
Making every question count: the impact of temporary donor deferral for suspected acute retroviral syndrome
Highlight box
Key findings
• Questioning and deferring donors for rash and lymphadenopathy [acute retroviral syndrome (ARS) symptoms] results in loss of donors who are unlikely to pose a threat to transfusion safety.
What is known and what is new?
• Early human immunodeficiency virus (HIV) infection can manifest as ARS, a nonspecific illness that occurs approximately 1–4 weeks following infection. Highly sensitive nucleic acid amplification tests can detect HIV prior to the development of ARS symptoms.
• Here, we demonstrate that questioning for ARS symptoms (rash and lymphadenopathy) does not effectively differentiate HIV-positive from HIV-negative donors and resulted in a loss of donors who were unlikely to pose a threat to transfusion safety.
What is the implication, and what should change now?
• This study is an evidence-based assessment and may trigger donor questionnaire modification to better reflect contemporary understanding of HIV.
Introduction
Donor questionnaires (DQs) are used by blood services worldwide to screen potential donors for possible threats to blood safety. People who present to donate blood can be deemed ineligible and deferred from donating temporarily or permanently. Donors are less likely to return to donate blood following deferral (1), leading to loss of donors, donations and blood supply sufficiency concerns (2). The risks and benefits of deferral criteria must be considered carefully.
Human immunodeficiency virus (HIV) is a leading cause of mortality and morbidity worldwide (3), and is a transfusion-transmissible infection (4). Comparatively, HIV prevalence is low in Australia with a prevalence of 0.1% (5). Early HIV infection can manifest as acute retroviral syndrome (ARS), a nonspecific illness that occurs approximately 1–4 weeks following infection (6). Clinical manifestations and their severity are variable and include fever, fatigue, myalgia, rash, headache, pharyngitis, lymphadenopathy, night sweats, arthralgia and diarrhoea (7). ARS also resembles diseases including Epstein-Barr virus, cytomegalovirus (CMV), influenza, systemic lupus erythematosus, vasculitis and drug reactions (8,9).
Despite the variable and non-specific nature of ARS, the Australian Red Cross Lifeblood (Lifeblood) DQ directly questions donors regarding ARS symptoms: “In the last 12 months have you had an illness with swollen glands and a rash, with or without a fever?”. If a potential donor answers yes, they are counselled by a medical officer and, if HIV infection is suspected based on the individual medical officer’s expertise, deferred for 12 months from the date of recovery. Donors were originally questioned about ARS as a means of detecting HIV infection before laboratory screening was available. All HIV tests have a window period, which represents the length of time between infection and ability to detect HIV. Early enzyme-linked immunosorbent assays (ELISA) had a long window period of 4–8 weeks (10), and deferring donors with ARS symptoms helped prevent donations during this window period (11). However, since the introduction of highly sensitive nucleic acid amplification testing (NAT), the window period has decreased to only 5.9 days (12). Therefore, contemporary tests can detect HIV prior to the development of ARS.
Pre-donation DQs worldwide are typically lengthy, designed to detect a variety of blood supply and donor safety risks. Research has indicated that donor satisfaction is likely to increase if questionnaires are made shorter (13). Removing questions with limited impact on safety would likely improve donor satisfaction, and perhaps compliance. It could also allow for introduction of potentially higher yield questions. Few studies have been conducted to investigate the yield of particular questions in detecting at risk donors and protecting the blood supply. Most recently, several countries have recently changed their blood donor history questionnaires and deferral policies with respect to men who have had sex with another man (MSM), moving towards more individual-based risk criteria (14).
Given the clinical manifestations of ARS are variable and non-specific and contemporary HIV testing methods can detect HIV prior to the development of ARS, we sought to provide an evidence-based assessment of the effectiveness of questioning donors for possible ARS-like symptoms in maintaining transfusion safety. This assessment will assist blood services worldwide in optimizing their pre-donation screening protocols to increase recruitment and retention of blood donors. We present this article in accordance with the STROBE reporting checklist (available at https://aob.amegroups.com/article/view/10.21037/aob-23-16/rc).
