
Immune thrombotic thrombocytopenic purpura: pathogenesis and novel therapies: a narrative review
Introduction
Immune thrombotic thrombocytopenic purpura (iTTP) is a rare, but potentially fatal blood disorder. It is caused by severe deficiency of a plasma metalloenzyme A Disintegrin And Metalloprotease with ThromboSpondin Type 1 Repeats, 13 (ADAMTS13) activity (1-3). The incidence of iTTP ranges from 3–6 cases per million residents per year (4,5). Immunoglobulin (Ig) G-type autoantibodies bind and inhibit plasma ADAMTS13 activity; additionally, immune complexes may be cleared from circulation resulting in significantly reduced ADAMTS13 protein in some cases (6-9). The severe deficiency of plasma ADAMTS13 activity renders an inability to cleave newly released ultra-large (UL) von Willebrand factor (vWF) multimers which are anchored on endothelial surface (10-13) and circulating in blood (14-17). This results in an enhanced platelet adhesion and aggregation, and the formation of occlusive thrombi in small arterioles and capillaries, leading to systemic organ damage and even death (18,19). Following acute episode, patients may experience disease relapses (20,21), cognitive decline, depression (22), and increased risk of cardiovascular diseases (23,24), etc., as part of long-term complications.
While the mechanism underlying the autoimmunity resulting in the formation of ADAMTS13 antibodies is still not known, limited data available to date have demonstrated that polyclonal IgG autoantibodies appear to bind multiple domains of ADAMTS13 with the cysteine-rich and spacer domains being the most frequent targets of all (25). Current treatment of iTTP includes therapeutic plasma exchange (TPE), caplacizumab, and immunosuppressives (such as rituximab, corticosteroids, or other regimens) (26,27). This triple therapy has significantly reduced the exacerbation, relapse, and in-hospital mortality of iTTP (28-31). The present review summarizes some of these progresses concerning the pathogenesis and novel therapeutics of iTTP. We present this article in accordance with the Narrative Review reporting checklist (available at https://aob.amegroups.com/article/view/10.21037/aob-22-29/rc).
Methods
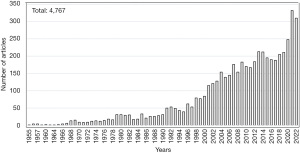
We conducted PubMed literature search using a combination of keywords thrombotic thrombocytopenic purpura and novel therapeutics or treatment from 1955 to November 2022. Of 4,767 articles published with full text, only small number of articles in English related to the topic were selected for further review and citation in this narrative review article (Table 1 and Figure 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | Nov. 19, 2022 |
| Databases and other sources searched | PubMed |
| Search terms used | Thrombotic, thrombocytopenic, purpura and pathogenesis, or thrombotic thrombocytopenic purpura and treatment |
| Timeframe | 1955–November 2022 |
| Inclusion and exclusion criteria | Only articles with full text in English are included for screening and small number of relevant articles are further reviewed |
| Selection process | First and senior authors |
Discussion
Pathophysiology
The pathophysiology of iTTP has been extensively studied since the identification and molecular cloning of ADAMTS13 (1,32-34) and the development of several rapid ADAMTS13 activity assays (35-39). The International Society of Thrombosis and Haemostasis (ISTH) guidelines for the diagnosis and management of TTP state that in a proper clinical context plasma ADAMTS13 activity less than 10 units/dL (or <10 percent of normal) (40) confirms the diagnosis of TTP; ADAMTS13 activity greater than 20 units/dL (or 20 percent of normal) essentially rules out the initial diagnosis of TTP; however, plasma ADAMTS13 activity between 10 and 20 units/dL or (10–20 percent of normal) is considered to be a broadline value (5,27).
The only known function of ADAMTS13 is to cleave newly releases UL-vWF multimers anchored on endothelial cells. This proteolytic cleavage is essential for normal hemostasis (Figure 2A) (11-13). ADAMTS13 may also cleave vWF multimers at the sites of vascular injury. This prevents the formation of occlusive thrombi but without affecting normal hemostasis (41-43). Furthermore, ADAMTS13 may cleave circulating vWF multimers when exposed to high fluidic shear, such as in the bifurcated or narrowed portions of blood vessels (44-46). If plasma ADAMTS13 protease is severely deficient, primarily resulting from autoantibodies-mediated inhibition or accelerated clearance of ADAMTS13, the UL-vWF multimers or strings accumulate on the endothelial surface (Figure 2B) or at the sites of vascular injury, which recruit flowing platelets and form occlusive thrombi.
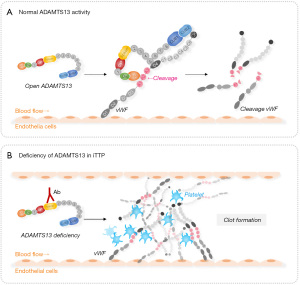
While severe deficiency of plasma ADAMTS13 activity may be necessary, it alone may not be sufficient to cause an acute episode of iTTP. Additional environmental or genetic factors may be required to trigger the disease. Infections (e.g., respiratory, or gastrointestinal), systemic inflammation (e.g., lupus), pregnancy (or postpartum), surgery (or trauma), and certain medications were often found to be associated with acute iTTP. The reason why a second hit is needed for a full-brown iTTP is not fully understood. It may be possible that a small amount of residual ADAMTS13 activity and/or other proteases such as neutrophil proteases (47) and plasmin (48) are sufficient to remove the UL-vWF strings from endothelial surface or at the sites of vascular injury at the basal levels. This proteolytic capacity may be overwhelmed when a large amount of vWF is secreted from endothelium as a result of an infection such as in the case of severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) infection (49-52) or trauma (53,54). Interestingly, in Baboon (55) or in some new born children (or neonates) (46,50,51,53,54,56), severe deficiency of plasma ADAMTS13 activity appears to be sufficient to cause an acute TTP, suggesting another potential genetic factor that might modify the susceptibility of TTP in these baboon or neonates.
