
THERAFLEX ultraviolet C (UVC)-based pathogen reduction technology for bacterial inactivation in blood components: advantages and limitations
Introduction
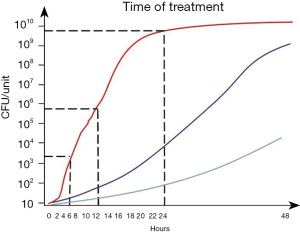
The risk of viral transfusion transmitted infection (TTI) could be dramatically decreased by different means, such as testing and donor deferral in at-risk cases. Apart from contamination levels below the detection limits, one can generally assume that if the donor tests negative, the blood product will also be negative. Nonetheless, bacterial contamination remains a constant threat in transfusion medicine (1-10) because TTIs cannot be prevented by simply testing blood donors for bacteria. Contaminating bacteria mainly enter into blood bags during venipuncture. Generally speaking, due to their initial low numbers they may not be detectable at the time when blood is drawn for the cultivation from blood components, such as red blood cells (RBCs) and platelets (PLT). By the time of transfusion, their numbers may have increased to levels capable of causing severe complications, including sepsis. Bacterial growth kinetics vary greatly with respect to strain, ambient temperature and type of blood component. Particularly when stored at room temperature, PLT preparations are at a very high risk of high-titer contamination. Bacterial growth curve simulations indicate that, from a low starting population of 10–100 colony-forming units (CFU) per unit, the maximum time between PLT product preparation and pathogen reduction technology (PRT) treatment, which must be observed in order to guarantee product safety and sterility, is 24 hours in the case of slowly growing bacteria and 12 hours for the majority of bacterial strains with an intermediate growth rate (Figure 1). However, if contaminated with fast-growing species (e.g., Escherichia coli or Staphylococcus aureus) that double in number every 60 minutes, earlier pathogen reduction (PR) is crucial to ensure product sterility: from a low initial count of 100 CFU/unit, fast-growing bacteria may increase to counts of 6.4×103 CFU per PLT unit within 6 hours, 4.1×105 CFU per PLT unit within 12 hours, and 1.7×109 CFU per unit within 24 hours (Figure 1). These simulations imply that PRT with a very high inactivation capacity of more than seven log10 reduction steps for the respective contaminating bacterial species may be able to achieve sufficient sterility if applied to the PLT within 12 hours, but would be overwhelmed by the bacterial load if used even slightly later. Consequently, the time point of PR is highly relevant to the bacterial safety of pathogen-reduced PLT. Therefore, the capacity of a PRT to inactivate bacteria in PLT concentrates strongly depends on the timing of treatment.
Different measures to prevent bacterial contamination are available. Sophisticated pre-transfusion testing strategies to prevent bacterial contamination of PLT concentrates have recently been published (11-14). Cold storage, for example, dramatically decreases the growth of bacteria that usually contaminate PLT preparations, but reduces PLT recovery and survival (15-19). Consequently, cold-storage PLT concentrates may be more suitable for therapeutic PLT transfusions than for prophylactic transfusions (20,21). PRTs offer a universal approach to reducing bacterial contamination. The PRT based on the addition of amotosalen followed by ultraviolet A (UVA) irradiation (320–400 nm) is a photochemical technology that works via the irreversible modification of nucleic acids in pathogens and leukocytes: Upon irradiation amotosalen is crosslinked between nucleic acid base pairs (INTERCEPT, Cerus Corp, Concord, CA, USA) (22-25).
The PRT based on the addition of riboflavin and subsequent UVA/ultraviolet B (UVB) irradiation (265–370 nm) is a photodynamic technology that works via the promotion of an irreversible modification of nucleic acids in pathogens and leukocytes in the presence of riboflavin (Mirasol, Terumo BCT, Lakewood, CO, USA) (25-28). Ultraviolet C (UVC)-based PRT (THERAFLEX UV-Platelets, Maco Pharma, Mouvaux, France), on the other hand is a purely physical PRT that uses UVC light (254 nm) alone, without a photosensitizing agent, to directly and irreversibly disrupt the integrity of nucleic acids (29). All of these technologies aim to inhibit nucleic acid amplification and to reduce or eliminate the infectivity of blood products due to disease-causing bacteria, viruses and protozoa.
THERAFLEX UV-Platelets UVC-based PRT
Effects of UVC irradiation
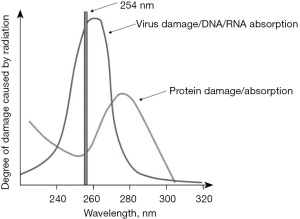
The germicidal effect of UVC light is already utilized for surface sterilization. When used to inactivate pathogens in blood products, however, one must strike a balance between exerting the germicidal properties of UVC and maintaining the functional integrity of the blood products. THERAFLEX UV-Platelets works by exposing blood products to UVC at a wavelength of 254 nm—a level that is close to the optimum wavelength for peak DNA/RNA absorption (260 nm) and the lowest possible protein absorption and damage (Figure 2). The latter property is crucial for conserving the delicate structure of PLT during UVC treatment (30,31).
UVC-induced chemical reactions in the nucleic acids of the contaminating pathogens result in the formation of cyclobutane-pyrimidine and pyrimidine-pyrimidone dimers, thus effectively blocking the elongation of nucleic acid transcripts (32,33). When cells are targeted, the number of alterations on bases may overwhelm the DNA repair capacity and lead to apoptosis and related programmed cell death processes (34,35). Because mature RBCs and mature PLT do not bear a nucleus that is required for cellular activity and function, disease-causing pathogens (e.g., bacteria, viruses and protozoa) in these blood products can be effectively inactivated while maintaining PLT and coagulation factor function (36,37). However, it is common to all available methods for PR of PLT that they cause or exert some effect on the integrity of PLT. In vitro studies have consistently shown increased metabolic activity and moderate activation of PLT after treatment with UVC and other PRT technologies (38-43). In addition, reduced recovery of PRT-treated PLT in transfused patients suggests an impact on PLT viability (44-52). Therefore, it is important for each PRT method to define the optimal conditions and protocols to find the right balance between effective pathogen inactivation and functional preservation (43). White blood cells (WBC), however, are susceptible to apoptosis, so leukocytes, which could cause graft-versus-host disease (GvHD) in vulnerable patients, are also inactivated by UVC (53-63).
