
Diagnosis and management of von Willebrand disease in Italy
Introduction
von Willebrand disease (VWD) is a frequent autosomally inherited bleeding disorder due to the reduction or functional abnormality of von Willebrand factor (VWF). Clinical and laboratory phenotypes associated with VWD are very heterogeneous and correct diagnosis is not always easily feasible unless the patient is referred to specialized centers (1,2). In Italy, early awareness of VWD led to relevant publications in the 70’s illustrating the different laboratory and clinical phenotypes associated with the disorder (3,4). This paved the way for the development of an established and qualified expertise in the diagnosis and management of this bleeding disorder in a few centers. In 1977, for the first time desmopressin, a pharmacological agent not derived from plasma fractionation, was used in Italy and demonstrated to be successful in preventing and/or treating bleeds in patients with VWD (5). In 1987, an Italian study for the first time demonstrated that the prevalence of VWD in the general population was significantly higher than previously believed (6).
However, despite improved knowledge about VWD and the guidelines published a few years ago (7-10), specific studies have mainly gathered well-characterized selected cases allowing the identification of predictors of bleeding according to type and laboratory characteristics (11-14). The large majority of cases classified as “mild” type 1 VWD patients remain often not well characterized with significant risk of mislabeling as VWD patients, subjects with low VWF and mild/negligible bleeding history, and without a true genetic disorder (15,16). As a whole, the National Italian registry of inherited bleeding disorders is particularly dedicated to patients with hemophilia and data on VWD are often not as accurate as those on hemophilia patients.
In Italy, an Institutional registry on inherited bleeding disorders has been established since the late 1980s. Furthermore, a retrospective registry on VWD, named the Registry of National von Willebrand disease (RENAWI) was organized in Italy in 2002. The results have been recently published (17) and this population represents the largest well characterized sample of Italian patients with VWD selected from the Italian registry on inherited bleeding disorders, and followed-up. In this paper, we will summarize the data from both registries to highlight achievements and unresolved issues related to VWD in Italy.
Patients’ registries
AICE registry
In Italy, the Italian Association of Hemophilia Centers (AICE) has maintained a general registry on hemophilia since the late 1980s and mainly focused on blood borne infections observed, especially in hemophiliacs, until late 1990s. Clinical and laboratory parameters on VWD have been collected during the same period from 48 Hemophilia Centers and general data on VWD have been published and updated within the registry on Hemophilia and Allied Disorders (18). Data on type of treatment and requirements for therapeutic plan are also included, but reporting type and frequency of bleeding episodes is left to the discretion of physician in charge. To compare the work in progress with the registry, a 2017 update is presented, along with that of 2010 (Table 1).
Table 1
| Patients and VWD type | RENAWI | AICE 2010 | AICE 2017 |
|---|---|---|---|
| Numbers of participating Hemophilia Centers | 16 | 48 | 48 |
| Patients enrolled in the registry | 1,234 | 2,285 | 3,196 |
| Classification of total VWD included (%) | |||
| VWD1 | 779 (63.1) | 1,773 (77.6) | 2,387 (74.7) |
| VWD2A | 87 (7.1) | 73 (3.2) | 307 (9.6) |
| VWD2B | 73 (5.9) | 113 (4.9) | 164 (5.1) |
| VWD2M | 219 (17.7) | 164 (7.2) | 172 (5.4) |
| VWD2N | 10 (0.8) | 40 (1.8) | 51 (1.6) |
| VWD3 | 66 (5.3) | 122 (5.3) | 115 (3.6) |
RENAWI
In the case of VWD patients, the Italian Ministry of Health fostered a specific registry to collect detailed information about VWD types requiring the use of therapeutic agents such as desmopressin (DDAVP) and/or VWF/FVIII concentrates. Data of RENAWI were collected from January 2002 throughout December 2005. Investigators of each participating Center received a computerized program and were trained to include VWD patients into the database. Among many other parameters, information was collected on: (I) VWD types with age-sex distribution; (II) type of bleeding episodes; (III) identification of VWD cases responsive to desmopressin; (IV) number of patients treated with DDAVP only and/or those with VWF/FVIII concentrates. 16/48 (33%) Centers enrolled patients into RENAWI. After careful evaluation of the patients registered and in some cases centralized retesting, 275 cases were excluded mainly because of incorrect diagnosis (n=226). Eventually in 2007, 1,234 cases were available for the second analysis and for testing VWF molecular defects. The number of participating centers and patients enrolled in these registries are summarized in Table 1.