Supporting this hypothesis comes from mutations in complement factor H (CFH) found in patients with hereditary TTP (57) or iTTP (8). A heterozygous or homozygous mutation in cfh and Adamts13 deficiency appear to play a synergistic role in causing thrombotic microangiopathy in mice, but either alone is not sufficient to cause a disease (6,58). In fact, elevated plasma levels of complement activation markers are commonly detected in patients with acute iTTP (7,9,59-63). Whether an inhibition of complement activation during acute iTTP would be therapeutically beneficial is yet to be determined. Anecdote case reports demonstrate the therapeutic efficacy of adding eculizumab to chronic relapsing iTTP (64,65).
Autoantibodies against ADAMTS13
Almost all adult iTTP patients are caused by IgG autoantibodies against ADAMTS13 (40,66), although the risk factors and underlying mechanism for the development of autoantibodies are not clear. Studies have shown that iTTP appears to be more common in young females, particularly African descents (67). The polyclonal anti-ADAMTS13 IgGs bind various domains of ADAMTS13 with the cysteine-rich and spacer domains being the most frequent targets. Additionally, approximately one third of patients may have IgG autoantibodies that bind other parts of ADAMTS13 protease. To date, there are a number of mouse and human monoclonal antibodies being developed or identified (Figure 3A). These are critical reagents for investigating the structure-function relationship and allosteric regulation of ADAMTS13 function.
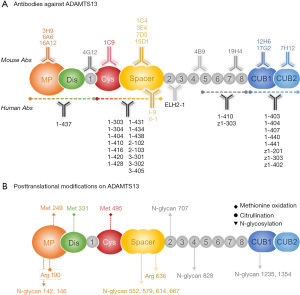
Most inhibitory antibodies appear to bind the spacer domain (68-71) although the mechanism of inhibition remains to be elucidated. Hydrogen-deuterium exchange plus mass spectrometric (HX-MS) analysis demonstrates that the anti-spacer domain antibodies, isolated from iTTP patients, bind 5 small flexible loops where vWF binds, suggesting that these antibodies may physically block the interaction between ADAMTS13 enzyme and its substrate vWF (69). An alternative hypothesis is also possible in which the binding of these antibodies to the spacer domain may result in a conformational change in the catalytic domain, thus affecting the cleavage efficiency of ADAMTS13 protease (72,73). More studies are needed to delineate the mechanism of antibody-mediated inhibition of ADAMTS13 activity.
Unlike anti-spacer antibody, the antibodies that bind the distal domains of ADAMTS13 such as T8-CUB (e.g., scFv4-3) or CUB (e.g., 17G2, 7H12 and 12H6) may induce conformational changes in ADAMTS13 (72,74,75), thus activating ADAMTS13 enzyme. However, how these polyclonal antibodies interact with ADAMTS13 resulting in inhibition of ADAMTS13 activity and/or accelerated clearance in vivo remains to be a subject of great interest for researchers.
Posttranslational modifications of ADAMTS13
Apart from vWF and antibodies targeting the C-terminal domains that activate ADAMTS13, post-translational modifications on ADAMTS13 protein may also impact its secretion and functions. ADAMTS13 may be modified at posttranslational levels by oxidation, glycosylation, citrullination, fucosylation or mannosylation (76-80), and possible methylation (Figure 3B). Glycosylation is a process by which a carbohydrate is covalently attached to the target protein. Glycosylation plays a variety of critical roles in many cellular events ranging from protein secretion, signaling, and protein-protein interaction (77,80,81). Changes in glycosylation can modulate inflammatory responses, enable viral immune escape, promote cancer cell progression or regulate cell death (82-84). N-glycosylation on ADAMTS13 has been shown to alter its secretion, conformation, and proteolytic activity (77,80). For instance, N146Q and N828Q mutants of ADAMTS13 exhibit a decreased secretion and proteolytic activity (77). It is also known that the loss of N-glycan in the T2-CUB region of ADAMTS13 where the molecular flexibility exists results in alteration of ADAMTS13 conformations (85). Citrullination may occur on arginine residues, which are converted to the citrullinated residues. This may impact ADAMTS13 protein structure, function, stability, and protein-protein interactions resulting from the loss of positive charges in the arginine residues. The citrullination process is catalyzed by an enzyme, peptidyl arginine deiminase-4 (PAD4) (80,83,84,86). Citrullination process is involved in pathology of many autoimmune disorders including rheumatoid arthritis and multiple sclerosis and other thrombo-inflammatory disorders such as sepsis and venous thrombosis (79,87,88). A recent study has demonstrated that ADAMTS13 can be citrullinated by PAD4 in vitro and in vivo (88). Mass spectrometry localized the citrullinated residues to arginine 190 and arginine 636 on ADAMTS13, which is within the metalloprotease and spacer domain, respectively. The citrullinated ADAMTS13 exhibits a reduced proteolytic activity towards vWF substrates (88).