PR procedure
THERAFLEX UV-Platelets is a PRT that is solely based on UVC light and does not utilize a photosensitizer, so that no additional steps for the removal of such substances are needed before transfusion of the treated blood components (64,65). In brief, whole blood-derived or apheresis PLT suspended in SSP+ Platelet Additive Solution (Maco Pharma, Mouvaux, France) are transferred to a UVC-permeable bag and irradiated with UVC light using an irradiation device (MACOTRONIC UV, Maco Pharma, Mouvaux, France). The large irradiation bag results in a small filling volume, providing a PLT concentrate with a small layer thickness and a big surface during light exposure. UVC light is applied from both sides at a dose of 0.2 J/cm2 while simultaneously agitating the bag. Due to the relatively low penetration depth of UVC light, vigorous mixing of the PLT unit is required to expose every blood compartment to UVC light at the surface of the bag. UVC exposure time is less than one minute. After UVC treatment, the PLT are transferred into a storage container, ready for transfusion without any further processing. In its current version, the UVC-based PRT process uses a plasma concentration of 30–40% for effective pathogen inactivation and maintenance of product quality.
Bacterial inactivation capacity
The standard method of determining the inactivation capacity of PRTs for PLT concentrates and other blood products is by measuring the reduction in virus titers achieved by the system (66). However, bacterial growth poses a different challenge for inactivation studies. Appropriate bacteria spike protocols have been established in previous studies with other PRT systems (67-69). In contrast to viral particles, bacteria can rapidly multiply in blood units and accumulate to titers that may overwhelm the capacity of the PRT within a relatively short time span (70-72). Consequently, recent recommendations for the validation of PRTs include the conditions of intended use, aiming at achieving a safe and sterile blood product through timely pathogen inactivation (73). These recommendations propose that validation tests be conducted using pooled PLT from at least three different donors to exclude donor-related effects, keeping the initial dose of bacteria as low as possible (1–100 CFU/unit) to mimic real contamination scenarios, and to use sufficiently large samples (>8 mL) for sterility testing after PR treatment.
Studies for evaluation of the inactivation capacity of the UVC-based PRT (THERAFLEX UV-Platelets, Maco Pharma) were assessed while strictly adhering to the recent recommendations. These tests were performed using a wide variety of transfusion-relevant bacteria, including the recently published and enlarged WHO reference repository of PLT transfusion-relevant bacterial reference strains (74-76). Moreover, different strains of the species were tested to check for potential intra-strain differences in UVC susceptibility, and clinical isolates from contaminated PLT concentrates were also tested. The results revealed that UVC treatment effectively inactivated all bacterial strains in the investigated PLT concentrates (Table 1). In brief, PLT concentrates of a volume between 325 and 375 mL were inoculated with the aforementioned bacterial suspensions. The yielded bacterial titers were between 105 CFU/mL and 107 CFU/mL. PLT concentrates were treated up to the full UVC light dose of 0.2 J/cm2. In parallel, “hold samples” that were inoculated with bacteria and left untreated did not show any bacteria inactivation by the blood product itself, excluding any kind of self-sterilizing effect (56).
Table 1
| Species | Strain | N | Log10 reduction factor at full UVC dose (0.2 J/cm²)* |
|---|---|---|---|
| Enterobacter cloacae | PEI-B-P-43 | 3 | 6 |
| Escherichia coli | PEI-B-P-19 | 6 | 7 |
| Klebsiella pneumoniae | PEI-B-P-08 | 6 | 6 |
| Morganella morganii | PEI-B-P-91 | 3 | 7 |
| Proteus mirabilis | PEI-B-P-55 | 3 | 7 |
| Pseudomonas fluorescens | PEI-B-P-77 | 3 | 7 |
| ATCC 17569 | 3 | ≥5 | |
| Serratia marcescens | PEI-B-P-56 | 3 | 6 |
| ATCC 43826 | 6 | ≥5 | |
| Staphylococcus aureus | PEI-B-P-63 | 3 | 6 |
| ATCC 25923 | 3 | 5 | |
| Clinical isolate | 3 | 4 | |
| Staphylococcus epidermidis | PEI-B-P-06 | 6 | 4 |
| Streptococcus bovis | PEI-B-P-61 | 3 | 7 |
| ATCC 33317 | 3 | 4 | |
| Streptococcus dysgalactiae | PEI-B-P-71 | 3 | 4 |
| ATCC 35666 | 3 | 4 | |
| Streptococcus pyogenes | PEI-B-P-20 | 4 | 4 |
| DSM 11728 | 6 | 4 | |
| DSM 25953 | 6 | 4 | |
| ATCC BAA-1064 | 6 | 5 | |
| Clinical isolate | 6 | 5 | |
| Listeria monocytogenes | ATCC 19115 | 6 | 5 |
| Acinetobacter baumannii | ATCC 17961 | 3 | 5 |
| Streptococcus agalactiae | ATCC 13813 | 3 | ≥6 |
| Streptococcus pneumoniae | ATCC 33400 | 3 | 5 |
| Bacillus cereus | PEI-B-P-57 | 3 | 3 |
| Bacillus thuringiensis | PEI-B-P-07 | 3 | 4 |
| Propionibacterium acnes | ATCC 6919 | 6 | 5 |
*, in some cases, the mean log10 reduction factors could not be exactly determined after full-dose UVC treatment with THERAFLEX UV-Platelets (and was thus expressed as “≥”) as the bacterial titers of some species reached the limit of detection of the plating assay at this UVC dose (56). UVC, ultraviolet C.