Laboratory methods
An array of tests are available in Italy to the laboratories diagnosing patients with VWD. Bleeding Time (BT) by the Simplate method has been widely used in the past but nowadays it is seldom used as a screening test. Factor VIII (FVIII) is assayed by one-stage coagulometric assay, VWF:Ag by commercial kits and home-made ELISA using polyclonal antibodies against VWF. VWF:RCo is either measured by aggregometry using formalin-fixed platelets from normal donors or by using the more sensitive ELISA-VWF:RCo assay. von Willebrand factor collagen binding assay (VWF:CB) with different in-house and/or commercial kits are available in a few centers. Ristocetin induced platelet aggregation (RIPA) is carried out in platelet rich plasma (PRP) by aggregometry and values are expressed in terms of the minimal concentration of ristocetin giving at least 30% increase in transmission (the normal range is usually 0.8–1.2 mg/mL ristocetin). VWF propeptide is seldom measured to identify patients with shortened VWF half-life. VWF multimeric pattern is evaluated in patients’ plasma by low-resolution gel electrophoresis and is available in 4 centers. Figure 1 summarizes the suggested diagnostic flow-chart in presence of all laboratory methods, available in few centers in Italy (Figure 1) (19).
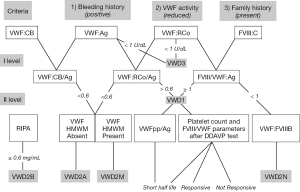
For molecular diagnosis, extracted genomic DNA undergoes to polymerase chain reaction (PCR) performed to amplify the whole VWF gene coding and intron-exon boundaries. Sequence analysis is usually performed by Sanger methodology. This diagnosis is currently available in four centers only.
Criteria for diagnosis of VWD types
AICE registry
No specific restrictive criteria are indicated for the diagnosis, made by each center and it is accepted bona fide without central checking unless specifically required for multicenter studies. However, the recommendations of International Society of Thrombosis and Haemostasis (ISTH) are suggested as a template for the diagnosis (1).
RENAWI
Patients were included if they fulfilled all the criteria recommended by the ISTH, translated into diagnostic flow chart in its updated version.
Diagnosis of VWD1 was based on the VWF:RCo (upper normal limit for non-O blood group =60 U/dL) and VWF:Ag plasma levels (upper normal limit for non-O blood group =68 U/dL) and a complete set of multimers in plasma in at least one member of the same family. According to definition of VWD1, characterized by reduced levels of normal VWF, VWF:RCo and VWF:Ag values were equally reduced with a VWF:RCo/Ag ratio >0.6, as determined in 1,200 normal individuals in the European Project entitled Molecular and Clinical Markers on Diagnosis and Management of type 1 VWD (20). VWD1 patients showed all the three criteria (bleeding history, familial bleeding history and lower VWF:RCo levels) recently proposed (21).
Participating Centers were requested to review their diagnosis several times by repeating assays at their local laboratories and in most cases the final diagnosis was eventually confirmed by the coordinating center by using also multimeric analysis and mutations of the VWF gene. DNA was available in all affected members of VWD2A, VWD2B, VWD2M, VWD2N and in most VWD3 (82%) and VWD1 (60%). Most identified mutations had been previously recorded in the ISTH VWF database (www.vwf.group.shef.ac.uk; accessed on 10 November 2017).
Use of DDAVP and/or VWF/FVIII concentrates
AICE registry and RENAWI
Information about the infusion trial with DDAVP and the use of DDAVP and/or VWF/FVIII concentrates are also included in the national Registry. Each VWD patient usually receives a therapeutic program indicating the dosage and brand name of the VWF/FVIII concentrate to be used for personal treatment and of desmopressin when indicated following the evaluation of an infusion-trial.