Methylation is another post-translational modification that may occur on arginine residues of ADAMTS13. This process is often catalyzed by protein arginine methylation transferase-1 (PRMT1), which catalyzes the transfer of a methyl group from a co-substrate S-adenosyl-L-methionine (SAM) to the guanidine nitrogen of a peptidyl arginine residue. PRMT1 performs over 80% of methylation activity in cells (89). Using MeMo and PRmePRed web tool (90,91), we estimate approximately 35 asymmetric di-methylated arginines and 35 mono-methylated arginines on ADAMTS13. Whether one or several arginines on ADAMTS13 are methylated and how this posttranslational modification affects ADAMTS13 secretion and proteolytic function are under intense investigation in our laboratory.
Novel therapeutics
ISTH published the first guidelines for the diagnosis and management of iTTP (5,27). When a patient with highly suspected or confirmed iTTP based on clinical presentation (e.g., severe thrombocytopenia, microangiopathic hemolytic anemia and organ injury) or ADAMTS13 activity less than 10 units/dL, a combination of TPE, caplacizumab, and immunosuppressive (e.g., corticosteroids, rituximab, etc.), known as the triple therapy, should be given as early as possible (5,27,92). Such a therapeutic modality has been shown to significantly reduce the exacerbation and in-hospital mortality (29,93-95). As soon as the clinical diagnosis is made, emergent TPE should be initiated, followed by early caplacizumab and corticosteroids.
TPE is presumably removes IgG autoantibodies against ADAMTS13 (92) and damage-associated molecular patterns (DAMPs) such as S100A8/9 (96) and histone-DNA complexes (96) while replenishing the deficient ADAMTS13 enzyme (Figure 4A). Longitudinal study demonstrated that TPE alone is not sufficient to remove all antibodies and supply sufficient amount of ADAMTS13 to overcome the inhibition, particularly in those with high-titer inhibitors (92).
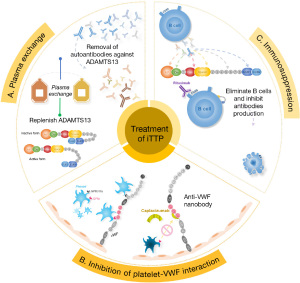
Caplacizumab, an anti-vWF nanobody, that binds vWF-A1 domain and inhibits the adhesion of platelets to UL-vWF, thus inhibiting thrombus formation (97,98) (Figure 4B). This prevents further tissue damage while plasma ADAMTS13 activity remains low and inhibitors are present. Despite of daily plasma exchange and early administration of rituximab, it may take weeks, if not months, to eliminate anti-ADAMTS13 IgG production (99,100).
Rituximab, an anti-CD20 monoclonal antibody, should be prescribed to patients as early as possible to halt the production of anti-ADAMTS13 autoantibodies (Figure 4C), which accelerates the recovery of ADAMTS13 and prevents exacerbation and relapses (101-103).
Other emergent therapeutic products, including recombinant ADAMTS13 (104-106), gain-of-functional (GoF) and antibody-resistant ADAMTS13 variants (107), and platelet-delivered ADAMTS13 (43,108), are all under development. All these approaches are expected to overcome or bypass the inhibitory antibodies against ADAMTS13 in patients with iTTP. The first in human trial for hereditary TTP has been completed and reported (104). An infusion of recombinant ADAMTS13 at 5, 20, and 40 IU/kg body weight resulted in a dose-dependent increase of plasma ADAMTS13 activity and an increase of platelet counts from the baseline. No significant adverse events were observed. A similar trial of recombinant ADAMTS13 for the treatment of acquired iTTP in conjunction with TPE and other immunosuppressive therapies has been closed but data are not published (NCT03922308). These well-designed clinical trials hope to demonstrate pharmacokinetics, therapeutic efficacy, and safety of these potential treatments for both hereditary TTP and iTTP. GoF-ADAMTS13 variants have been created and shown to have an increased specific activity and significant resistance to autoantibody-mediated inhibition, which may be explored for therapeutic purpose (107,109). Alternative strategy to avoid autoantibody-mediated inhibition of recombinant ADAMTS13 is to pack recombinant ADAMTS13 inside platelet α-granules through genetic engineering (43) or in vitro uptake (108). In this case, autoantibody does not have a chance to interact with recombinant ADAMTS13 inside platelets. Upon activation, platelets release recombinant ADAMTS13 at the site where proteolysis of UL-vWF is most needed (43,108).
Conclusions
In summary, tremendous progresses have been made in past decades in our understandings of pathogenesis, diagnosis, and management of iTTP. However, the mechanisms underlying anti-ADAMTS13 autoantibody production and inhibition remain to be elucidated. Additionally, the mechanisms of allosteric or posttranslational regulations of ADAMTS13 activity continue to be the hot areas for future investigation in the field. Triple therapy including TPE, caplacizumab, and immunosuppression has significantly reduced exacerbation and mortality. However, the long-term effects of this novel therapeutic strategy on cardiovascular complications, neurocognitive and behavioral changes, and quality of life are still not known.
Acknowledgments
Funding: This work was supported by grants from National Institutes of Health (Nos. HL126724, HL144552, and HL157975-01A1) and Answering TTP Foundation (to XL Zheng).