Bacterial titers can develop very differently within a PLT concentrate, depending on the inherent growth characteristics of the bacterial species and its response to the blood component and the additive solution. Some strains are highly susceptible to this environment and show self-sterilizing behavior, whereas some sterilizing effects may also be attributed to donor-related characteristics (37,77). Other strains grow slowly and reach significant titers only at the end of their shelf life. The most dangerous strains are those that are nearly undetectable at the time of a preparation and then rapidly grow to numbers that overwhelm the inactivation capacity of a PRT (Figure 1). Thus, it had to be determined what interval between preparation and UVC illumination safely and effectively ensures the sterility of pathogen-reduced PLT concentrates over the entire storage period (56,65).
In brief, in these time-to-treatment experiments two PLT concentrates were pooled and then inoculated with the respective bacterial suspension using a target titer of 100 CFU/unit. This concentrate was then split into a control and a test PLT concentrate. From the test PLT concentrate bacterial titers were determined after 6 or 8 hours and the PLT concentrate was then subjected to UVC-irradiation according to the THERAFLEX-UV-Platelets protocol. Sterility testing was performed during storage time, using a culture system (BacT/ALERT, bioMérieux, Marcy l'Etoile, France) (56).
The time-to-treatment experiments showed that UVC treatment consistently achieved PLT concentrate sterility for up to 7 days of storage when performed within 6 hours after spiking. In order to identify the possible limits of this PRT, titers of fast-growing bacteria—Escherichia coli, Klebsiella pneumoniae, Staphylococcus epidermidis and Streptococcus pyogenes—were also determined in PLT concentrates UVC-treated 8 hours after preparation. This additional time before pathogen inactivation impaired the sterility of the PLT concentrate in single cases: 11 out of 12 Escherichia coli and Streptococcus pyogenes samples were still sterile 7 days after PRT. The above data demonstrate that timely inactivation is the key to preventing the transmission of bacteria to PLT recipients (Table 2).
Table 2
| Species | Origin/strain | Sterility of UVC-treated PLT concentrates (N/N) depending on time to treatment after spiking | |
|---|---|---|---|
| 6 hours | 8 hours | ||
| Bacillus cereus† | PEI-B-P-57 | 12/12 | n.t. |
| Bacillus thuringiensis† | PEI-B-P-07 | 12/12 | n.t. |
| Escherichia coli | PEI-B-P-19 | 12/12 | 11/12 |
| Klebsiella pneumoniae | PEI-B-P-19 | 12/12 | 12/12 |
| Enterobacter cloacae | PEI-B-P-43 | 12/12 | n.t. |
| Morganella morganii | PEI-B-P-91 | 12/12 | n.t. |
| Proteus mirabilis | PEI-B-P-55 | 12/12 | n.t. |
| Pseudomonas fluorescens | PEI-B-P-77 | 12/12 | n.t. |
| Serratia marcescens | PEI-B-P-56 | 12/12 | n.t. |
| Staphylococcus aureus | PEI-B-P-63 | 12/12 | n.t. |
| Staphylococcus epidermidis | PEI-B-P-06 | 12/12 | 12/12 |
| Streptococcus bovis | PEI-B-P-61 | 12/12 | n.t. |
| Streptococcus pyogenes | PEI-B-P-20 | 12/12 | 11/12 |
| Streptococcus dysgalactiae | PEI-B-P-71 | 12/12 | n.t. |
These experiments were done with 12 replicates (two series of six) for each bacterial strain. †, vegetative forms only. n.t., not tested; UVC, ultraviolet C; PLT, platelet.
Advantages and limitations
THERAFLEX UV-Platelets has an easy and quick procedure. Because it does not require any photosensitizers, it has a short hands-on time along with a short overall processing time. Since UVC treatment is performed after standard PLT collection and preparation, it can easily be integrated into the blood product supply chain without altering the manufacturing process significantly. According to the current specifications, the THERAFLEX UV-Platelets technology must be applied to plasma-reduced PLT for efficient PR.
When PLT are stored, bacteria may adhere to blood bag and tubing systems via fibrin caps or other organized biofilms (78-82). Penetration of photosensitizers used for PRT may then be impaired, making subsequent UV treatment ineffective, leading to break-through contamination. THERAFLEX UV-Platelets uses different bags in sequence, so the initial adherence of bacteria to the bag cannot impact the final preparation. The PLT are transferred from a first storage bag to an irradiation bag, and then to a final storage bag. Moreover, THERAFLEX UV-Platelets only uses UVC light for PR, making the accessibility of a biofilm to a photosensitizer irrelevant. The system uses relatively large irradiation bags, which are agitated rapidly during the irradiation process to ensure efficient mixing and complete penetration of UVC light. The bag system is designed so as to prevent niches or reservoirs where pathogens could collect and escape from UVC light exposure.
THERAFLEX UV-Platelets is so far the only system that provides a systematic evaluation of the timing of bacterial PR needed to ensure product sterility (Table 2). Data show that this system not only has a high capacity to effectively inactivate transfusion-relevant bacteria in PLT concentrates, but also ensures the sterility of PLT concentrates contaminated with less PI-sensitive bacteria when applied early after PLT preparation (56).
Leukocyte and parasite inactivation experiments have shown that UVC light effectively damages ribonucleic acid inside blood cells (36,59). The high capacity of UVC to inactivate leukocytes and intracellular parasites like Plasmodium falciparum suggests that the THERAFLEX UV-Platelets system may also be effective against intracellular bacteria and viruses, although this remains to be proved in scientific experiments.
