these effects has yet to be elucidated since there does not appear to be a correlation with platelet counts or VEGF, PDGF, and EGF levels (26). Jones et al. [2018] outlined relevant meta-analyses that have been published on musculoskeletal applications of PRP (17). There is some support for improved pain and function with PRP injections in osteoarthritis (OA) based on meta-analytical studies with more favorable results with knee, but not hip, OA but these trends have not been replicated in all analyses (17). These have shown modest benefits for some indications including tendon or ligament injuries and knee OA with undetermined clinical significance. Meta-analyses of mixed orthopedic injuries, including muscle injuries, have not found quality trials and even those that have been conducted have not shown benefit. Additionally, when some of these analyses excluded trials that were not blinded, purported benefits were no longer statistically significant. Setayesh et al.’s 2018 review supports the lack of convincing data on effectiveness of PRP therapy for muscle injury buntial improvement in return to sport (24).
Dermatology and plastic surgery
Human studies have shown intradermal and subdermal injection of PRP matrix formed through activation of PRP with calcium chloride to increase angiogenesis, collagenesis, fibroblast activation, and adipocyte stimulation on skin biopsy in healthy volunteers (27). These have led to interest in the regenerative capabilities of PRP for cosmetic enhancements and therapeutic interventions such as skin rejuvenation, treatment of alopecia, scars, and striae distensae in the fields of dermatology and plastic surgery (5,22). PRP has been administered topically, as well as intradermally and many interventions include the addition of PRP to other dermatologic procedures such as microneedling or laser therapy (28-30). Outcome measures have included pathologic specimen evaluation, before and after photographic comparison, and split side controls to control for individual differences (22,26,31).
In efforts to counter the effects of aging and skin damage, the use of PRP with laser therapy have been shown to improve skin elasticity and reduce erythema (32). Similarly, studies have shown a synergistic effect of intradermal and topical PRP with fractional carbon dioxide laser therapy for acne scars (29,30). Beyond elective aesthetic enhancements, PRP has also been studied in hard-to-heal acute and chronic wounds. Dermatology and plastic surgery colleagues are often consulted for reconstructing non-healing wounds which may be a result of underlying diseases or surgery such as cutaneous ulcers and wound dehiscence. Carter et al. [2011] published a meta-analysis on PRP-containing gel on wound healing and found that many studies have found this topical form of PRP to be associated with improved wound healing and potentially reduced wound infection rates (33).
In the area of hair restoration, Takikawa et al. [2011] demonstrated improvement in hair growth following subcutaneous injection of PRP and potential accentuated effect when used in conjunction with dalteparin and ptomaine microparticle carriers (31). Rodrigues et al. [2018] also demonstrated improvement in male-pattern alopecia with PRP injection (26). In contrast, Marwah et al. [2014] demonstrated greater improvement in subjective rather than objective measures of hair restoration thereby recommending against use of PRP as stand-alone treatment (34).
Although these reviews demonstrate the promise of PRP in many dermatologic applications, the heterogeneity of studies leaves many questions unanswered.
Controversies
Despite the accumulating body of research on PRP and its applications, the majority of studies are underpowered and unblinded and address efficacy in various ways. There is also a notable lack of standardization of instrumentation, platelet concentration, application methods, and control groups. Additionally, combination therapies such as radiofrequency, fat grafting, and laser therapy make it hard to determine the magnitude of benefit afforded by PRP, especially in studies with no control groups (22). Current trial data is mixed regarding effectiveness of PRP applications and the existing heterogeneity between studies further muddles interpretation of findings. Moreover, sources of variability in the final product can occur at the collection, processing, and administration stages (17).
There remains much to be elucidated about applications for PRP. This includes appropriate dosage, route of administration, and specific conditions for which PRP formulations may be beneficial. Castillo et al. [2011] showed a range of required starting whole blood volume from 18 to 55 mL to obtain approximately the same volume of PRP (6 to 7.5 mL) when comparing three commercial PRP systems (35). Further heterogeneity exists based on individual differences in platelet concentration and activity status of donor blood. Intra-individual variation has also been documented in samples obtained from the same individual at different time points despite consistent cell counts (12).
Although the low-risk designation of these products has exempted them from regulation thus far, the provision of therapies of unknown efficacy continues to have ethical and financial implications for patients. PRP treatment costs can vary widely with estimates of $500–$2,500 USD per treatment making PRP therapies more expensive than other evidence-based treatments such as steroid injections with costs often not covered by insurance (17,18). Reimbursement structure for these therapies has also not been delineated (18).
Although the majority of studies assessing efficacy have reported no adverse events, significant adverse events have resulted from PRP use in the clinical setting including transmission of HIV infection during treatment from unlicensed professionals without established universal precautions to sterilize equipment and prevent the spread of blood-borne pathogens (36).