Methods
General information
All blood donations within Australia are voluntary, and received entirely by Lifeblood. Lifeblood is funded by the Australian government and is a not-for-profit organisation receiving approximately 1.6 million donations annually from over 70 collection centres. Each year, Australian industries and organisations will organise small and large blood drives to encourage donations to Lifeblood. Following donation, donors receive updates via text message and/or email regarding the status of their donation (for example, the hospital that the blood has been distributed to) and a reminder regarding when their deferral is ending or when they are next eligible to donate (15).
Data sources
Multiple Lifeblood national databases were assessed in this paper including the Medical Officer Database (MODB), the National Blood Management System (NBMS), and the Risk Factor Database (RFDB). A brief explanation of each database is included in Table 1.
Table 1
| Database | Data availability dates | Description of data available |
|---|---|---|
| MODB | July 2014–February 2016 | Utilised to extract data regarding donors that answered ‘yes’ to the ARS question but were not deferred, and why |
| NBMS | January 2000–March 2015 | Utilised to extract data regarding donors that answered ‘yes’ to the ARS question but were deferred |
| Utilised to extract data regarding donors that returned to donate, and when | ||
| RFDB | January 2001–May 2016 | Utilised to extra data on risk factors for donors that tested HIV positive upon attempted donation |
MODB, Medical Officer Database; NBMS, National Blood Management System; RFDB, Risk Factor Database; ARS, acute retroviral syndrome; HIV, human immunodeficiency virus.
Any prospective donor that declares symptoms of ARS on the DQ is counselled by a medical officer and, if HIV infection is suspected based on the individual medical officer’s expertise, deferred for 12 months from the date of recovery. The MODB was used to identify donors who declared possible ARS, but were not deferred The MODB had a limited dataset available, including donors who presented between July 2014 and February 2016. For the same time period, the NBMS was used to identify donors who declared possible ARS, but were deferred.
NBMS was used to identify donors who declared possible ARS and were deferred for 12 months. NBMS was also used to identify donors deferred for 12 months due to other risk factors. These 12-month deferrals, grouped by similarity, are detailed in Table 2. Data was extracted regarding donors who were deferred between January 2000 and March 2015.
Table 2
| Group | Deferral | Description |
|---|---|---|
| ARS | Acute retroviral syndrome | Illness with rash and lymphadenopathy, with or without fever |
| Sexual | Sex with endemic partner | Donor had sex overseas in HIV risk area with resident of area |
| Suspicious sexual contact | Donor states had sex with person with a possible blood-borne virus | |
| Sex worker contact | Donor had sex with sex worker | |
| Sex worker | Donor accepted payment for sex in money, gifts or drugs | |
| HIV close contact | Donor has close household/sexual/mucosal contact with known HIV/AIDS person | |
| Hepatitis C close contact | Donor has close household/sexual/mucosal contact with known hepatitis C person | |
| HTLV close contact | Donor has close household/sexual/mucosal contact with known human T-lymphotropic virus person | |
| Gonorrhoea | Donor infected with gonorrhoea | |
| Transsexual sex with male | Transsexual donor has sex with a male | |
| Female sex with bisexual male | Female donor had sex with male who has sex with males | |
| Sex with recipient of factor VIII/IX | Sexual partner of a person being treated with blood-derived coagulation factor | |
| MSM | MSM | Male donor had oral or anal sex with a man |
| Medical | Hepatitis/jaundice event | Donor had hepatitis or jaundice of unknown cause |
| Glandular fever with hepatitis | Donor had glandular fever with hepatitis | |
| CMV with hepatitis | Donor had cytomegalovirus induced hepatitis | |
| Allogeneic blood transfusion | Donor received blood transfusion from another individual | |
| Transplant recipient | Donor received an allogeneic bone, tendon or skin graft | |
| Acute PE | Donor had pulmonary embolism | |
| GBS | Donor had Guillain-Barrè syndrome | |
| Short term coagulation treatment | Donor had short-term treatment with blood-derived coagulation factor | |
| Prison | Prison | Donor held in prison, lock-up or detention center for >72 hours |
| Bite/scratch | Bite (rabies) | Bitten or scratched by animal in rabies-endemic area, not pre-immunized against rabies |
| Bat scratch/bite | Bitten or scratched by bat |
ARS, acute retroviral syndrome; HIV, human immunodeficiency virus; AIDS, acquired immunodeficiency syndrome; HTLV, human T-lymphotropic virus; MSM, men who have sex with men; CMV, cytomegalovirus; PE, pulmonary embolism; GBS, Guillain-Barrè syndrome.