After implementation of the INTERCEPT PR method for PLT concentrates in routine use in the US, isolated cases of sepsis after transfusion of pathogen-reduced PLT concentrates have been reported over the last years, and some even had a fatal outcome (83-85). While it must be underscored that hemovigilance data do not provide evidence of systemic flaws, the investigators demonstrated that the patients in question did contract the bacteria from the PLT concentrates, and it is striking that the bacteria involved in the cases with a fatal outcome (Acinetobacter baumanii complex, Leclercia adecarboxylata and Staphylococcus saphrophyticus) were effectively inactivated by seven log10 steps in inactivation experiments. In view of the fact that Acinetobacter baumanii and Staphylococcus saphrophyticus are environmental strains rather than part of the usual skin flora, contamination after amotosalen/UV light inactivation seemed likely. Later analysis of the bags revealed invisible leaks that could only be detected by air pressure tests. Abrasion-related damage to the bags during transport or agitation was determined to be the most likely cause of the undetected leaks. The same bacteria, Acinetobacter baumanii and Staphylococcus saphrophyticus, were also implicated in three previous cases of sepsis after transfusion of pathogen-reduced PLT. Since PR was performed after 16 hours in these cases, high bacterial contamination with titers above the inactivation capacity of the PR system could be discussed as a cause. On the other hand, the amotosalen/UV-based system has a complex bag system consisting of one bag for the photosensitizer amotosalen, one bag for illumination, one bag for the removal of amotosalen and photoproducts, and one final storage bag for the PLT concentrate, making this system vulnerable to handling, transportation and storage-related damage. The UVC-based PRT system requires only the PLT concentrate to be UVC-treated, a big irradiation bag and the final storage bag. The irradiation process itself takes less than one minute. Afterwards, the UVC-treated PLT are transferred into the storage bag. The UVC-based PRT does neither require addition nor removal of photoactive substances. While post-PRT damage can occur on any PLT bags of any PRT system, it seems plausible that the THERAFLEX UV-Platelets system’s simple procedure may be less susceptible to material damage and production errors that could cause undetectable leakage.
All PRTs for blood components are limited in their efficacy to inactivate spores. Previous studies have shown that UVC inactivates vegetative bacteria much better than spores (64,86,87). Thus, PLT concentrates contaminated by spore-forming bacteria (e.g., Bacillus spp.) may contain viable spores after PR. Surviving spores in PLT concentrates could then develop into vegetative forms and grow to clinically relevant numbers during storage (88). However, the relevance and levels of spores in bacterially contaminated PLT concentrates are largely unknown. Storage of PLT products at room temperature provides good growth conditions for bacteria, but may not favor the production of high numbers of spores. More research on the sporulation of bacteria in PLT concentrates is required to better address potential safety issues related to the insufficient inactivation of spores by a PRT.
As already emphasized above, high titers of bacteria can overwhelm the inactivation capacity of the THERAFLEX UV-Platelets system at the time of treatment. This problem is a significant challenge to all PRTs that can only be adequately resolved by treating PLT concentrates very early after preparation.
The preparation of pooled PLT concentrates from whole blood donations usually takes more time from blood collection to PR than the preparation of apheresis PLT concentrates. Therefore, bacteria infiltrating the collection bag during whole blood donation may have more time to adjust to the additive solution and environment within the blood bag and multiply than bacteria contaminating apheresis PLT concentrates. However, current data suggests that the initial bacterial burden of pooled PLT concentrates at the time of preparation is comparable to that of apheresis PLT concentrates after donation. Previous studies have shown that bacteria are significantly eliminated by the preparation procedure for random donor PLT concentrates (77,89-91). Therefore, it might be reasonable to define the maximum allowable time between PLT collection and PR treatment in terms of the time of preparation of the respective pooled or apheresis PLT concentrate. Time-to-treatment experiments using blood banking conditions and bacterial contamination levels that mimic routine use have shown that a maximal interval of 6 hours between PLT preparation and UVC treatment is sufficient to guaranty sterility for both PLT product types. It would, of course, be desirable to have more experimental data on the growth kinetics and location of bacteria during the manufacturing process of whole blood-derived pool PLT concentrates and on the inactivation capacities of the available PRTs for contaminated pooled PLT concentrates under different PLT concentrate production, bacterial contamination and treatment timing conditions. However, controlling the experimental settings of such studies is very complex because additional confounding factors, such as donor-specific interactions, elimination of bacteria by WBCs and the choice of additive solution, need to be considered.
Perspectives
The investigated UVC light-based PRT effectively inactivates viruses, bacteria, parasites and alloreactive T-cells in PLT concentrates while maintaining PLT function (54-58,60,63,92-95). A phase I study of the safety and tolerability of autologous UVC-irradiated PLT concentrates in healthy volunteers did not reveal any adverse reactions or immunization against treated PLT concentrates, among other clinical parameters (96). Recently, the CAPTURE study (Clinical Assessment of Platelets Treated with UVC in Relation to Established Preparations), a phase III randomized, double-blind, parallel controlled non-inferiority trial comparing pathogen-reduced pooled and apheresis PLT with conventional pooled and apheresis PLT was completed (97). The application for marketing authorization of the THERAFLEX UV-Platelets system is currently under evaluation by the responsible authority in Germany.
The THERAFLEX technology was originally developed for PLT but is also suitable for plasma, RBCs and whole blood. Proof of principle of UVC treatment for the inactivation of pathogens in plasma units and RBCs has been demonstrated (98,99). PRTs for the treatment of whole blood would be a major step towards increasing the bacterial safety of blood components. Whole blood could be pathogen-reduced early in the manufacturing process, thus significantly reducing the time for contaminating bacterial growth. Situations in which the bacterial load could exceed the inactivation capacity of the PRT would then be less likely to occur. Moreover, such technology would have significant practical and economic advantages because the treatment of a single unit of whole blood could yield up to three different pathogen-reduced blood components: plasma, PLT and RBCs.
Bacterial inactivation capacity research conducted in the course of new PRT systems development or existing systems modification should include other tests in addition to the classical experiments for the determination of log10 reduction capacity. To be meaningful, the study design must also consider the clinical setting. In accordance with recommendations of the Transfusion Transmitted Infectious Disease Bacteria Working Party of the ISBT (TTID-B), focused research should be conducted under conditions which simulate routine clinical use while the sterility of the investigated blood products is the only clinically relevant outcome. Key elements of this approach include the use of transfusion-relevant bacteria (ideally, WHO International Reference Repository of Platelet Transfusion-Relevant Bacterial Reference Strains), different sources of blood products to balance the influence of donor-specific parameters, and sensitive tests for the detection of residual bacteria (73,100,101).