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Blood for the series “Thrombotic Thrombocytopenic Purpura”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aob.amegroups.com/article/view/10.21037/aob-22-29/rc
Peer Review File: Available at https://aob.amegroups.com/article/view/10.21037/aob-22-29/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://aob.amegroups.com/article/view/10.21037/aob-22-29/coif). The series “Thrombotic Thrombocytopenic Purpura” was commissioned by the editorial office without any funding or sponsorship. XLZ served as the unpaid Guest Editor of the series and serves as an unpaid Editorial Board member of Annals of Blood from March 2022 to February 2024. XLZ receives consulting fees from Alexion, Sanofi and Takeda, and receives grants from National Institutes of Health (Nos. HL126724, HL144552, and HL157975-01A1) and Answering TTP Foundation. XLZ is a consultant or a member of advisory boards for Alexion, Sanofi, and Takeda, as well as a co-founder of Clotsolution. XLZ also serves in several journal editorial boards (CJTH, JCTP, Arch Path Lab Med, Genomics, JCM, Diagnostics, Genomics and World Federation of Chinese Med). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zheng X, Chung D, Takayama TK, et al. Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J Biol Chem 2001;276:41059-63. [Crossref] [PubMed]
- Tsai HM, Lian EC. Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N Engl J Med 1998;339:1585-94. [Crossref] [PubMed]
- Moake JL. Thrombotic thrombocytopenic purpura: the systemic clumping "plague". Annu Rev Med 2002;53:75-88. [Crossref] [PubMed]
- Terrell DR, Williams LA, Vesely SK, et al. The incidence of thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: all patients, idiopathic patients, and patients with severe ADAMTS-13 deficiency. J Thromb Haemost 2005;3:1432-6. [Crossref] [PubMed]
- Zheng XL, Vesely SK, Cataland SR, et al. ISTH guidelines for the diagnosis of thrombotic thrombocytopenic purpura. J Thromb Haemost 2020;18:2486-95. [Crossref] [PubMed]
- Zheng L, Zhang D, Cao W, et al. Synergistic effects of ADAMTS13 deficiency and complement activation in pathogenesis of thrombotic microangiopathy. Blood 2019;134:1095-105. [Crossref] [PubMed]
- Noris M, Mescia F, Remuzzi G. STEC-HUS, atypical HUS and TTP are all diseases of complement activation. Nat Rev Nephrol 2012;8:622-33. [Crossref] [PubMed]
- Chapin J, Eyler S, Smith R, et al. Complement factor H mutations are present in ADAMTS13-deficient, ticlopidine-associated thrombotic microangiopathies. Blood 2013;121:4012-3. [Crossref] [PubMed]
- Westwood JP, Langley K, Heelas E, et al. Complement and cytokine response in acute Thrombotic Thrombocytopenic Purpura. Br J Haematol 2014;164:858-66. [Crossref] [PubMed]
- Chauhan AK, Kisucka J, Brill A, et al. ADAMTS13: a new link between thrombosis and inflammation. J Exp Med 2008;205:2065-74. [Crossref] [PubMed]
- Dong JF, Moake JL, Nolasco L, et al. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood 2002;100:4033-9. [Crossref] [PubMed]
- Dong JF, Moake JL, Bernardo A, et al. ADAMTS-13 metalloprotease interacts with the endothelial cell-derived ultra-large von Willebrand factor. J Biol Chem 2003;278:29633-9. [Crossref] [PubMed]
- López JA, Dong JF. Cleavage of von Willebrand factor by ADAMTS-13 on endothelial cells. Semin Hematol 2004;41:15-23. [Crossref] [PubMed]
- Sadler JE. A new name in thrombosis, ADAMTS13. Proc Natl Acad Sci U S A 2002;99:11552-4. [Crossref] [PubMed]
- Moake JL, Rudy CK, Troll JH, et al. Unusually large plasma factor VIII:von Willebrand factor multimers in chronic relapsing thrombotic thrombocytopenic purpura. N Engl J Med 1982;307:1432-5. [Crossref] [PubMed]
- Tsai HM. Shear stress and von Willebrand factor in health and disease. Semin Thromb Hemost 2003;29:479-88. [Crossref] [PubMed]
- Tsai HM. von Willebrand factor, shear stress, and ADAMTS13 in hemostasis and thrombosis. ASAIO J 2012;58:163-9. [Crossref] [PubMed]
- Bell WR, Braine HG, Ness PM, et al. Improved survival in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Clinical experience in 108 patients. N Engl J Med 1991;325:398-403. [Crossref] [PubMed]
- Rock GA, Shumak KH, Buskard NA, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian Apheresis Study Group. N Engl J Med 1991;325:393-7. [Crossref] [PubMed]
- Sun L, Mack J, Li A, et al. Predictors of relapse and efficacy of rituximab in immune thrombotic thrombocytopenic purpura. Blood Adv 2019;3:1512-8. [Crossref] [PubMed]