In accordance with Lifeblood protocol, donors testing HIV-positive were contacted and offered further testing, confidential interview, counselling and referral to an appropriate clinician. All interviews were conducted by trained medical officers. The donor’s answers to standardized risk factor questions were recorded. Data regarding donors testing HIV-positive between 2001–2015 were compiled using a combination of: transfusion transmission surveillance reports (5,12,16-18), original risk factor forms from the RFDB, and Lifeblood infectious disease statistics. Donors who declined interview or did not answer all risk factor questions were excluded.
Statistical analyses
Definitions
We restricted our analysis of donors to whole blood donors only.
A donor return was defined as a donor that had returned to Lifeblood to attempt donation, regardless of their eligibility to donate upon return or donation success.
Comparison of deferred and non-deferred donors who declared ARS
The records of blood donors who declared possible ARS were examined, and the proportion deferred calculated. Non-deferred donors were followed until May 2016 to assess the proportion who returned to donate and median return time. Return time was calculated with day one following the standard 12-week inter-donation interval and included any return attempt irrespective of donation success. The follow-up period was up to 579 days.
ARS-deferred donors were then compared to donors who declared possible ARS but were not deferred. Due to differences in database management, ARS-deferred donor data were extracted from a different data source to donors who declared possible ARS but were not deferred. To allow for comparison between data from different sources, follow up of ARS-deferred donors was cut-off at 579 days post-deferral expiry to match the non-deferred group, with last follow-up ending in March 2016. Comparison of the proportion of donors who returned was made using the Chi-squared test. Return time data normality was assessed using the D’Agostino & Pearson omnibus test. The data was not normally distributed, and was thus compared using a Mann-Whitney U test (GRAPHPAD Prism; GraphPad® Software, La Jolla, CA, USA).
Comparison of donors deferred for possible ARS with other 12-month deferrals
Data were collected regarding all other 12-month deferrals. Deferrals were grouped based on similarity into the following categories: possible ARS, MSM, other sexual risk factors, medical, bite/scratch (Table 2). For each deferral category, the proportion of donors who returned and return time were determined. Note that deferral periods started on the date the deferrable behaviour or exposure occurred, as opposed to the date the donor presented to donate. Donors were followed until March 2016, with donors not returning by this date classified as having not returned. All returning donors were included regardless of their eligibility to donate on return.
The proportions of donors returning in each deferral category were compared to those returning following the ARS deferral using the Chi-squared test. Return time data normality was assessed as described previously and was not normally distributed. Comparisons of return time between deferrals were then made using the Kruskal-Wallis test with Dunn’s multiple comparisons test.
Analysis of HIV markers in donors declaring possible ARS
Donors who declared possible ARS and donated without deferral or were deferred but later returned to donate were followed to assess HIV status until May 2016. Blood donation testing protocol is constantly evolving. In Australia, all donations are routinely tested for HIV using the following tests for anti-HIV-1, anti-HIV-2 and p24 Ag: Abbott PRISM (Abbott Diagnostics, Wiesbaden-Delkenheim, Germany) Chemiluminescent Immunoassay system until October 2020, then subsequently Abbott Alinity S (Abbott Diagnostics) Chemiluminescent Immunoassay system. They are also tested for HIV-1 and HIV-2 RNA by the Chiron Procleix HIV-1/HCV (Multiplex) Assay (San Diego, CA, USA), and the HIV-1 and HCV Discriminatory Assays (Chiron Blood Testing, Emeryville, CA, USA) from June 2000 until July 2010, then subsequently by Novartis HIV-1/HCV/HBV Procleix Ultrio assay (Emeryville, CA, USA) using a fully automated testing system (Procleix Tigris). The Ultrio assay was replaced by Grifols/Hologic HIV-1&2/HCV/HBV Procleix Ultrio Plus assay (Emeryville, CA, USA) in August 2013. The Ultrio Plus assay was replaced by the Grifols/Hologic HIV-1/2/HCV/HBV Procleix Ultrio Elite assay (Emeryville, CA, USA) in May 2021 (5).