Acknowledgments
The authors would like to thank Anke Wenk for her assistance in the preparation of the manuscript.
Funding: TJS, UG and AS received grants from the Research Foundation of the German Red Cross Blood Services (Deutsche Forschungsgemeinschaft der Blutspendedienste des Deutschen Roten Kreuzes) and Maco Pharma for development of the investigated UVC-based PI technology for platelets.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Sandra Ramirez-Arcos) for the series “Bacterial Contamination of Platelet Components” published in Annals of Blood. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aob.amegroups.com/article/view/10.21037/aob-21-44/coif). The series “Bacterial Contamination of Platelet Concentrates” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the manuscript and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Heroes AS, Ndalingosu N, Kalema J, et al. Bacterial contamination of blood products for transfusion in the Democratic Republic of the Congo: temperature monitoring, qualitative and semi-quantitative culture. Blood Transfus 2020;18:348-58. [PubMed]
- Brecher ME, Hay SN. Bacterial contamination of blood components. Clin Microbiol Rev 2005;18:195-204. [Crossref] [PubMed]
- Depcik-Smith ND, Hay SN, Brecher ME. Bacterial contamination of blood products: factors, options, and insights. J Clin Apher 2001;16:192-201. [Crossref] [PubMed]
- Seghatchian J. Bacterial contamination of blood components. Transfus Apher Sci 2001;25:147-50. [Crossref] [PubMed]
- Blajchman MA, Goldman M. Bacterial contamination of platelet concentrates: incidence, significance, and prevention. Semin Hematol 2001;38:20-6. [Crossref] [PubMed]
- Brecher ME, Blajchman MA, Yomtovian R, et al. Addressing the risk of bacterial contamination of platelets within the United States: a history to help illuminate the future. Transfusion 2013;53:221-31. [Crossref] [PubMed]
- Hong H, Xiao W, Lazarus HM, et al. Detection of septic transfusion reactions to platelet transfusions by active and passive surveillance. Blood 2016;127:496-502. [Crossref] [PubMed]
- Ramirez-Arcos S, DiFranco C, McIntyre T, et al. Residual risk of bacterial contamination of platelets: six years of experience with sterility testing. Transfusion 2017;57:2174-81. [Crossref] [PubMed]
- Abela MA, Fenning S, Maguire KA, et al. Bacterial contamination of platelet components not detected by BacT/ALERT®. Transfus Med 2018;28:65-70. [Crossref] [PubMed]
- McDonald C, Allen J, Brailsford S, et al. Bacterial screening of platelet components by National Health Service Blood and Transplant, an effective risk reduction measure. Transfusion 2017;57:1122-31. [Crossref] [PubMed]
- Levy JH, Neal MD, Herman JH. Bacterial contamination of platelets for transfusion: strategies for prevention. Crit Care 2018;22:271. [Crossref] [PubMed]
- Blajchman MA. Bacterial contamination of blood products and the value of pre-transfusion testing. Immunol Invest 1995;24:163-70. [Crossref] [PubMed]
- Ramirez-Arcos S, Evans S, McIntyre T, et al. Extension of platelet shelf life with an improved bacterial testing algorithm. Transfusion 2020;60:2918-28. [Crossref] [PubMed]
- Ramírez-Arcos S, Jenkins C, Dion J, et al. Canadian experience with detection of bacterial contamination in apheresis platelets. Transfusion 2007;47:421-9. [Crossref] [PubMed]
- Marini I, Aurich K, Jouni R, et al. Cold storage of platelets in additive solution: the impact of residual plasma in apheresis platelet concentrates. Haematologica 2019;104:207-14. [Crossref] [PubMed]
- Braathen H, Sivertsen J, Lunde THF, et al. In vitro quality and platelet function of cold and delayed cold storage of apheresis platelet concentrates in platelet additive solution for 21 days. Transfusion 2019;59:2652-61. [Crossref] [PubMed]
- Wood B, Johnson L, Hyland RA, et al. Maximising platelet availability by delaying cold storage. Vox Sang 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Murphy S, Gardner FH. Effect of storage temperature on maintenance of platelet viability--deleterious effect of refrigerated storage. N Engl J Med 1969;280:1094-8. [Crossref] [PubMed]
- Hornsey VS, Drummond O, McMillan L, et al. Cold storage of pooled, buffy-coat-derived, leucoreduced platelets in plasma. Vox Sang 2008;95:26-32. [Crossref] [PubMed]
- Scorer T, Williams A, Reddoch-Cardenas K, et al. Manufacturing variables and hemostatic function of cold-stored platelets: a systematic review of the literature. Transfusion 2019;59:2722-32. [Crossref] [PubMed]
- Mack JP, Miles J, Stolla M. Cold-Stored Platelets: Review of Studies in Humans. Transfus Med Rev 2020;34:221-6. [Crossref] [PubMed]
- Lin L, Cook DN, Wiesehahn GP, et al. Photochemical inactivation of viruses and bacteria in platelet concentrates by use of a novel psoralen and long-wavelength ultraviolet light. Transfusion 1997;37:423-35. [Crossref] [PubMed]
- Lin L, Dikeman R, Molini B, et al. Photochemical treatment of platelet concentrates with amotosalen and long-wavelength ultraviolet light inactivates a broad spectrum of pathogenic bacteria. Transfusion 2004;44:1496-504. [Crossref] [PubMed]
- Lin L, Hanson CV, Alter HJ, et al. Inactivation of viruses in platelet concentrates by photochemical treatment with amotosalen and long-wavelength ultraviolet light. Transfusion 2005;45:580-90. [Crossref] [PubMed]
- Grass JA, Hei DJ, Metchette K, et al. Inactivation of leukocytes in platelet concentrates by photochemical treatment with psoralen plus UVA. Blood 1998;91:2180-8. [Crossref] [PubMed]