Results
Demographic, clinical and laboratory data of the VWD cohort
AICE Registry
A total of 3,196 patients with VWD are included in the Registry as of October 2017 (Table 1). VWD types have been classified (%) as follows: VWD1=2,387 (74.7), VWD2A=307 (9.6), VWD2B=164 (5.1), VWD2M=172 (5.4), VWD2N=51 (1.6) and VWD3=115 (3.6). The demographic, clinical and lab data of the 3,196 cases included in the update version of the Italian Registry are summarized in Table 2. Females are more prevalent (58% vs. 42%), with the largest difference being observed for VWD1 and VWD2B, while they are equal to men in VWD2M. Median age are similar across the different types. All the 48 centers contributed to registry. Laboratory data substantially overlapped those reported also in the RENAWI, with some patients tested during prophylactic treatment and thus showing higher levels of VWF especially (Table 2).
Table 2
| Characteristics | VWD1 | VWD2A | VWD2B | VWD2M | VWD2N | VWD3 |
|---|---|---|---|---|---|---|
| Case number (%) | 2,387 | 307 | 164 | 172 | 51 | 115 |
| Gender M/F (%) | 41/59 | 48/52 | 40/60 | 50/50 | 42/58 | 43/57 |
| Median age [range] | 43 [1–95] | 46 [2–96] | 45 [2–91] | 51 [6–95] | 46 [8–84] | 40 [1–84] |
| VWF: RCo (U/dL) | 12 [1–137] | 6 [2–59] | 26 [6–125] | 7 [1–73] | 84 [20–156] | <3 [<3–30] |
| VWF:Ag (U/dL) | 39 [2–183] | 29 [2–105] | 43 [4–160] | 20 [1–123] | 91 [36–165] | 1 [<1–47] |
| FVIII:C (U/dL) | 35 [1–138] | 27 [2–110] | 28 [6–78] | 12 [2–46] | 31 [1–118] | 2 [<1–21] |
RENAWI
The data obtained by RENAWI have been collected using a specific database in only 16/48 Centers Italian Centers currently participating to the AICE registry, although these were following the majority of the Italian VWD population (at that time 1,529/2,420, 63%). A total of 1,234 patients have been centrally and carefully re-evaluated for VWD diagnosis in the RENAWI (Table 1). VWD types could be identified (family n; %) as follows: VWD1=779 (279; 63), VWD2A=87 (31; 7), VWD2B=73 (22; 6), VWD2M=219 (67; 18), VWD2N=10 (5; 1) and VWD3=66 (47; 5). Women were more frequently diagnosed among all VWD (58%), especially in milder types (VWD1=60%) while they were equal to men in VWD2A (48%), VWD2N (50%) and VWD3 (51%). Patients had a median age of 32 years even though VWD2N (20 yrs) and VWD3 (28 yrs) cases were diagnosed at younger ages. All these data have been previously published (17).
Clinical use of DDAVP and/or VWF/FVIII concentrates
AICE registry
Information was collected about the number of patients exposed to DDAVP and VWF/FVIII concentrates during the last 5 years, along with therapeutic plans released to the patients for home treatment or treatment at other sites (Table 3). Desmopressin was largely used in VWD1 and in very few cases of VWD2A, VWD2M, and VWD2N. VWF/FVIII concentrates were largely used in VWD3 and VWD2. Interestingly, at least 17 patients with VWD3, but also 20 with VWD1 and to a lesser extent patients with all VWD2 except VWD2N underwent secondary prophylaxis during the 5-year observation period. Only three patients with VWD3 started primary prophylaxis during the same period.
Table 3
| VWD types | VWF/FVIII concentrates | VWF/FVIII concentrates + DDAVP | DDAVP Only | Total | Therapeutic plans |
|---|---|---|---|---|---|
| vWD 1 | 60 | 57 | 126 | 243 | 582 (on demand) 24 (prophylaxis) |
| vWD 2A | 31 | 10 | 4 | 45 | 81 (on demand) 11 (prophylaxis) |
| vWD 2B | 21 | – | 1 | 22 | 48 (on demand) 7 (prophylaxis) |
| vWd 2M | 4 | 10 | 3 | 17 | 29 (on demand) 3 (prophylaxis) |
| vWd 2N | 2 | – | 3 | 5 | 15 (on demand) |
| vWD 3 | 37# | – | – | 37 | 30 (on demand) 20 (prophylaxis) |
#, 1 patient with inhibitor treated with recombinant FVIII.