Analysis of donors who tested HIV-positive
The proportion of HIV-positive donors who experienced ARS symptoms was calculated. Donors for whom no risk factor information was available were excluded from analyses. The symptoms of ARS reported by HIV-positive donors were tabulated, with prevalence of these symptoms in this population compared using exact 95% confidence intervals (CIs).
Ethical approval
All data were de-identified (anonymized). Ethical approval was obtained from the Lifeblood Human Research Ethics Committee (reference No. 2015#15) and the University of Queensland School of Medicine Low Risk Ethics Review Panel (clearance No. 2015-SOMILRE-0149). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Every DQ includes a declaration from the donor consenting to the use of their information and donation for research purposes once approved by a relevant Human Research Ethics Committee.
Results
Between July 2014 and February 2016, 90 people declared possible ARS on the DQ. Of these individuals, 59 were deferred (65.56%, 95% CI: 55.74–75.37%), 26 donated whole blood (28.89%, 95% CI: 19.52–38.25%), 4 donated plasma (4.44%, 95% CI: 0.19–8.70%) and one attempted donation but was unsuccessful (1.11%, 95% CI: 1.05–3.28%). Of those that were not deferred, the reasons reported for this were medical officer discretion (29.0%, n=9/31; 95% CI: 13.05–45.01%), alternative infection reported by patient as cause of symptoms (48.39%, n=15/31; 95% CI: 30.80–65.98%), allergic reaction (12.9%, n=4/31; 95% CI: 1.10–24.70%), patient did not have a rash (6.45%, n=2/31; 95% CI: 0.00–15.10%), or an alternative cause for the rash was identified i.e. eczema (3.23%; n=1/31; 95% CI: 0.00–9.45%).
Return parameters for the ARS-declaring donors who successfully donated whole blood (n=26) were compared to a larger cohort of donors (donating between January 2000 and March 2015) who declared possible ARS but were deferred (n=273), over the same follow up period of 579 days. Deferred donors were significantly less likely to return during follow-up (22.34%, n=61/273; 95% CI: 17.40–27.29%) than those not deferred (50.00%, n=13/26; 95% CI: 30.78–69.22%) (P<0.005), although there was no difference in their time to return to donate {median 85 days [interquartile range (IQR) 23] if not deferred, 80 days (IQR 251) if deferred} (P=0.53). This is outlined in Figure 1.
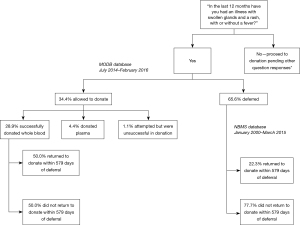
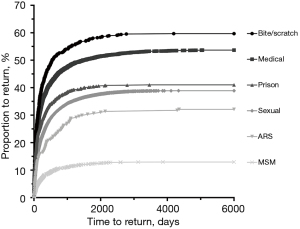
Deferral type was found to be an important predictor of donor return (Table 3; Figure 2). Notably, 32.23% (n=88/273; 95% CI: 26.69–37.78%) of donors deferred for possible ARS returned when followed from deferral until the cut-off date March 2016, which is slightly more than when followed for 579 days as demonstrated previously. Donors deferred for ARS were less likely to return than those deferred due to incarceration in prison (P<0.01), sexual reasons (P<0.05), medical reasons (P<0.0001) and bite or scratch (P<0.0001). However, donors deferred for ARS were significantly more likely to return than those deferred for MSM (P<0.0001). Those deferred due to bite or scratch were the most likely to return (P<0.005). Furthermore, ARS-deferred donors took longer to return than those deferred for incarceration (P<0.005) or bite or scratch (P<0.05). Interestingly, no donors declaring ARS tested HIV positive on initial or subsequent donations at any point during the study period.