- Falter T, Herold S, Weyer-Elberich V, et al. Relapse Rate in Survivors of Acute Autoimmune Thrombotic Thrombocytopenic Purpura Treated with or without Rituximab. Thromb Haemost 2018;118:1743-51. [Crossref] [PubMed]
- Han B, Page EE, Stewart LM, et al. Depression and cognitive impairment following recovery from thrombotic thrombocytopenic purpura. Am J Hematol 2015;90:709-14. [Crossref] [PubMed]
- Brodsky MA, Sukumar S, Selvakumar S, et al. Major adverse cardiovascular events in survivors of immune-mediated thrombotic thrombocytopenic purpura. Am J Hematol 2021;96:1587-94. [Crossref] [PubMed]
- Balasubramaniyam N, Yandrapalli S, Kolte D, et al. Cardiovascular Complications and Their Association With Mortality in Patients With Thrombotic Thrombocytopenic Purpura. Am J Med 2021;134:e89-97. [Crossref] [PubMed]
- Thomas MR, de Groot R, Scully MA, et al. Pathogenicity of Anti-ADAMTS13 Autoantibodies in Acquired Thrombotic Thrombocytopenic Purpura. EBioMedicine 2015;2:942-52. [Crossref] [PubMed]
- Chan M, Zhao X, Zheng XL. Low ADAMTS-13 predicts adverse outcomes in hospitalized patients with suspected heparin-induced thrombocytopenia. Res Pract Thromb Haemost 2021;5:e12581. [Crossref] [PubMed]
- Zheng XL, Vesely SK, Cataland SR, et al. ISTH guidelines for treatment of thrombotic thrombocytopenic purpura. J Thromb Haemost 2020;18:2496-502. [Crossref] [PubMed]
- Picod A, Veyradier A, Coppo P. Should all patients with immune-mediated thrombotic thrombocytopenic purpura receive caplacizumab? J Thromb Haemost 2021;19:58-67. [Crossref] [PubMed]
- Coppo P, Bubenheim M, Azoulay E, et al. A regimen with caplacizumab, immunosuppression, and plasma exchange prevents unfavorable outcomes in immune-mediated TTP. Blood 2021;137:733-42. [Crossref] [PubMed]
- Cid J, Pérez-Valencia AI, Torrente MÁ, et al. Successful management of three patients with autoimmune thrombotic thrombocytopenic purpura with paradigm-changing therapy: Caplacizumab, steroids, plasma exchange, rituximab, and intravenous immunoglobulins (CASPERI). Transfus Apher Sci 2021;60:103011. [Crossref] [PubMed]
- Völker LA, Kaufeld J, Miesbach W, et al. Real-world data confirm the effectiveness of caplacizumab in acquired thrombotic thrombocytopenic purpura. Blood Adv 2020;4:3085-92. Erratum in: Blood Adv 2022 Apr 12;6(7):2434. [Crossref] [PubMed]
- Gerritsen HE, Robles R, Lämmle B, et al. Partial amino acid sequence of purified von Willebrand factor-cleaving protease. Blood 2001;98:1654-61. [Crossref] [PubMed]
- Fujikawa K, Suzuki H, McMullen B, et al. Purification of human von Willebrand factor-cleaving protease and its identification as a new member of the metalloproteinase family. Blood 2001;98:1662-6. [Crossref] [PubMed]
- Plaimauer B, Zimmermann K, Völkel D, et al. Cloning, expression, and functional characterization of the von Willebrand factor-cleaving protease (ADAMTS13). Blood 2002;100:3626-32. [Crossref] [PubMed]
- Kokame K, Nobe Y, Kokubo Y, et al. FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br J Haematol 2005;129:93-100. [Crossref] [PubMed]
- Kato S, Matsumoto M, Matsuyama T, et al. Novel monoclonal antibody-based enzyme immunoassay for determining plasma levels of ADAMTS13 activity. Transfusion 2006;46:1444-52. [Crossref] [PubMed]
- Nakashima MO, Zhang X, Rogers HJ, et al. Validation of a panel of ADAMTS13 assays for diagnosis of thrombotic thrombocytopenic purpura: activity, functional inhibitor, and autoantibody test. Int J Lab Hematol 2016;38:550-9. [Crossref] [PubMed]
- Valsecchi C, Mirabet M, Mancini I, et al. Evaluation of a New, Rapid, Fully Automated Assay for the Measurement of ADAMTS13 Activity. Thromb Haemost 2019;119:1767-72. [Crossref] [PubMed]
- Stratmann J, Ward JN, Miesbach W. Evaluation of a rapid turn-over, fully-automated ADAMTS13 activity assay: a method comparison study. J Thromb Thrombolysis 2020;50:628-31. [Crossref] [PubMed]
- George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med 2014;371:654-66. [Crossref] [PubMed]
- Chauhan AK, Motto DG, Lamb CB, et al. Systemic antithrombotic effects of ADAMTS13. J Exp Med 2006;203:767-76. [Crossref] [PubMed]
- Banno F, Chauhan AK, Kokame K, et al. The distal carboxyl-terminal domains of ADAMTS13 are required for regulation of in vivo thrombus formation. Blood 2009;113:5323-9. [Crossref] [PubMed]
- Pickens B, Mao Y, Li D, et al. Platelet-delivered ADAMTS13 inhibits arterial thrombosis and prevents thrombotic thrombocytopenic purpura in murine models. Blood 2015;125:3326-34. [Crossref] [PubMed]
