
Cardiovascular genetics: a super-bright or over-hyped perspective?
Cardiovascular disease (CVD), which conventionally includes the three major ischemic pathologies ischemic heart disease (IHD), stroke and peripheral occlusive artery disease (PAOD), is still the first cause of mortality and morbidity worldwide. According to the Mortality Database of the World Health Organization (WHO) (1), IHD and stroke account for up to 27% of all deaths around the world, with a mortality rate that is approximately 5-fold higher than the second cause (i.e., lower respiratory infections; 5.7%). Even more importantly, although the worldwide annual death rate has increased by 8% during the past 15 years (i.e., from to 52.1 to 56.4 million deaths), the annual mortality rate for these two conditions has instead dramatically risen by 22% (i.e., from 12.3 to 15.0 million deaths).
The pathogenesis of CVD is complex and multifaceted, involving a kaleidoscope of genetic, epigenetic, phenotypic and environmental factors. Although the last update was published more than 15 years ago, the Adult Treatment Panel (ATP) of the National Cholesterol Education Program (NCEP) is the most widely used document providing scientific rationale for CVD prevention (2). The ATP III identified a number of major risk factors, including high values of total cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, and low values of high-density lipoprotein (HDL) cholesterol, along with hypertension, cigarette smoking, advanced age, metabolic syndrome and premature history of IHD, which should all be especially targeted for lowering the risk of CVD events. Minor risk factors, which can only enhance the baseline CVD risk in specific subsets of subjects include lipoprotein remnants, lipoprotein(a), small LDL particles, HDL subspecies, homocysteine, thrombogenic/hemostatic factors. Although the NECP recognizes that genetic factors may play an important role in modulating the CVD, no specific recommendations were made regarding the role that genetic testing may have in influencing risk assessment, despite the potential that it may indeed have a pivotal role. Interestingly, the US Preventive Services Task Force has also concluded that the current evidence is not sufficient for weighting the balance between benefits and harms of analysing nontraditional markers of IHD, thus including genetic testing (3).
Although the conclusions of these two important expert panels are seemingly in accordance to discourage widespread and deregulated genetic testing for cardiovascular risk assessment, they both recognize that positive “family history” remains a major risk factor. A large part of the family history is seemingly influenced by a plethora of environmental aspects (i.e., diet, lifestyle, environmental pollution). However, we also cannot discount the fact that genetics will define many biological signatures, which can be inherited from parents and the transferred to their children. Therefore, it is now virtually undisputable that genetics may indeed play a certain role in modulating individual cardiovascular risk (4).
In a recent article published by Jaiswal et al. in the New England Journal of Medicine (5) and including four case-control studies totaling 4,726 patients with IHD and 3,529 controls, the authors found that subjects with clonal hematopoiesis of indeterminate potential (CHIP) have a 4-fold higher risk of myocardial infarction than noncarriers. CHIP reflects a disorder of hematopoietic cells caused by somatic mutations acquired during aging, which then promote clonal expansion up to development of myeloid or lymphoid leukaemias (6). The somatic mutations most frequently associated with CHIP are those involving the ASXL1, DNMT3A, TET2, TP53, BCORL1, GNAS, SF3B1 and JAK2 genes, with those occurring in the first three such genes being detected in the vast majority (i.e., approximately 95%) of CHIP cases. A reasonable perspective has also been provided to justify the association between CHIP and CVD, entailing an increased production of some proinflammatory cytokines (e.g., interleukin-8 and interleukin-1β) by macrophages bearing somatic mutations in CHIP-associated genes, which would then promote the adhesion of mononuclear cells to the inflamed endothelium and thus fostering atherogenesis (7). This plausible theory would lead to plan development of future studies with anti-inflammatory drugs in patients with CHIP, to define whether targeting pro-inflammatory pathways may be beneficial in affected patients.
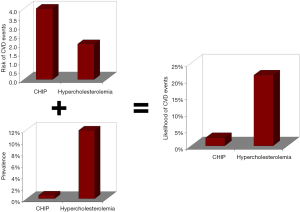
Albeit we may be persuaded to think that the results published by Jaiswal et al. (5) should be regarded as “glittering” findings, since factors associated with a 4-fold enhanced risk of developing future CVD events are perceptively remarkable (e.g., hypercholesterolemia is associated with a ~2-fold enhanced risk of IHD) (2), a detailed analysis of their findings should on the other hand lead to dampen this enthusiasm. In fact, although the 4-fold enhanced risk of CVD events attributable to CHIP remains a virtually unquestionable milestone, the prevalence of this condition is extremely low in both IHD patients (i.e., 2.1%) and in the general population (i.e., 0.6%). Quite different figures have been recently published by the American Heart Association (AHA) regarding hypercholesterolemia, which has an estimated prevalence of ~12% in the US general population (8). If we simply translate these data into post-test probabilities, the likelihood of developing CVD events remains nearly 10-fold higher for hypercholesterolemia than for CHIP (i.e., 21.3% versus 2.4%) (Figure 1). This pragmatic analysis fosters the question as to whether screening for CHIP, along with other putative genetic determinants of CVD, is actually clinically reasonable and economically sustainable by many healthcare systems still plagued by shortage of public funding. Essentially, whilst the biological and clinical effectiveness of managing hypercholesterolemia with statins or other drugs is now straightforward and proven effective when targeting the right population (9), the clinical management of patients with CHIP remains unexplored territory to date. To put it simply, will cardiovascular genetics foster a super-bright perspective, especially considering the discovery new triggers of CVD, or is this news simply an over-hyped perspective in the ongoing challenge toward establishing effective CVD prevention? Only time will tell.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Blood. The article did not undergo external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob.2018.02.03). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the manuscript and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organization. WHO Mortality Database. Available online: http://www.who.int/healthinfo/mortality_data/en/
- . National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143-421. [PubMed]
- U.S. Preventive Services Task Force. Using nontraditional risk factors in coronary heart disease risk assessment: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2009;151:474-82. [Crossref] [PubMed]
- Montagnana M, Danese E, Lippi G. Genetic risk factors of atherothrombosis. Pol Arch Med Wewn 2014;124:474-82. [PubMed]
- Jaiswal S, Natarajan P, Silver AJ, et al. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N Engl J Med 2017;377:111-21. [Crossref] [PubMed]
- Steensma DP, Bejar R, Jaiswal S, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 2015;126:9-16. [Crossref] [PubMed]
- Keaney JF Jr. CHIP-ping Away at Atherosclerosis. N Engl J Med 2017;377:184-5. [Crossref] [PubMed]
- Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017;135:e146-e603. Erratum in: Circulation 2017;135:e646 Circulation 2017;136:e196. [Crossref] [PubMed]
- Lippi G, Plebani M. Statins for Primary Prevention of Cardiovascular Disease. Trends Pharmacol Sci 2017;38:111-2. [Crossref] [PubMed]
Cite this article as: Lippi G, Favaloro EJ. Cardiovascular genetics: a super-bright or over-hyped perspective? Ann Blood 2018;3:18.