Table 3
| Group | Total donors to return after deferral | Time to return (days) | ||||||
|---|---|---|---|---|---|---|---|---|
| Deferred | No. returning (%) | 95% CI (%) | P valuea | No. | Median [IQR] | P valuea | ||
| ARS | 273 | 88 (32.23) | 26.69–37.78 | – | 88 | 225 [729.30] | – | |
| Prison | 1,063 | 436 (41.02) | 38.06–43.97 | <0.01 | 436 | 89 [306] | <0.005 | |
| Sexual | 18,416 | 7,160 (38.88) | 38.18–39.58 | <0.05 | 7,160 | 172 [470] | >0.99 | |
| Medical | 6,225 | 3,341 (53.67) | 52.43–54.91 | <0.0001 | 3,341 | 128 [387] | 0.16 | |
| Bite/scratch | 665 | 397 (59.70) | 55.97–63.43 | <0.0001 | 397 | 117 [325.50] | <0.05 | |
| MSM | 2,361 | 306 (12.96) | 11.61–14.32 | <0.0001 | 306 | 203 [511.55] | >0.99 | |
Specific deferrals included in the groups are defined in Table 2. a, P value compared to ARS deferral group. ARS, acute retroviral syndrome; MSM, men who have sex with men; IQR, interquartile range; CI, confidence interval.
Donors who tested HIV-positive by Lifeblood donation screening were identified. Between 2001 and 2015, 67 donors tested positive for HIV, with 65 included in analyses. Despite no donor who reported possible ARS on the DQ testing HIV-positive, 56.92% (n=37/65; 95% CI: 44.88–68.96%) of donors who tested HIV-positive reported experiencing at least one symptom consistent with ARS on follow-up interview (Table 4). Fever was the most common symptom experienced (18.46%, n=12/65; 95% CI: 9.03–27.89%). No donors reported the specific combination of rash and lymphadenopathy.
Table 4
| Symptom | Number (n=65) | Proportion of donors % (95% CI) |
|---|---|---|
| Fever | 12 | 18.46 (9.03 to 27.89) |
| Rash/pruritus | 10 | 15.38 (6.61 to 24.16) |
| Lethargy | 9 | 13.85 (5.45 to 22.24) |
| Flu to like illness | 8 | 12.31 (4.32 to 20.29) |
| Unknowna | 7 | 10.77 (3.23 to 18.31) |
| Lymphadenopathy | 6 | 9.23 (2.19 to 16.27) |
| Anorexia/weight loss | 6 | 9.23 (2.19 to 16.27) |
| Pharyngitis | 5 | 7.69 (1.21 to 14.17) |
| GIT symptomsb | 5 | 7.69 (1.21 to 14.17) |
| Myalgia | 4 | 6.15 (0.31 to 12.00) |
| Sweats | 3 | 4.62 (−0.49 to 9.72) |
| Headache | 3 | 4.62 (−0.49 to 9.72) |
| Photophobia | 1 | 1.54 (−1.45 to 4.53) |
a, the donor is recorded as having experienced an ARS-like syndrome, although the exact signs and symptoms were not recorded. b, GIT, gastrointestinal tract symptoms including nausea, vomiting, diarrhoea or abdominal pain. HIV, human immunodeficiency virus; CI, confidence interval; GIT, gastrointestinal tract.
Discussion
The effectiveness of pre-donation screening in ensuring donor and recipient safety must be balanced against blood supply sufficiency. This study is the first to evaluate the role of questioning for ARS, specifically the combination of rash and lymphadenopathy, as a screening tool for HIV infection. We found that no donors who declared possible ARS and subsequently donated tested HIV-positive; and no donor testing HIV-positive who experienced ARS reported rash and lymphadenopathy in combination. Fever was the most commonly reported symptom but is too non-specific to be considered for inclusion in the DQ. These results suggest questioning donors for possible ARS has limited utility in the Australian population. Questioning for ARS does not effectively detect HIV, does not protect the blood supply from HIV, and also has a small negative impact on blood supply sufficiency.