- Gandhi C, Khan MM, Lentz SR, et al. ADAMTS13 reduces vascular inflammation and the development of early atherosclerosis in mice. Blood 2012;119:2385-91. [Crossref] [PubMed]
- Jin SY, Tohyama J, Bauer RC, et al. Genetic ablation of Adamts13 gene dramatically accelerates the formation of early atherosclerosis in a murine model. Arterioscler Thromb Vasc Biol 2012;32:1817-23. [Crossref] [PubMed]
- Gandhi C, Ahmad A, Wilson KM, et al. ADAMTS13 modulates atherosclerotic plaque progression in mice via a VWF-dependent mechanism. J Thromb Haemost 2014;12:255-60. [Crossref] [PubMed]
- Raife TJ, Cao W, Atkinson BS, et al. Leukocyte proteases cleave von Willebrand factor at or near the ADAMTS13 cleavage site. Blood 2009;114:1666-74. [Crossref] [PubMed]
- Tersteeg C, de Maat S, De Meyer SF, et al. Plasmin cleavage of von Willebrand factor as an emergency bypass for ADAMTS13 deficiency in thrombotic microangiopathy. Circulation 2014;129:1320-31. [Crossref] [PubMed]
- Rodríguez Rodríguez M, Castro Quismondo N, Zafra Torres D, et al. Increased von Willebrand factor antigen and low ADAMTS13 activity are related to poor prognosis in covid-19 patients. Int J Lab Hematol 2021;43:O152-5. [Crossref] [PubMed]
- Philippe A, Chocron R, Gendron N, et al. Circulating Von Willebrand factor and high molecular weight multimers as markers of endothelial injury predict COVID-19 in-hospital mortality. Angiogenesis 2021;24:505-17. [Crossref] [PubMed]
- Ward SE, Curley GF, Lavin M, et al. Von Willebrand factor propeptide in severe coronavirus disease 2019 (COVID-19): evidence of acute and sustained endothelial cell activation. Br J Haematol 2021;192:714-9. [Crossref] [PubMed]
- Beaulieu MC, Mettelus DS, Rioux-Massé B, et al. Thrombotic thrombocytopenic purpura as the initial presentation of COVID-19. J Thromb Haemost 2021;19:1132-4. [Crossref] [PubMed]
- Russell RT, McDaniel JK, Cao W, et al. Low Plasma ADAMTS13 Activity Is Associated with Coagulopathy, Endothelial Cell Damage and Mortality after Severe Paediatric Trauma. Thromb Haemost 2018;118:676-87. [Crossref] [PubMed]
- Kumar M, Cao W, McDaniel JK, et al. Plasma ADAMTS13 activity and von Willebrand factor antigen and activity in patients with subarachnoid haemorrhage. Thromb Haemost 2017;117:691-9. [Crossref] [PubMed]
- Feys HB, Roodt J, Vandeputte N, et al. Thrombotic thrombocytopenic purpura directly linked with ADAMTS13 inhibition in the baboon (Papio ursinus). Blood 2010;116:2005-10. [Crossref] [PubMed]
- Fujimura Y, Kokame K, Yagi H, et al. Hereditary deficiency of ADAMTS13 activity: Upshaw-Schulman syndrome. In: Rodgers GM, editor. ADAMTS13 Biology and Disease. Cham, Heidlberg, New York, Dordrecht, London: Springer, 2015:73-90.
- Noris M, Bucchioni S, Galbusera M, et al. Complement factor H mutation in familial thrombotic thrombocytopenic purpura with ADAMTS13 deficiency and renal involvement. J Am Soc Nephrol 2005;16:1177-83. [Crossref] [PubMed]
- Ueda Y, Mohammed I, Song D, et al. Murine systemic thrombophilia and hemolytic uremic syndrome from a factor H point mutation. Blood 2017;129:1184-96. [Crossref] [PubMed]
- Ruiz-Torres MP, Casiraghi F, Galbusera M, et al. Complement activation: the missing link between ADAMTS-13 deficiency and microvascular thrombosis of thrombotic microangiopathies. Thromb Haemost 2005;93:443-52. [Crossref] [PubMed]
- Réti M, Farkas P, Csuka D, et al. Complement activation in thrombotic thrombocytopenic purpura. J Thromb Haemost 2012;10:791-8. [Crossref] [PubMed]
- Wu TC, Yang S, Haven S, et al. Complement activation and mortality during an acute episode of thrombotic thrombocytopenic purpura. J Thromb Haemost 2013;11:1925-7. [Crossref] [PubMed]
- Cao WJ, Pham HP, Williams LA, et al. Plasma Levels of Human Neutrophil Peptides and Complement Activation Markers in Patients with Acquired Autoimmune Thrombotic Thrombocytopenic Purpura. Blood 2015;126:1147. [Crossref]
- Staley EM, Cao W, Pham HP, et al. Clinical factors and biomarkers predict outcome in patients with immune-mediated thrombotic thrombocytopenic purpura. Haematologica 2019;104:166-75. [Crossref] [PubMed]
- Chapin J, Weksler B, Magro C, et al. Eculizumab in the treatment of refractory idiopathic thrombotic thrombocytopenic purpura. Br J Haematol 2012;157:772-4. [Crossref] [PubMed]