- Marschner S, Goodrich R. Pathogen Reduction Technology Treatment of Platelets, Plasma and Whole Blood Using Riboflavin and UV Light. Transfus Med Hemother 2011;38:8-18. [Crossref] [PubMed]
- Goodrich RP, Edrich RA, Li J, et al. The Mirasol PRT system for pathogen reduction of platelets and plasma: an overview of current status and future trends. Transfus Apher Sci 2006;35:5-17. [Crossref] [PubMed]
- Kumar V, Lockerbie O, Keil SD, et al. Riboflavin and UV-light based pathogen reduction: extent and consequence of DNA damage at the molecular level. Photochem Photobiol 2004;80:15-21. [Crossref] [PubMed]
- Schmidt S, Kauling J. Process and Laboratory Scale UV Inactivation of Viruses and Bacteria Using an Innovative Coiled Tube Reactor. Chem Eng Technol 2007;30:945-50. [Crossref]
- Terpstra FG, van 't Wout AB, Schuitemaker H, et al. Potential and limitation of UVC irradiation for the inactivation of pathogens in platelet concentrates. Transfusion 2008;48:304-13. [PubMed]
- Verhoeven AJ, Verhaar R, Gouwerok EG, et al. The mitochondrial membrane potential in human platelets: a sensitive parameter for platelet quality. Transfusion 2005;45:82-9. [Crossref] [PubMed]
- Cadet J, Sage E, Douki T. Ultraviolet radiation-mediated damage to cellular DNA. Mutat Res 2005;571:3-17. [Crossref] [PubMed]
- Sinha RP, Häder DP. UV-induced DNA damage and repair: a review. Photochem Photobiol Sci 2002;1:225-36. [Crossref] [PubMed]
- Douki T, Laporte G, Cadet J. Inter-strand photoproducts are produced in high yield within A-DNA exposed to UVC radiation. Nucleic Acids Res 2003;31:3134-42. [Crossref] [PubMed]
- Rodrigo G, Roumagnac S, Wold MS, et al. DNA replication but not nucleotide excision repair is required for UVC-induced replication protein A phosphorylation in mammalian cells. Mol Cell Biol 2000;20:2696-705. [Crossref] [PubMed]
- Castro E, González LM, Rubio JM, et al. The efficacy of the ultraviolet C pathogen inactivation system in the reduction of Babesia divergens in pooled buffy coat platelets. Transfusion 2014;54:2207-16. [Crossref] [PubMed]
- Castro E, Girones N, Guerro N, et al. Poster session 1: Apheresis: The effectiveness of UVC pathogen inactivation system on reducing the Trypanosoma Cruzi and Leishmania Infantum burden in platelets. Vox Sanguinis 2008;95:74-326. [Crossref]
- Verhaar R, Dekkers DW, De Cuyper IM, et al. UV-C irradiation disrupts platelet surface disulfide bonds and activates the platelet integrin alphaIIbbeta3. Blood 2008;112:4935-9. [Crossref] [PubMed]
- Apelseth TØ, Bruserud Ø, Wentzel-Larsen T, et al. In vitro evaluation of metabolic changes and residual platelet responsiveness in photochemical treated and gamma-irradiated single-donor platelet concentrates during long-term storage. Transfusion 2007;47:653-65. [Crossref] [PubMed]
- Apelseth TO, Hervig TA, Wentzel-Larsen T, et al. Cytokine accumulation in photochemically treated and gamma-irradiated platelet concentrates during storage. Transfusion 2006;46:800-10. [Crossref] [PubMed]
- Ruane PH, Edrich R, Gampp D, et al. Photochemical inactivation of selected viruses and bacteria in platelet concentrates using riboflavin and light. Transfusion 2004;44:877-85. [Crossref] [PubMed]
- van Rhenen DJ, Vermeij J, Mayaudon V, et al. Functional characteristics of S-59 photochemically treated platelet concentrates derived from buffy coats. Vox Sang 2000;79:206-14. [Crossref] [PubMed]
- Mohr H, Steil L, Gravemann U, et al. A novel approach to pathogen reduction in platelet concentrates using short-wave ultraviolet light. Transfusion 2009;49:2612-24. [Crossref] [PubMed]
- van Rhenen D, Gulliksson H, Cazenave JP, et al. Transfusion of pooled buffy coat platelet components prepared with photochemical pathogen inactivation treatment: the euroSPRITE trial. Blood 2003;101:2426-33. [Crossref] [PubMed]
- McCullough J, Vesole DH, Benjamin RJ, et al. Therapeutic efficacy and safety of platelets treated with a photochemical process for pathogen inactivation: the SPRINT Trial. Blood 2004;104:1534-41. [Crossref] [PubMed]
- Mirasol Clinical Evaluation Study Group. A randomized controlled clinical trial evaluating the performance and safety of platelets treated with MIRASOL pathogen reduction technology. Transfusion 2010;50:2362-75. [Crossref] [PubMed]
- Kerkhoffs JL, van Putten WL, Novotny VM, et al. Clinical effectiveness of leucoreduced, pooled donor platelet concentrates, stored in plasma or additive solution with and without pathogen reduction. Br J Haematol 2010;150:209-17. [Crossref] [PubMed]
- Janetzko K, Cazenave JP, Klüter H, et al. Therapeutic efficacy and safety of photochemically treated apheresis platelets processed with an optimized integrated set. Transfusion 2005;45:1443-52. [Crossref] [PubMed]
- Lozano M, Knutson F, Tardivel R, et al. A multi-centre study of therapeutic efficacy and safety of platelet components treated with amotosalen and ultraviolet A pathogen inactivation stored for 6 or 7 d prior to transfusion. Br J Haematol 2011;153:393-401. [Crossref] [PubMed]
- Rebulla P, Vaglio S, Beccaria F, et al. Clinical effectiveness of platelets in additive solution treated with two commercial pathogen-reduction technologies. Transfusion 2017;57:1171-83. [Crossref] [PubMed]
- Sigle JP, Infanti L, Studt JD, et al. Comparison of transfusion efficacy of amotosalen-based pathogen-reduced platelet components and gamma-irradiated platelet components. Transfusion 2013;53:1788-97. [Crossref] [PubMed]