Ways to ensure efficacy and safety while prioritizing innovation, such as those being developed for regenerative medicine therapies, have been proposed and may be appropriate for the study and regulation of PRP (19). The AABB guidelines, as well as transfusion medicine expertise, would be valuable in assisting with standardization of processing and storage practices. Furthermore, clear discussion with patients about the lack of evidence for these therapies and the resulting lack of data on efficacy, procedure details and risks, and lack of guidelines on recommended frequency of administration are crucial components of informed consent for these therapies (20). Emphasis on adequate and standardized reporting of PRP characteristics as outlined by Fadadu et al. [2019] who describe minimal parameters of platelet concentration, relative concentration compared to whole blood, WBC concentration and differential, and growth factor concentrations, as well as specific procedures including baseline platelet count, centrifugal force and time, and use of activators are also necessary to ensure useful data collection that can be used to guide future use (3).
Conclusions
The use of PRP is pervasive in academic and community practice, and often without involvement of transfusion medicine or laboratory services. Some studies show promise but further investigation into the precise mechanisms of PRP activity and its effects is warranted. Being an autologous product, PRP has a favorable safety profile but it deserves further research with oversight, standardization, and quality control to fortify evidence of any potential benefits or lack thereof. This is an opportunity for transfusion or laboratory medicine specialists to engage with colleagues who want to perform research or administer PRP to ensure patient safety and benefit, as well as prevent harm.
Acknowledgments
This topic, and part of this manuscript, was presented by Dr. Theresa N. Kinard at the 2019 AABB Annual Meeting.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Paul D. Mintz) for the series “Transfusion Therapy: Principles and Practices” published in Annals of Blood. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://aob.amegroups.com/article/view/10.21037/aob-21-57/coif). The series “Transfusion Therapy: Principles and Practices” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the manuscript and ensures that the questions related to the accuracy or integrity of any part of the work are appropriately investigated and reported.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Waller C. Case of Uterine Hemorrhage, in Which the Operation of Transfusion Was Successfully Performed. Lond Med Phys J 1825;54:273-7. [PubMed]
- Dhurat R, Sukesh M. Principles and Methods of Preparation of Platelet-Rich Plasma: A Review and Author's Perspective. J Cutan Aesthet Surg 2014;7:189-97. [Crossref] [PubMed]
- Fadadu PP, Mazzola AJ, Hunter CW, et al. Review of concentration yields in commercially available platelet-rich plasma (PRP) systems: a call for PRP standardization. Reg Anesth Pain Med 2019; [Crossref] [PubMed]
- Hsu WK, Mishra A, Rodeo SR, et al. Platelet-rich plasma in orthopaedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg 2013;21:739-48. [Crossref] [PubMed]
- Samadi P, Sheykhhasan M, Khoshinani HM. The Use of Platelet-Rich Plasma in Aesthetic and Regenerative Medicine: A Comprehensive Review. Aesthetic Plast Surg 2019;43:803-14. [Crossref] [PubMed]
- Foster TE, Puskas BL, Mandelbaum BR, et al. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med 2009;37:2259-72. [Crossref] [PubMed]
- Borrione P, Gianfrancesco AD, Pereira MT, et al. Platelet-rich plasma in muscle healing. Am J Phys Med Rehabil 2010;89:854-61. [Crossref] [PubMed]
- Gawaz M, Vogel S. Platelets in tissue repair: control of apoptosis and interactions with regenerative cells. Blood 2013;122:2550-4. [Crossref] [PubMed]
- Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent 2001;10:225-8. [Crossref] [PubMed]
- Harmon K, Hanson R, Bowen J, et al. Guidelines for the use of platelet rich plasma. The International Cellular Medical Society. Available online: https://www.scribd.com/document/159334949/206-ICMS-Guidelines-for-the-Use-of-Platelet-Rich-Plasma-Draftob-oasbonasdandbowndoww
- Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol 2009;27:158-67. [Crossref] [PubMed]
- Mazzocca AD, McCarthy MB, Chowaniec DM, et al. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am 2012;94:308-16. [Crossref] [PubMed]