- Tsai E, Chapin J, Laurence JC, et al. Use of eculizumab in the treatment of a case of refractory, ADAMTS13-deficient thrombotic thrombocytopenic purpura: additional data and clinical follow-up. Br J Haematol 2013;162:558-9. [Crossref] [PubMed]
- Blombery P, Scully M. Management of thrombotic thrombocytopenic purpura: current perspectives. J Blood Med 2014;5:15-23. [PubMed]
- Terrell DR, Vesely SK, Kremer Hovinga JA, et al. Different disparities of gender and race among the thrombotic thrombocytopenic purpura and hemolytic-uremic syndromes. Am J Hematol 2010;85:844-7. [Crossref] [PubMed]
- Zheng XL, Wu HM, Shang D, et al. Multiple domains of ADAMTS13 are targeted by autoantibodies against ADAMTS13 in patients with acquired idiopathic thrombotic thrombocytopenic purpura. Haematologica 2010;95:1555-62. [Crossref] [PubMed]
- Casina VC, Hu W, Mao JH, et al. High-resolution epitope mapping by HX MS reveals the pathogenic mechanism and a possible therapy for autoimmune TTP syndrome. Proc Natl Acad Sci U S A 2015;112:9620-5. [Crossref] [PubMed]
- Pos W, Luken BM, Kremer Hovinga JA, et al. VH1-69 germline encoded antibodies directed towards ADAMTS13 in patients with acquired thrombotic thrombocytopenic purpura. J Thromb Haemost 2009;7:421-8. [Crossref] [PubMed]
- Pos W, Sorvillo N, Fijnheer R, et al. Residues Arg568 and Phe592 contribute to an antigenic surface for anti-ADAMTS13 antibodies in the spacer domain. Haematologica 2011;96:1670-7. [Crossref] [PubMed]
- Schelpe AS, Petri A, Roose E, et al. Antibodies that conformationally activate ADAMTS13 allosterically enhance metalloprotease domain function. Blood Adv 2020;4:1072-80. [Crossref] [PubMed]
- Halkidis K, Zheng XL. ADAMTS13 conformations and mechanism of inhibition in immune thrombotic thrombocytopenic purpura. J Thromb Haemost 2022;20:2197-203. [Crossref] [PubMed]
- Muia J, Zhu J, Gupta G, et al. Allosteric activation of ADAMTS13 by von Willebrand factor. Proc Natl Acad Sci U S A 2014;111:18584-9. [Crossref] [PubMed]
- Halkidis K, Siegel DL, Zheng XL. A human monoclonal antibody against the distal carboxyl terminus of ADAMTS13 modulates its susceptibility to an inhibitor in thrombotic thrombocytopenic purpura. J Thromb Haemost 2021;19:1888-95. [Crossref] [PubMed]
- Sorvillo N, Kaijen PH, Matsumoto M, et al. Identification of N-linked glycosylation and putative O-fucosylation, C-mannosylation sites in plasma derived ADAMTS13. J Thromb Haemost 2014;12:670-9. [Crossref] [PubMed]
- Zhou W, Tsai HM. N-Glycans of ADAMTS13 modulate its secretion and von Willebrand factor cleaving activity. Blood 2009;113:929-35. [Crossref] [PubMed]
- Verbij FC, Stokhuijzen E, Kaijen PH, et al. Identification of glycans on plasma-derived ADAMTS13. Blood 2016;128:e51-8. [Crossref] [PubMed]
- Ciesielski O, Biesiekierska M, Panthu B, et al. Citrullination in the pathology of inflammatory and autoimmune disorders: recent advances and future perspectives. Cell Mol Life Sci 2022;79:94. [Crossref] [PubMed]
- Nowak AA, O'Brien HER, Henne P, et al. ADAMTS-13 glycans and conformation-dependent activity. J Thromb Haemost 2017;15:1155-66. [Crossref] [PubMed]
- Dennis JW, Granovsky M, Warren CE. Protein glycosylation in development and disease. Bioessays 1999;21:412-21. [Crossref] [PubMed]
- Marth JD, Grewal PK. Mammalian glycosylation in immunity. Nat Rev Immunol 2008;8:874-87. [Crossref] [PubMed]
- Dalziel M, Crispin M, Scanlan CN, et al. Emerging principles for the therapeutic exploitation of glycosylation. Science 2014;343:1235681. [Crossref] [PubMed]
- Mammadova-Bach E, Jaeken J, Gudermann T, et al. Platelets and Defective N-Glycosylation. Int J Mol Sci 2020;21:5630. [Crossref] [PubMed]
- Deforche L, Roose E, Vandenbulcke A, et al. Linker regions and flexibility around the metalloprotease domain account for conformational activation of ADAMTS-13. J Thromb Haemost 2015;13:2063-75. [Crossref] [PubMed]
- Wong SL, Wagner DD. Peptidylarginine deiminase 4: a nuclear button triggering neutrophil extracellular traps in inflammatory diseases and aging. FASEB J 2018;32:fj201800691R. [Crossref] [PubMed]
- Martinod K, Demers M, Fuchs TA, et al. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci U S A 2013;110:8674-9. [Crossref] [PubMed]
- Sorvillo N, Mizurini DM, Coxon C, et al. Plasma Peptidylarginine Deiminase IV Promotes VWF-Platelet String Formation and Accelerates Thrombosis After Vessel Injury. Circ Res 2019;125:507-19. [Crossref] [PubMed]
- Tang J, Frankel A, Cook RJ, et al. PRMT1 is the predominant type I protein arginine methyltransferase in mammalian cells. J Biol Chem 2000;275:7723-30. [Crossref] [PubMed]