The purpose of the ARS question and subsequent deferral is to prevent transfusion-transmission of HIV. The likelihood of acute HIV infection in people with ARS-like symptoms is low, ranging between 1–2.5% in USA (19) and UK (20) studies. Given the Australian prevalence of HIV (0.1%) is lower than in the USA or UK (21), and is even lower in Australian blood donors (1.05 per 100,000 donations), the likelihood of acute HIV infection in Australian blood donors with ARS-like symptoms is negligible. Based on our analyses, the majority of donors receiving temporary deferrals for ARS-like symptoms are highly unlikely to pose a threat to blood transfusion safety in Australia. We could postulate that the ARS question may be of greater relevance in populations with a higher prevalence of HIV, or in countries without access to HIV NAT.
Most HIV-related questions on the DQ identify high-risk behaviours for HIV exposure. The ARS question is an exception, instead identifying symptoms of possible infection. Consistent with previous research, our results show that signs and symptoms of ARS cannot reliably identify donors with acute HIV infection (19). Combined with the knowledge that contemporary HIV testing methods can detect HIV prior to ARS symptoms developing, there is strong evidence to support removal of the question (6,12).
Understanding the impact of deferral on donor return is important. This study found a small number of donors declare ARS-like symptoms on the DQ, with more than half of these deferred, leading to a small loss of donors. This is consistent with prior research showing deferrals negatively impact future blood donation, and temporarily deferred donors often never donate again (1,2,22,23). This study grouped similar deferrals to assess the relationship between deferral type and donor return. It was found that donors deferred for medical reasons or a bite or scratch were the most likely to return. ARS-deferred donors were significantly less likely to return to donate than all other deferral groups with the exception of MSM. Despite no studies having compared these specific deferral groups previously, the result that “health incident” related deferrals (i.e., medical, bite or scratch) are more likely to return than potentially modifiable “social risk factor” related deferrals (i.e., incarceration in prison, sexual risk factors) is in keeping with previous studies comparing different deferral types (1,2,23). Reasons for non-return after temporary deferral have been considered previously (24), although it is unclear why donors deferred for social risk factors are less likely to return than other deferral types. We could speculate that social risk factors are more likely to reoccur. For example, MSM-deferred donors may be less likely to return due to ongoing MSM behaviour and self-deferral. However, the reoccurrence of ARS-like symptoms seems unlikely. We propose that social stigma and feelings of deferral unjustness may play a role in boycotting donation in ARS-deferred donors. Other reasons for non-return include that donors may misinterpret the ARS deferral as permanent, misinterpret that any illness requires self-deferral, or may actually have HIV-infection and self-defer.
This study has limitations. Firstly, demographic factors that may influence donation patterns, including donor status, age, education and nationality were not assessed. Secondly, an unavoidable consequence of the study design prevented follow-up of ARS-deferred donors who did not return. It is possible these donors did not return due to HIV infection. Thirdly, due to differences in databases and data availability, deferred and non-deferred donors who declared ARS were compared over different donation periods. This may have introduced potential confounding factors, for example shorter follow up data from the MODB may have resulted in underestimation of the return rate of donors not deferred for ARS (although this group already had a higher return rate). Finally, an analysis of other predictors for HIV infection was not conducted, as no data were available for risk factors in donors without HIV. This type of analysis could be conducted in the future to investigate whether new questions reflecting emerging risk factors for HIV infection would increase the sensitivity of the DQ.