- van der Bijl I, Vlig M, Middelkoop E, et al. Allogeneic platelet-rich plasma (PRP) is superior to platelets or plasma alone in stimulating fibroblast proliferation and migration, angiogenesis, and chemotaxis as relevant processes for wound healing. Transfusion 2019;59:3492-500. [Crossref] [PubMed]
- Graziani F, Ivanovski S, Cei S, et al. The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin Oral Implants Res 2006;17:212-9. [Crossref] [PubMed]
- Kakudo N, Minakata T, Mitsui T, et al. Proliferation-promoting effect of platelet-rich plasma on human adipose-derived stem cells and human dermal fibroblasts. Plast Reconstr Surg 2008;122:1352-60. [Crossref] [PubMed]
- Peng GL. Platelet-Rich Plasma for Skin Rejuvenation: Facts, Fiction, and Pearls for Practice. Facial Plast Surg Clin North Am 2019;27:405-11. [Crossref] [PubMed]
- Jones IA, Togashi RC, Thomas Vangsness C Jr. The Economics and Regulation of PRP in the Evolving Field of Orthopedic Biologics. Curr Rev Musculoskelet Med 2018;11:558-65. [Crossref] [PubMed]
- Harm SK, Fung MK. Platelet-rich plasma injections: out of control and on the loose? Transfusion 2015;55:1596-8. [Crossref] [PubMed]
- Marks P, Gottlieb S. Balancing Safety and Innovation for Cell-Based Regenerative Medicine. N Engl J Med 2018;378:954-9. [Crossref] [PubMed]
- Beitzel K, Allen D, Apostolakos J, et al. US definitions, current use, and FDA stance on use of platelet-rich plasma in sports medicine. J Knee Surg 2015;28:29-34. [Crossref] [PubMed]
- Cohn CS, Lockhart E, McCullough JJ. The use of autologous platelet-rich plasma in the orthopedic setting. Transfusion 2015;55:1812-20. [Crossref] [PubMed]
- Leo MS, Kumar AS, Kirit R, et al. Systematic review of the use of platelet-rich plasma in aesthetic dermatology. J Cosmet Dermatol 2015;14:315-23. [Crossref] [PubMed]
- Rachul C, Rasko JEJ, Caulfield T. Implicit hype? Representations of platelet rich plasma in the news media. PLoS One 2017;12:e0182496. [Crossref] [PubMed]
- Setayesh K, Villarreal A, Gottschalk A, et al. Treatment of Muscle Injuries with Platelet-Rich Plasma: a Review of the Literature. Curr Rev Musculoskelet Med 2018;11:635-42. [Crossref] [PubMed]
- Chen P, Huang L, Ma Y, et al. Intra-articular platelet-rich plasma injection for knee osteoarthritis: a summary of meta-analyses. J Orthop Surg Res 2019;14:385. [Crossref] [PubMed]
- Rodrigues BL, Montalvão SAL, Cancela RBB, et al. Treatment of male pattern alopecia with platelet-rich plasma: A double-blind controlled study with analysis of platelet number and growth factor levels. J Am Acad Dermatol 2019;80:694-700. [Crossref] [PubMed]
- Sclafani AP, McCormick SA. Induction of dermal collagenesis, angiogenesis, and adipogenesis in human skin by injection of platelet-rich fibrin matrix. Arch Facial Plast Surg 2012;14:132-6. [Crossref] [PubMed]
- Hashim PW, Levy Z, Cohen JL, et al. Microneedling therapy with and without platelet-rich plasma. Cutis 2017;99:239-42. [PubMed]
- Lee JW, Kim BJ, Kim MN, et al. The efficacy of autologous platelet rich plasma combined with ablative carbon dioxide fractional resurfacing for acne scars: a simultaneous split-face trial. Dermatol Surg 2011;37:931-8. [Crossref] [PubMed]
- Gawdat HI, Hegazy RA, Fawzy MM, et al. Autologous platelet rich plasma: topical versus intradermal after fractional ablative carbon dioxide laser treatment of atrophic acne scars. Dermatol Surg 2014;40:152-61. [Crossref] [PubMed]
- Takikawa M, Nakamura S, Nakamura S, et al. Enhanced effect of platelet-rich plasma containing a new carrier on hair growth. Dermatol Surg 2011;37:1721-9. [Crossref] [PubMed]
- Shin MK, Lee JH, Lee SJ, et al. Platelet-rich plasma combined with fractional laser therapy for skin rejuvenation. Dermatol Surg 2012;38:623-30. [Crossref] [PubMed]
- Carter MJ, Fylling CP, Parnell LK. Use of platelet rich plasma gel on wound healing: a systematic review and meta-analysis. Eplasty 2011;11:e38. [PubMed]
- Marwah M, Godse K, Patil S, et al. Is there sufficient research data to use platelet-rich plasma in dermatology? Int J Trichology 2014;6:35-6. [Crossref] [PubMed]
- Castillo TN, Pouliot MA, Kim HJ, et al. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med 2011;39:266-71. [Crossref] [PubMed]
- New Mexico Office of the Attorney General. Attorney General Balderas Announces Indictment of Former VIP Salon Owner Who Unlawfully Performed ‘Vampire Facial’ Medical Procedures, NMAGO File #: 201905-00043. 2021 [cited 2021 Aug 12]. Available online: https://www.nmag.gov/uploads/PressRelease/48737699ae174b30ac51a7eb286e661f/Attorney_General_Balderas_Announces_Indictment_of_Former_VIP_Salon_Owner.pdf
Cite this article as: Yaman R, Kinard TN. Platelet rich plasma: hope or hype? Ann Blood 2022;7:6.