- van der Meer PF, Ypma PF, van Geloven N, et al. Hemostatic efficacy of pathogen-inactivated vs untreated platelets: a randomized controlled trial. Blood 2018;132:223-31. [Crossref] [PubMed]
- Seltsam A, Müller TH. UVC Irradiation for Pathogen Reduction of Platelet Concentrates and Plasma. Transfus Med Hemother 2011;38:43-54. [Crossref] [PubMed]
- Eickmann M, Gravemann U, Handke W, et al. Inactivation of Ebola virus and Middle East respiratory syndrome coronavirus in platelet concentrates and plasma by ultraviolet C light and methylene blue plus visible light, respectively. Transfusion 2018;58:2202-7. [Crossref] [PubMed]
- Faddy HM, Fryk JJ, Prow NA, et al. Inactivation of dengue, chikungunya, and Ross River viruses in platelet concentrates after treatment with ultraviolet C light. Transfusion 2016;56:1548-55. [Crossref] [PubMed]
- Gravemann U, Handke W, Müller TH, et al. Bacterial inactivation of platelet concentrates with the THERAFLEX UV-Platelets pathogen inactivation system. Transfusion 2019;59:1324-32. [Crossref] [PubMed]
- Fryk JJ, Marks DC, Hobson-Peters J, et al. Reduction of Zika virus infectivity in platelet concentrates after treatment with ultraviolet C light and in plasma after treatment with methylene blue and visible light. Transfusion 2017;57:2677-82. [Crossref] [PubMed]
- Steinmann E, Gravemann U, Friesland M, et al. Two pathogen reduction technologies--methylene blue plus light and shortwave ultraviolet light--effectively inactivate hepatitis C virus in blood products. Transfusion 2013;53:1010-8. [Crossref] [PubMed]
- Pohler P, Müller M, Winkler C, et al. Pathogen reduction by ultraviolet C light effectively inactivates human white blood cells in platelet products. Transfusion 2015;55:337-47. [Crossref] [PubMed]
- Praditya D, Friesland M, Gravemann U, et al. Hepatitis E virus is effectively inactivated in platelet concentrates by ultraviolet C light. Vox Sang 2020;115:555-61. [Crossref] [PubMed]
- Rustanti L, Hobson-Peters J, Colmant AMG, et al. Inactivation of Japanese encephalitis virus in plasma by methylene blue combined with visible light and in platelet concentrates by ultraviolet C light. Transfusion 2020;60:2655-60. [Crossref] [PubMed]
- Faddy HM, Fryk JJ, Hall RA, et al. Inactivation of yellow fever virus in plasma after treatment with methylene blue and visible light and in platelet concentrates following treatment with ultraviolet C light. Transfusion 2019;59:2223-7. [Crossref] [PubMed]
- Eickmann M, Gravemann U, Handke W, et al. Inactivation of three emerging viruses - severe acute respiratory syndrome coronavirus, Crimean-Congo haemorrhagic fever virus and Nipah virus - in platelet concentrates by ultraviolet C light and in plasma by methylene blue plus visible light. Vox Sang 2020;115:146-51. [Crossref] [PubMed]
- Seltsam A, Müller TH. Update on the use of pathogen-reduced human plasma and platelet concentrates. Br J Haematol 2013;162:442-54. [Crossref] [PubMed]
- Müller TH, Montag T, Seltsam AW. Laboratory Evaluation of the Effectiveness of Pathogen Reduction Procedures for Bacteria. Transfus Med Hemother 2011;38:242-50. [Crossref] [PubMed]
- World Health Organization. Guidelines on viral inactivation and removal procedures intended to assure the viral safety of human blood plasma products. Annex 4. WHO Technical Report, Series No. 924, 2004.
- Goodrich RP, Gilmour D, Hovenga N, et al. A laboratory comparison of pathogen reduction technology treatment and culture of platelet products for addressing bacterial contamination concerns. Transfusion 2009;49:1205-16. [Crossref] [PubMed]
- Goodrich RP, Custer B, Keil S, et al. Defining "adequate" pathogen reduction performance for transfused blood components. Transfusion 2010;50:1827-37. [Crossref] [PubMed]
- Bah A, Cardoso M, Seghatchian J, et al. Reflections on the dynamics of bacterial and viral contamination of blood components and the levels of efficacy for pathogen inactivation processes. Transfus Apher Sci 2018;57:683-8. [Crossref] [PubMed]
- Schmidt M, Hourfar MK, Sireis W, et al. Evaluation of the effectiveness of a pathogen inactivation technology against clinically relevant transfusion-transmitted bacterial strains. Transfusion 2015;55:2104-12. [Crossref] [PubMed]
- Wagner SJ, Benjamin RJ, Hapip CA, et al. Investigation of bacterial inactivation in apheresis platelets with 24 or 30 hours between inoculation and inactivation. Vox Sang 2016;111:226-34. [Crossref] [PubMed]
- McDonald CP, Bearne J, Aplin K, et al. Assessing the inactivation capabilities of two commercially available platelet component pathogen inactivation systems: effectiveness at end of shelf life. Vox Sang 2021;116:416-24. [Crossref] [PubMed]
- Benjamin RJ, Wagner SJ. Bacterial pathogen reduction requires validation under conditions of intended use. Transfusion 2015;55:2060-3. [Crossref] [PubMed]
- Benjamin RJ, Dy B, Perez J, et al. Bacterial culture of apheresis platelets: a mathematical model of the residual rate of contamination based on unconfirmed positive results. Vox Sang 2014;106:23-30. [Crossref] [PubMed]
- Störmer M, Arroyo A, Brachert J, et al. Establishment of the first international repository for transfusion-relevant bacteria reference strains: ISBT working party transfusion-transmitted infectious diseases (WP-TTID), subgroup on bacteria. Vox Sang 2012;102:22-31. [Crossref] [PubMed]