RENAWI
The data reported in the RENAWI were not significantly different, but they were more accurate owing to the specific aims of the registry. In particular, 463/1234 (36%) were treated during the 12 months prior to enrollment with VWF/FVIII concentrates and 376/1234 (30%) with desmopressin (17). From this original population, 796 cases were followed-up to assess the influence of bleeding history and laboratory parameters in predicting clinical outcomes and the need for replacement therapy (14).
Molecular diagnosis of VWD patients
The most frequent molecular defects found in Italian VWD patients are summarized in Table 4. As far as VWD1 is concerned, a relatively large number of patients (at least 127) carry the mutation R1205H, typically observed in VWD1 “Vicenza”. Among other frequent gene defects observed in the Italian VWD1 the following molecular abnormalities could be identified: small deletion or insertions (D1277/E78delinsE), missense (C1130F/R, A1716P, V1822G, R2318H, C2362F, S2469P) or nonsense (Q1475X, R1779X) mutations. In VWD3, the molecular abnormalities were heterogeneous and large deletions, small deletions and insertions, nonsense, splice site were all documented in the Italian population of VWD patients, along with some missense mutations. In general, no particular founder effect is evident in Italian type 3 patients, although a few with mutations reported in North Europe as founder mutations have been identified (e.g., 2435delC, Arg2535*) (22). This pattern refers to patients with VWF:Ag <1 U/dL as a result of the effect of null alleles. Of note, seven VWD3 patients characterized by homozygous large gene deletions had at least one episode of life-threatening anaphylactic reaction in their lifetime (23-25). Interestingly, a number of patients were identified who do not strictly fulfill the criteria for VWD3 diagnosis, although previously reported in this category (Table 5), but with a clear recessive inheritance and relevant bleeding history from early infancy (26,27).
Table 4
| VWD types | Localization of VWF defects | VWF Mutations associated with specific types |
|---|---|---|
| VWD2N | D’–D3 domains | R816W/Q; R854Q/W; R924Q; C1060R |
| VWD1 Vicenza | D3 domain | R1205H (M740I) |
| VWD2A (formerly IIE) | C1130F/R; C1143Y; C1173R | |
| VWD2B | A1 domain | P1266L/Q; H1268D; R1306Q/L/W; R1308C/P; I1309V; V1316M; P1337L; R1341Q/W |
| VWD2M/2A | A1 domain | R1315C/L; Y1321C/W; R1374C/H |
| VWD2A | A2 domain | S1506L; S1543F; R1597W; V1607D; G1629R; G1631D; V1665E; Y1584C |
| VWD1 | A3 domain | A1716P; V1822G |
| VWD1 | CK and other domains | R2313H; C2362F; S2469P Q2520P |
| VWD3# | Whole subunit | Gene deletion, IVS46+1G>T; delCTCTc.2016-219; c.2435del C; c.6182delT aa206Frameshift; c.2908delC; c.4470delC; R1659*; Q2544*; R2535*; IVS25+1 G>T; c.3179delG; c.4944Del T / IVS 18-1 G>C |
#, For type 3 VWD are summarized “null” mutation detected in at least 3 patients.