- Chen H, Xue Y, Huang N, et al. MeMo: a web tool for prediction of protein methylation modifications. Nucleic Acids Res 2006;34:W249-53. [Crossref] [PubMed]
- Kumar P, Joy J, Pandey A, et al. PRmePRed: A protein arginine methylation prediction tool. PLoS One 2017;12:e0183318. [Crossref] [PubMed]
- Zheng XL, Kaufman RM, Goodnough LT, et al. Effect of plasma exchange on plasma ADAMTS13 metalloprotease activity, inhibitor level, and clinical outcome in patients with idiopathic and nonidiopathic thrombotic thrombocytopenic purpura. Blood 2004;103:4043-9. [Crossref] [PubMed]
- Zheng XL. The standard of care for immune thrombotic thrombocytopenic purpura today. J Thromb Haemost 2021;19:1864-71. [Crossref] [PubMed]
- Coppo P, Cuker A, George JN. Thrombotic thrombocytopenic purpura: Toward targeted therapy and precision medicine. Res Pract Thromb Haemost 2019;3:26-37. [Crossref] [PubMed]
- Knoebl P, Cataland S, Peyvandi F, et al. Efficacy and safety of open-label caplacizumab in patients with exacerbations of acquired thrombotic thrombocytopenic purpura in the HERCULES study. J Thromb Haemost 2020;18:479-84. [Crossref] [PubMed]
- Sui J, Lu R, Halkidis K, et al. Plasma levels of S100A8/A9, histone/DNA complexes, and cell-free DNA predict adverse outcomes of immune thrombotic thrombocytopenic purpura. J Thromb Haemost 2021;19:370-9. [Crossref] [PubMed]
- Schleinitz N, Ebbo M, Mazodier K, et al. Rituximab as preventive therapy of a clinical relapse in TTP with ADAMTS13 inhibitor. Am J Hematol 2007;82:417-8. [Crossref] [PubMed]
- Bresin E, Gastoldi S, Daina E, et al. Rituximab as pre-emptive treatment in patients with thrombotic thrombocytopenic purpura and evidence of anti-ADAMTS13 autoantibodies. Thromb Haemost 2009;101:233-8. [Crossref] [PubMed]
- Scully M, McDonald V, Cavenagh J, et al. A phase 2 study of the safety and efficacy of rituximab with plasma exchange in acute acquired thrombotic thrombocytopenic purpura. Blood 2011;118:1746-53. [Crossref] [PubMed]
- Peyvandi F, Scully M, Kremer Hovinga JA, et al. Caplacizumab for Acquired Thrombotic Thrombocytopenic Purpura. N Engl J Med 2016;374:511-22. [Crossref] [PubMed]
- Scully M, Cataland SR, Peyvandi F, et al. Caplacizumab Treatment for Acquired Thrombotic Thrombocytopenic Purpura. N Engl J Med 2019;380:335-46. [Crossref] [PubMed]
- Froissart A, Buffet M, Veyradier A, et al. Efficacy and safety of first-line rituximab in severe, acquired thrombotic thrombocytopenic purpura with a suboptimal response to plasma exchange. Experience of the French Thrombotic Microangiopathies Reference Center. Crit Care Med 2012;40:104-11. [Crossref] [PubMed]
- McDonald V, Manns K, Mackie IJ, et al. Rituximab pharmacokinetics during the management of acute idiopathic thrombotic thrombocytopenic purpura. J Thromb Haemost 2010;8:1201-8. [Crossref] [PubMed]
- Scully M, Knöbl P, Kentouche K, et al. Recombinant ADAMTS-13: first-in-human pharmacokinetics and safety in congenital thrombotic thrombocytopenic purpura. Blood 2017;130:2055-63. [Crossref] [PubMed]
- Plaimauer B, Schiviz A, Kaufmann S, et al. Neutralization of inhibitory antibodies and restoration of therapeutic ADAMTS-13 activity levels in inhibitor-treated rats by the use of defined doses of recombinant ADAMTS-13. J Thromb Haemost 2015;13:2053-62. [Crossref] [PubMed]
- Tersteeg C, Schiviz A, De Meyer SF, et al. Potential for Recombinant ADAMTS13 as an Effective Therapy for Acquired Thrombotic Thrombocytopenic Purpura. Arterioscler Thromb Vasc Biol 2015;35:2336-42. [Crossref] [PubMed]
- Jian C, Xiao J, Gong L, et al. Gain-of-function ADAMTS13 variants that are resistant to autoantibodies against ADAMTS13 in patients with acquired thrombotic thrombocytopenic purpura. Blood 2012;119:3836-43. [Crossref] [PubMed]
- Abdelgawwad MS, Cao W, Zheng L, et al. Transfusion of Platelets Loaded With Recombinant ADAMTS13 (A Disintegrin and Metalloprotease With Thrombospondin Type 1 Repeats-13) Is Efficacious for Inhibiting Arterial Thrombosis Associated With Thrombotic Thrombocytopenic Purpura. Arterioscler Thromb Vasc Biol 2018;38:2731-43. [Crossref] [PubMed]
- South K, Luken BM, Crawley JT, et al. Conformational activation of ADAMTS13. Proc Natl Acad Sci U S A 2014;111:18578-83. [Crossref] [PubMed]
Cite this article as: Liu S, Zheng XL. Immune thrombotic thrombocytopenic purpura: pathogenesis and novel therapies: a narrative review. Ann Blood 2023;8:26.