Conclusions
This study is the first to comprehensively evaluate the role of questioning for ARS as a screening mechanism for HIV infection. Pre-donation health questioning is integral in protecting blood recipients, however this must be balanced against the demand for blood. It is predicted that worldwide blood supply shortages will become more common in future (25,26), driven by the aging population (27). Innovation of blood service protocols worldwide is currently being considered, and will ensure that blood donation safety and supply is optimized to prevent blood shortages. This study demonstrates little remaining utility of continuing to question donors for ARS-like symptoms, particularly in countries like Australia where the incidence of HIV is low. Given the effectiveness of the current DQ in Australia, any changes require careful consideration. However, the continued inclusion of the ARS question on the DQ is unlikely to be of benefit. Future research could focus on quantifying the impact of other deferral types on blood safety and supply and optimizing pre-donation screening methods to prevent blood shortages.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://aob.amegroups.com/article/view/10.21037/aob-23-16/rc
Data Sharing Statement: Available at https://aob.amegroups.com/article/view/10.21037/aob-23-16/dss
Peer Review File: Available at https://aob.amegroups.com/article/view/10.21037/aob-23-16/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aob.amegroups.com/article/view/10.21037/aob-23-16/coif). R.L.P.F. serves as an unpaid editorial board member of Annals of Blood from May 2022 to April 2024. H.M.F. serves as an unpaid editorial board member of Annals of Blood from February 2022 to January 2024. C.E.S. reports to be an associate investigator on a partnership grant between the Kirby Institute UNSW and Australian Red Cross Lifeblood. This analysis, project and manuscript is not covered by the grant. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All data were de-identified (anonymized). Ethical approval was obtained from the Lifeblood Human Research Ethics Committee (reference No. 2015#15) and the University of Queensland School of Medicine Low Risk Ethics Review Panel (clearance No. 2015-SOMILRE-0149). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Every DQ includes a declaration from the donor consenting to the use of their information and donation for research purposes once approved by a relevant Human Research Ethics Committee.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Custer B, Schlumpf KS, Wright D, et al. Donor return after temporary deferral. Transfusion 2011;51:1188-96. [Crossref] [PubMed]
- Custer B, Chinn A, Hirschler NV, et al. The consequences of temporary deferral on future whole blood donation. Transfusion 2007;47:1514-23. [Crossref] [PubMed]
- Wood E, Kerr T, Rowell G, et al. Does this adult patient have early HIV infection?: The Rational Clinical Examination systematic review. JAMA 2014;312:278-85. [Crossref] [PubMed]
- Shaw GM, Hunter E. HIV transmission. Cold Spring Harb Perspect Med 2012;2:a006965. [Crossref] [PubMed]
- Kirby Institute, UNSW Sydney, Australian Red Cross Lifeblood. Transfusion-transmissible infections in Australia: 2022 Surveillance Report. 2022. Available online: https://www.kirby.unsw.edu.au/sites/default/files/documents/Transfusion-transmissible-infections-in-Australia-Surveillance-Report-2022.pdf
- Schacker T, Collier AC, Hughes J, et al. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med 1996;125:257-64. [Crossref] [PubMed]
- Vanhems P, Routy JP, Hirschel B, et al. Clinical features of acute retroviral syndrome differ by route of infection but not by gender and age. J Acquir Immune Defic Syndr 2002;31:318-21. [Crossref] [PubMed]
- Chu C, Selwyn PA. Diagnosis and initial management of acute HIV infection. Am Fam Physician 2010;81:1239-44. [PubMed]
- Kassutto S, Rosenberg ES. Primary HIV type 1 infection. Clin Infect Dis 2004;38:1447-53. [Crossref] [PubMed]
- Wylie BR. Transfusion transmitted infection: viral and exotic diseases. Anaesth Intensive Care 1993;21:24-30. [Crossref] [PubMed]