Table 5
| Family | Age at diagnosis, years | VIII:C, IU/dL | VWF:Ag, IU/dL | VWF:RCo, IU/dL | Bleeding time (min) | Blood group | VWF mutation |
|---|---|---|---|---|---|---|---|
| T | 3 | 20–31 | 4–6 | <3–6 | >30 | A | Q77X/1534-3 C>A splice site intron13 |
| C | 2 | 7–11 | 4–7 | <3 | > 15 | O | R365X/S1731T |
| G | 2 | 5–16 | 3–5 | <3 | >15 | A | R1315C |
| S | 4 | 25–37 | 10–12 | <3 | 15– >20 | O | 4699delG/Y1584C |
| M | 7 | 19–44 | 2–5 | <3 | >30 | A | C2362F/C2362F |
| Va | 1 | 12–27 | 3–5 | <3 | ND | O | C2362F/C2362F |
| B | 1 | 8–21 | 0.5–2 | <3 | >30 | O | C2362F/7375insC |
| V | 1 | 10–21 | 1–5 | <3 | >30 | O | C2362F/1534-3 C>A splice site intron 13 |
| DZ | 3 | 18–24 | 5–7 | <3–6 | >15 | B | C2362F/1534-3 C>A splice site intron 13 |
| DZ | 1 | 18–19 | 6–7 | <3–6 | >15 | A | C2362F/1534-3 C>A splice site intron13 |
| F | 1 | 25 | 8 | 6 | >15 | O | C2362F/1534-3 C>A splice site intron13 |
| Z | 1 | 8–21 | 1–4 | <3 | >30 | A | C2362F/R2535X |
| P | 1 | 14–21 | 3–8 | <3 | >15 | B | C2362F/2908del C |
| A | 9 | 10–19 | 1.8–3 | <3 | >30 | O | C2671Y/gene deletion |
| I | 4 | 18–33 | 5–6 | <3 | 20 | O | C2671Y/W2193R |
| Normal range | – | 52–173 | 47–165 | 51–188 | <7.5 | – | NO |
Discussion
The AICE registry is still increasingly reporting new patients with VWD. This is due to several reasons, in particular because of priority assigned in the past to hemophilia patients, the paucity of symptoms in the majority of VWD patients so that they are registered at time of bleeding, and the incomplete widespread laboratory methods availability to classify correctly VWD patients. No attempt to validate the diagnosis made at individual center has been made for cases reported to AICE Registry and this could prevent to achieve an accurate figure of the different type prevalence across the Country. In the RENAWI, 400 patients who showed borderline VWF levels in plasma were re-tested using not only more standardized assays for VWF:RCo and VWF:Ag but also multimer analyses and VWF:FVIIIB assays as recommended. After this re-evaluation, 295/1,529 (19%) did not meet criteria for VWD diagnosis and therefore were excluded from any further analyses (14). These findings suggest that a significant proportion of patients with type 1 VWD included in the AICE registry could have their diagnosis not confirmed, thus reducing especially the prevalence of type 1 VWD, as observed in the RENAWI register (Table 1) after detailed re-evaluation (74.7% vs. 63%) (17).
The most severe VWF:RCo/Ag ratio reduction was observed as a whole in VWD2A patients and VWD2M (Table 2), but no data are available for VWF:CB in the AICE registry. Thus, we cannot exclude some misdiagnosis of VWD2A instead of VWD2M considering the relatively high number of VWD2A in the AICE registry compared to the RENAWI (Table 2). In the RENAWI all the VWD types were identified appropriately using VWF:RCo/Ag ratios, multimeric analyses and genotypes and this is not easily available in all Italian Centers. VWD2N showed low levels of FVIII with FVIII/VWF:Ag ratio <1 but these were confirmed by the specific VWF:FVIIIB assay. Patients with VWD3 showed undetectable levels of VWF:Ag and very low levels of FVIII. Seven patients from 3 different families developed in their life anti-VWF inhibitors with life-threatening reactions (23,25).
In the RENAWI, mucosal bleedings such as epistaxis, menorrhagia, bleeding from minor wounds, gums bleeds) were more frequently observed than hematomas (13%) and joint bleeding (6%) (14,17). A population of 796 patients from the RENAWI has been followed-up for 1 year by using semi-quantitative evaluation of bleeding symptoms and bleeding history at enrollment was superior to FVIII and VWF assays in predicting clinical outcomes and the need for replacement therapy (14). These results extend those previously obtained in a population of patients with VWD2A and VWD2M (13). Unfortunately, this analysis is not feasible for the AICE registry.