- Spindler-Raffel E, Benjamin RJ, McDonald CP, et al. Enlargement of the WHO international repository for platelet transfusion-relevant bacteria reference strains. Vox Sang 2017;112:713-22. [Crossref] [PubMed]
- Mohr H, Bayer A, Gravemann U, et al. Elimination and multiplication of bacteria during preparation and storage of buffy coat-derived platelet concentrates. Transfusion 2006;46:949-55. [Crossref] [PubMed]
- Greco-Stewart VS, Brown EE, Parr C, et al. Serratia marcescens strains implicated in adverse transfusion reactions form biofilms in platelet concentrates and demonstrate reduced detection by automated culture. Vox Sang 2012;102:212-20. [Crossref] [PubMed]
- Loza-Correa M, Ayala JA, Perelman I, et al. The peptidoglycan and biofilm matrix of Staphylococcus epidermidis undergo structural changes when exposed to human platelets. PLoS One 2019;14:e0211132. [Crossref] [PubMed]
- Loza-Correa M, Yousuf B, Ramirez-Arcos S. Staphylococcus epidermidis undergoes global changes in gene expression during biofilm maturation in platelet concentrates. Transfusion 2021;61:2146-58. [Crossref] [PubMed]
- Percival SL, Suleman L, Vuotto C, et al. Healthcare-associated infections, medical devices and biofilms: risk, tolerance and control. J Med Microbiol 2015;64:323-34. [Crossref] [PubMed]
- Hadjesfandiari N, Schubert P, Fallah Toosi S, et al. Effect of texture of platelet bags on bacterial and platelet adhesion. Transfusion 2016;56:2808-18. [Crossref] [PubMed]
- Jones SA, Jones JM, Leung V, et al. Sepsis Attributed to Bacterial Contamination of Platelets Associated with a Potential Common Source - Multiple States, 2018. MMWR Morb Mortal Wkly Rep 2019;68:519-23. [Crossref] [PubMed]
- Fridey JL, Stramer SL, Nambiar A, et al. Sepsis from an apheresis platelet contaminated with Acinetobacter calcoaceticus/baumannii complex bacteria and Staphylococcus saprophyticus after pathogen reduction. Transfusion 2020;60:1960-9. [Crossref] [PubMed]
- Fadeyi EA, Wagner SJ, Goldberg C, et al. Fatal sepsis associated with a storage container leak permitting platelet contamination with environmental bacteria after pathogen reduction. Transfusion 2021;61:641-8. [Crossref] [PubMed]
- Setlow P. Resistance of spores of Bacillus species to ultraviolet light. Environ Mol Mutagen 2001;38:97-104. [Crossref] [PubMed]
- Mohr H, Gravemann U, Bayer A, et al. Sterilization of platelet concentrates at production scale by irradiation with short-wave ultraviolet light. Transfusion 2009;49:1956-63. [Crossref] [PubMed]
- Störmer M, Vollmer T, Kleesiek K, et al. Spore-forming organisms in platelet concentrates: a challenge in transfusion bacterial safety. Transfus Med 2008;18:371-6. [Crossref] [PubMed]
- Benjamin RJ, McDonald CPISBT Transfusion Transmitted Infectious Disease Bacterial Workgroup. The international experience of bacterial screen testing of platelet components with an automated microbial detection system: a need for consensus testing and reporting guidelines. Transfus Med Rev 2014;28:61-71. [Crossref] [PubMed]
- Pearce S, Rowe GP, Field SP. Screening of platelets for bacterial contamination at the Welsh Blood Service. Transfus Med 2011;21:25-32. [Crossref] [PubMed]
- Murphy WG, Foley M, Doherty C, et al. Screening platelet concentrates for bacterial contamination: low numbers of bacteria and slow growth in contaminated units mandate an alternative approach to product safety. Vox Sang 2008;95:13-9. [Crossref] [PubMed]
- Gravemann U, Handke W, Lambrecht B, et al. Ultraviolet C light efficiently inactivates nonenveloped hepatitis A virus and feline calicivirus in platelet concentrates. Transfusion 2018;58:2669-74. [Crossref] [PubMed]
- Johnson L, Hyland R, Tan S, et al. In vitro Quality of Platelets with Low Plasma Carryover Treated with Ultraviolet C Light for Pathogen Inactivation. Transfus Med Hemother 2016;43:190-7. [Crossref] [PubMed]
- Gravemann U, Pohler P, Mueller TH, et al. In vitro quality of platelets treated in the THERAFLEX UV-Platelets system is well preserved during storage. Vox Sang 2013;105:abstr P-208.
- Seltsam A, Pohler P, Mueller M, et al. Inactivation of human white blood cells in platelet concentrates after pathogen reduction by UVC-light in the THERAFLEX UV-Platelets system. Vox Sang 2012;103:abstr 4C-S30-06.
- Thiele T, Pohler P, Kohlmann T, et al. Tolerance of platelet concentrates treated with UVC-light only for pathogen reduction--a phase I clinical trial. Vox Sang 2015;109:44-51. [Crossref] [PubMed]
- Brixner V, Bug G, Pohler P, et al. Efficacy of UVC-treated, pathogen-reduced platelets versus untreated platelets: a randomized controlled non-inferiority trial. Haematologica 2021;106:1086-96. [Crossref] [PubMed]
- Mohr H, Gravemann U, Müller TH. Inactivation of pathogens in single units of therapeutic fresh plasma by irradiation with ultraviolet light. Transfusion 2009;49:2144-51. [Crossref] [PubMed]
- 29th Regional Congress of the ISBT, Basel, Switzerland, 22-26 June 2019. Vox Sang 2019;114:5-240. [PubMed]
- Benjamin RJ. Pathogen inactivation - defining ‘adequate’ bacterial protection. ISBT Science Series 2014;9:124-30. [Crossref]
- Heddle NM, Cardoso M, van der Meer PF. Revisiting study design and methodology for pathogen reduced platelet transfusions: a round table discussion. Transfusion 2020;60:1604-11. [Crossref] [PubMed]
Cite this article as: Schulze TJ, Gravemann U, Seltsam A. THERAFLEX ultraviolet C (UVC)-based pathogen reduction technology for bacterial inactivation in blood components: advantages and limitations. Ann Blood 2022;7:28.