- Klarkowski D, O'Brien DP, Shanks L, et al. Causes of false-positive HIV rapid diagnostic test results. Expert Rev Anti Infect Ther 2014;12:49-62. [Crossref] [PubMed]
- The Kirby Institute. Transfusion-Transmissible Infections in Australia: 2014 Surveillance Report. The Kirby Institute, The University of New South Wales and Australian Red Cross Blood Service; 2014. Available online: https://www.kirby.unsw.edu.au/sites/default/files/documents/SERP_2014-Australian-Blood-Donors-Surveillance-Report.pdf
- Kamel HT, Bassett MB, Custer B, et al. Safety and donor acceptance of an abbreviated donor history questionnaire. Transfusion 2006;46:1745-53. [Crossref] [PubMed]
- Schroyens N, Borra V, Compernolle V, et al. Men who have sex with men and risk for transfusion-transmissible infections in blood donors in Western countries: A systematic review update. Vox Sang 2023;118:709-20. [Crossref] [PubMed]
- Gemelli CN, Thijsen A, Van Dyke N, et al. Notifying donors when their deferral is ending: An effective donor retention strategy. Transfusion 2021;61:2930-40. [Crossref] [PubMed]
- The Kirby Institute. Safe blood – a focus on education, epidemiology and testing. Transfusion-transmissible infections in Australia: 2011 Surveillance Report. The University of New South Wales and Australian Red Cross Blood Service; 2011. Available online: https://www.lifeblood.com.au/sites/default/files/resource-library/2021-12/185.-safe_blood_a_focus_on_educationepidemiology_and_testing_8940.pdf
- The Kirby Institute, The University of New South Wales and Australian Red Cross Blood Service. Transfusion-transmissible infections in Australia: 2012 surveillance report. 2012. Available online: https://www.kirby.unsw.edu.au/sites/default/files/documents/SERP_2012-Australian-Blood-Donors-Surveillance-Report.pdf
- The Kirby Institute. Transfusion-transmissible infections in Australia: 2013 Surveillance Report. The University of New South Wales and Australian Red Cross Blood Service; 2013. Available online: https://www.kirby.unsw.edu.au/sites/default/files/documents/SERP_2013-Australian-Blood-Donors-Surveillance-Report.pdf
- Pincus JM, Crosby SS, Losina E, et al. Acute human immunodeficiency virus infection in patients presenting to an urban urgent care center. Clin Infect Dis 2003;37:1699-704. [Crossref] [PubMed]
- Hsu DT, Ruf M, O'Shea S, et al. Diagnosing HIV infection in patients presenting with glandular fever-like illness in primary care: are we missing primary HIV infection? HIV Med 2013;14:60-3. [Crossref] [PubMed]
- The Kirby Institute. HIV, viral hepatitis and sexually transmissible infections in Australia Annual Surveillance Report 2015. Sydney, NSW, Australia: The Kirby Institute, UNSW; 2015. Available online: https://www.fast-trackcities.org/sites/default/files/Kirby%20Institute%202015%20Annual%20Surveillance%20Report%20of%20HIV,%20Viral%20Hepatitis,%20STIs.pdf
- Halperin D, Baetens J, Newman B. The effect of short-term, temporary deferral on future blood donation. Transfusion 1998;38:181-3. [Crossref] [PubMed]
- Zou S, Musavi F, Notari EP, et al. Donor deferral and resulting donor loss at the American Red Cross Blood Services, 2001 through 2006. Transfusion 2008;48:2531-9. [Crossref] [PubMed]
- Hillgrove TL, Doherty KV, Moore VM. Understanding non-return after a temporary deferral from giving blood: a qualitative study. BMC Public Health 2012;12:1063. [Crossref] [PubMed]
- Ali A, Auvinen MK, Rautonen J. The aging population poses a global challenge for blood services. Transfusion 2010;50:584-8. [Crossref] [PubMed]
- Williamson LM, Devine DV. Challenges in the management of the blood supply. Lancet 2013;381:1866-75. [Crossref] [PubMed]
- Greinacher A, Weitmann K, Lebsa A, et al. A population-based longitudinal study on the implications of demographics on future blood supply. Transfusion 2016;56:2986-94. [Crossref] [PubMed]
Cite this article as: Colbran RE, Dean MM, Harley RJ, Flower RLP, Shuttleworth G, Styles CE, Faddy HM. Making every question count: the impact of temporary donor deferral for suspected acute retroviral syndrome. Ann Blood 2024;9:2.