Molecular diagnosis was performed in the majority of affected members with VWD types in the RENAWI and most are included in the AICE registry and the results obtained were useful to determine family groups with specific genotype. We found a very high frequency of the mutation R1205H, with or without M740I typically observed in VWD1 “Vicenza” (28,29) in at least 127 patients. No other mutations seem to have such high frequency in the Italian population. In case of VWD3 patients the molecular abnormalities were heterogeneous and no significant founder effect was identified. However, 7 patients belonging to three different families with large deletion of VWF gene showed the presence of anti-VWF inhibitors, with life-threatening anaphylactic reactions (23-25). Interestingly, it is now clear that several recessive patients with severely reduced but measurable FVIII and VWF levels are present who show milder but still relevant bleeding histories. They usually are homozygous for missense mutations or compound heterozygous for null and missense mutations and may partially respond to desmopressin (26,27) (Table 5). Importantly, at variance with what occurs in VWD3, these patients may have a significant increase of FVIII after desmopressin, which has been successfully used to prevent bleeding during minor surgery (26).
As to the treatment of Italian VWD, the results of RENAWI and AICE registries showed that DDAVP is widely used in most VWD1 patients. Nevertheless, even though DDAVP trial showed a relatively high rate of biological response in the RENAWI registry, the data from AICE registry confirms that not all these patients are treated with DDAVP to manage or prevent bleeding episodes, probably especially in surgical settings where DDAVP protracted administration is accompanied by the risk of tachyphylaxis (30). This appears to represent an important aspect of the management of patients with increased VWF clearance after desmopressin who may need VWF/FVIII concentrates when sustained levels are recommended, as for surgery, despite very good short-lived response to desmopressin (12).
Compared to hemophiliacs, VWD patients certainly have less frequently severe bleeding episodes if not on prophylaxis with the missing factor and thus only a small percentage of VWD cases usually are treated with VWF/FVIII concentrates following spontaneous and/or trauma induced bleedings or for surgery. Nevertheless, a significant proportion of patients with VWD3 were on prophylaxis during the last five years (Table 3) in the AICE registry, in keeping with the significant larger consumption of VWF/FVIII concentrates over a year reported in the RENAWI in these patients as a whole (56% used >50,000 IU VWF:RCo) (17). Type 2A and, to a lesser extent, VWD2B also may use great amounts of concentrates because of prophylaxis. This pattern was especially associated with the presence of recurrent gastrointestinal bleeding, particularly frequent in VWD2A, while joint bleeding was the most prevalent indication in VWD3 (13,31-35 and data not shown). The predictors of bleeds in patients with VWD3 and the need of DDAVP and VWF/FVIII concentrates are currently under evaluation in another prospective study (34).
In conclusion, the information gathered in AICE and RENAWI registries represent important advancements in the knowledge of VWD distribution in Italy. Furthermore, the results of clinical studies deriving from the well characterized population in the RENAWI and by the high participation to the Italian AICE registry, have allowed to understand better the relationships between the type of VWD and the severity of bleeding tendency. However, appropriate phenotypic and genotypic tests are not available in most hemophilia centers and some laboratories are being incorporated in general laboratories. Thus, the risk exists of losing the ability to perform the correct identification and classification of VWD type. The aim of the National Societies dealing with diagnosis and treatment of inherited bleeding disorders is to maintain a high attention to the diagnostic and not only therapeutic problems in this area, to foster multicenter standardization and proficiency testing and to share with patient associations the common scope to prevent that such rare disorders are not timely and adequately diagnosed, treated, and followed-up.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Emmanuel J. Favaloro) for the series “Diagnosis and Management of von Willebrand Disease: Diverse Approaches to a Global and Common Bleeding Disorder” published in Annals of Blood. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob.2018.03.03). The series “Diagnosis and Management of von Willebrand Disease: Diverse Approaches to a Global and Common Bleeding Disorder” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the manuscript and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sadler JE, Budde U, Eikenboom JC, et al. Update on the pathophysiology and classification of von Willebrand disease: a report of the Subcommittee on von Willebrand Factor. J. Thromb Haemost 2006;4:2103-14. [Crossref] [PubMed]
- Federici AB, Mannucci PM. Management of inherited von Willebrand disease in 2007. Ann Med 2007;39:346-58. [Crossref] [PubMed]
- Dini E, Barbui T, Chisesi T, et al. von Willebrand’s disease in Italy. A study of 13 families from a small area in the province of Vicenza. Acta Haematol 1974;52:29-39. [Crossref] [PubMed]
- . Spectrum of von Willebrand’s disease: a study of 100 cases. Br J Haematol 1977;35:101-12. [Crossref] [PubMed]
- Mannucci PM, Ruggeri ZM, Pareti FI, et al. 1-deamino-8-D-arginine vasopressin: a new pharmacological approach to the management of haemophilia and von Willebrand’s disease. Lancet 1977;1:869-72. [Crossref] [PubMed]
- Rodeghiero F, Castaman G, Dini E. Epidemiological investigation of the prevalence of von Willebrand’s disease. Blood 1987;69:454-9. [PubMed]
- Pasi KJ, Collins PW, Keeling DM, et al. The diagnosis of von Willebrand disease: a guideline from the UK Haemophilia Centre Doctors’ Organization. Haemophilia 2004;10:218-31. [Crossref] [PubMed]
- Nichols WL, Hulting MB, James AH, et al. Von Willebrand disease (VWD): evidence-based diagnosis and management guidelines, the national Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA). Haemophilia 2008;14:171-232. [Crossref] [PubMed]
- Mannucci PM, Franchini M, Castaman G, et al. Evidence-based recommendations on the treatment of von Willebrand disease in Italy. Blood Transfus 2009;7:117-26. [PubMed]
- Castaman G, Goodeve A. Eikenboom J on behalf of the European Group on von Willebrand disease (EUVWD). Principles of care for diagnosis and treatment of von Willebrand disease. Haematologica 2013;98:667-74. [Crossref] [PubMed]
- Federici AB, Mannucci PM, Castaman G, et al. Clinical and molecular predictors of thrombocytopenia and risk of bleeding in patients with von Willebrand disease type 2B: a cohort study of 67 patients. Blood 2009;113:526-34. [Crossref] [PubMed]
- Castaman G, Tosetto A, Federici AB, et al. Bleeding tendency and efficacy of anti-haemorrhagic treatments in patients with type 1 von Willebrand disease and increased von Willebrand factor clearance. Thromb Haemost 2011;105:647-54. [Crossref] [PubMed]
- Castaman G, Federici AB, Tosetto A, et al. Different bleeding risk in type 2A and 2M von Willebrand disease: a 2-year prospective study in 107 patients. J Thromb Haemost 2012;10:632-8. [Crossref] [PubMed]
- Federici AB, Bucciarelli P, Castaman G, et al. The bleeding score predicts clinical outcomes and replacement therapy in adults with von Willebrand disease. Blood 2014;123:4037-44. [Crossref] [PubMed]
- Castaman G, Eikenboom JC, Bertina RM, et al. Inconsistency of association between type 1 von Willebrand disease phenotype and genotype in families identified in an epidemiological investigation. Thromb Haemost 1999;82:1065-70. [Crossref] [PubMed]
- Sadler JE. Low von Willebrand factor: sometimes a risk factor and sometimes a disease. Hematology Am Soc Hematol Educ Program 2009;106-12. [Crossref] [PubMed]
- Federici AB, Bucciarelli P, Castaman G, et al. Management of inherited von Willebrand disease in Italy: results from the retrospective study on 1234 patients. Semin Thromb Hemost 2011;37:511-21. [Crossref] [PubMed]
- Iorio A, Oliovecchio E, Morfini M, et al. Italian Registry of Haemophilia and Allied Disorders. Objectives, methodology and data analysis. Haemophilia 2008;14:444-53. [Crossref] [PubMed]
- Federici AB. Towards a more automatic and rapid laboratory diagnosis of von Willebrand disease. Thromb Res 2016;141:198-201. [Crossref] [PubMed]
- Goodeve A, Eikenboom J, Castaman G, et al. Phenotype and genotype of a cohort of families historically diagnosed with type 1 von Willebrand disease in the European study, Molecular and Clinical Markers for the Diagnosis and Management of Type 1 von Willebrand Disease (MCMDM-1VWD). Blood 2007;109:112-21. [Crossref] [PubMed]
- Tosetto A, Castaman G, Rodeghiero F. Evidence-based diagnosis of type 1 von Willebrand disease: a Bayes theorem approach. Blood 2008;111:3998-4003. [Crossref] [PubMed]
- Schneppenheim R, Budde U. Phenotypic and genotypic diagnosis of von Willebrand disease: a 2004 update. Semin Hematol 2005;42:15-28. [Crossref] [PubMed]
- Shelton-Inloes BB, Chehab FF, Mannucci PM, et al. Gene deletion correlates with the 21 development of alloantibodies in von Willebrand disease. J Clin Invest 1987;79:1459-65. [Crossref] [PubMed]
- Mannucci PM, Tamaro G, Narchi G, et al. Life-threatening reaction to factor VIII concentrate in a patient with severe von Willebrand disease and alloantibodies to von Willebrand factor. Eur J Haematol 1987;39:467-70. [Crossref] [PubMed]
- Bergamaschini L, Mannucci PM, Federici AB, et al. Posttransfusion anaphylactic reactions in a patient with severe von Willebrand disease: role of complement and alloantibodies to von Willebrand factor. J Lab Clin Med 1995;125:348-55. [PubMed]
- Castaman G, Lattuada A, Mannucci PM, et al. Factor VIII:C increases after desmopressin in a subgroup of patients with autosomal recessive severe von Willebrand disease. Br J Haematol 1995;89:147-51. [Crossref] [PubMed]
- Castaman G, Giacomelli S, Rodeghiero F. Autosomal recessive von Willebrand disease type 1 or 2 due to homozygous or compound heterozygous mutations in the von Willebrand factor gene. A single center experience on molecular heterogeneity and laboratory features in 12 families. Acta Haematol 2009;121:106-10. [Crossref] [PubMed]
- Schneppenheim R, Federici AB, Budde U, et al. Von Willebrand Disease type 2M "Vicenza" in Italian and German patients: identification of the first candidate mutation (G3864A; R1205H) in 8 families. Thromb Haemost 2000;83:136-40. [Crossref] [PubMed]
- Castaman G, Missiaglia E, Federici AB, et al. An additional unique candidate mutation (G2470A; M740I) in the original families with von Willebrand disease type 2 M Vicenza and the G3864A (R1205H) mutation. Thromb Haemost 2000;84:350-1. [Crossref] [PubMed]
- Mannucci PM, Bettega D, Cattaneo M. Patterns of development of tachyphylaxis in patients with haemophilia and von Willebrand disease after repeated doses of desmopressin (DDAVP). Br J Haematol 1992;82:87-93. [Crossref] [PubMed]
- Federici AB, Castaman G, Franchini M, et al. Clinical use of Haemate P in inherited von Willebrand's disease: a cohort study on 100 Italian patients. Haematologica 2007;92:944-51. [Crossref] [PubMed]
- Federici AB. Highly purified VWF/FVIII concentrates in the treatment and prophylaxis of von Willebrand disease: the PRO.WILL Study. Haemophilia 2007;13:15-24. [Crossref] [PubMed]
- Federici AB, Barillari G, Zanon E, et al. Efficacy and safety of highly purified, doubly virus-inactivated VWF/FVIII concentrates in inherited von Willebrand’s disease: results of an Italian cohort study on 120 patients characterized by bleeding severity score. Haemophilia 2010;16:101-10. [Crossref] [PubMed]
- Castaman G, Coppola A, Zanon E, et al. Efficacy and safety during formulation switch of a pasteurized VWF/FVIII concentrate: results from an Italian prospective observational study in patients with von Willebrand disease. Haemophilia 2013;19:82-8. [Crossref] [PubMed]
- See data on line: www.vwd-3winters-ips.com, accessed on November 22, 2017.
Cite this article as: Castaman G, Oliovecchio E, Federici AB. Diagnosis and management of von Willebrand disease in Italy. Ann Blood 2018;3:28.


