
Clinical use and laboratory testing of oral anticoagulation therapy: experience from Finland
Introduction
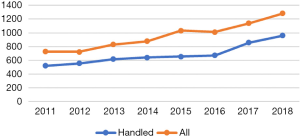
Our Coagulation Disorders Unit was founded 20 years ago. The unit is a tertiary care center within the Helsinki University Hospital, combining clinical and coagulation laboratory services and serving the Helsinki and Uusimaa Hospital District. The unit handles consultation cases for severe thrombosis and/or bleeding tendency, also offering consultation services nationwide. While hemophilias, von Willebrand disease and other bleeding disorders constitute the major part of patient visits to the clinic, thrombosis and consultations regarding the safe use of anticoagulants are frequent and increasing (Figure 1). In a population of around 2 million inhabitants, more than 900,000 coagulation tests, of which 60% represent INR, were performed in our laboratories (HUSLAB) in 2018.
When the direct oral anticoagulants (DOACs) became available from 2008 onwards, initially, we were not confident on the overall safety of these new drugs, in the absence of the monitoring of the drug effect. In the phase III trials of the DOACs, relevant patient groups, such as patients with kidney or liver failure, patients having antiplatelet therapy, patients encountering bleeds and patients with active malignancy were excluded (1). Nevertheless, questions regarding anticoagulation of these vulnerable patients are often encountered, and we could not recommend DOACs for these patients at this early stage. We also, to increase the national awareness, authored an article in the Finnish Medical Society Journal to encourage rational introduction of these new drugs (2), and national web-based guidelines have been gathered (accessible at www.hematology.fi, in Finnish). We were the first to set up specific laboratory assays for DOACs in our country, for dabigatran in 2011, for rivaroxaban in 2012 and for apixaban in 2015. In original studies, we have found that dabigatran may even paradoxically increase thrombin generation (TG) in certain situations, while rivaroxaban and apixaban differ in their TG response, mirroring the increasing DOAC concentration—i.e., once- vs. twice-daily dosing (3,4).
DOACs are gaining new indications, rivaroxaban for prevention of atherothrombosis in coronary artery disease, and several DOACs are being considered in cancer patients as well (5-7). While the use of DOACs is increasing, clinicians will encounter many patients switching from traditional vitamin K antagonists (VKAs) to DOACs, and broad education is required (8). To respond to the challenge of increased repertoire of anticoagulants in patients with risk factors for both thrombosis and bleeding, e.g., elderly patients undergoing vascular surgery, a special anticoagulation clinic was launched in conjunction with the Coagulation Disorders Unit in 2015. One physician and a registered nurse have the primary responsibility of responding to clinical consultations in anticoagulation-related questions in both in- and out-patient settings. A warfarin self-management program was launched as well in collaboration with the laboratory at that time, to provide warfarin guidance and establishing safe therapy in patients with mechanical valvular replacement.
Oral anticoagulants
Direct oral anticoagulants
Dabigatran was the first DOAC to come on market, for thromboprophylaxis after orthopedic surgery with European Medicines Agency (EMA) approval in 2008. Orally administered direct factor Xa inhibitors (FXaI) include rivaroxaban, apixaban, edoxaban and betrixaban (but not in the EU for the last agent). At present, the indications for DOACs dabigatran, rivaroxaban and apixaban include thromboprophylaxis after knee and hip replacement orthopedic surgery, stroke prevention in conjunction with non-valvular atrial fibrillation (AF), treatment of deep venous thrombosis (DVT) or uncomplicated, hemodynamically stable pulmonary embolism (PE) (9). Uniquely for rivaroxaban, prevention of atherothrombotic events in coronary artery disease or peripheral arterial occlusive disease in conjunction with acetylsalicylic acid (ASA) is also recently indicated (5,10). Edoxaban was approved by the EMA for stroke prevention with AF and treatment of DVT and uncomplicated PE in 2015 (11). Betrixaban is not currently available in the European market, but is the sole DOAC with the indication of thromboprophylaxis for medically ill patients (12). The distribution of anticoagulation use is presented in Figure 2. Rivaroxaban is the most used DOAC in Finland at the moment. Recently dabigatran was found to be non-inferior to warfarin in the treatment of cerebral sinus venous thrombosis (RESPECT_CVT, submitted 2019).
Monitoring of DOAC anticoagulation effect is not recommended for routine use, while liver or kidney dysfunction, thrombosis or bleeding breakthrough during treatment, extremes in body weight, suspicion of non-compliance or overdose are situations where measurement is useful. Routine coagulation tests such as prothrombin time (PT) and activated partial thromboplastin time (APTT) are unreliable in assessment of DOAC effect, and their sensitivity depends on laboratory and reagents used (13-16). However, the baseline assessment of PT and APTT are valuable screening tests to obtain an understanding of the liver function prior to initiation of DOACs. Also, in case of severe accumulation of DOAC, both tests depict prolonged coagulation times. The effect and interference of DOACs on the other coagulation factor testing is under continuous discussion and education between clinic and laboratory. Some neutralization strategies to better interpret laboratory tests may be needed.
Specific assays for DOACs are available and in regular use nowadays (Table 1). Thrombin time (TT) is extremely sensitive to dabigatran and can be diluted and calibrated with known drug concentrations, one modification of such is used in our laboratory. Ecarin clotting assay (ECA) based methods have also proven to be accurate (14).
Table 1
| Anticoagulant | Indication | Advantages | Disadvantages | Laboratory measurement |
|---|---|---|---|---|
| Unfractionated heparin, UFH | Anticoagulation during surgical procedures, intensive care, resolution of hemodynamically unstable pulmonary embolism | High therapeutic doses possible, antidote protamine sulfate available | Intravenous or subcutaneous dosing, highest risk of heparin-induced thrombocytopenia (HIT) | APTT, TT, Anti-Xa, ACT |
| Low-molecular weight heparin, LMWH | Anticoagulation during hospitalization, in active cancer, bridging treatment with warfarin, thrombophilias | Dosed subcutaneously, easier to use than unfractionated heparin, lower risk of HIT, antidote protamine sulfate available, although less effective than for UFH | Parenteral subcutaneous dosing | Anti-Xa |
| Warfarin, VKA | Almost all long-term situations requiring anticoagulation, including artificial heart valve, thrombophilia and cancer | Long experience on clinical use, readily monitored with INR, vitamin K readily available as antidote, for urgent reversal, PCC | Regular visits to the laboratory required for INR checks | INR |
| Dabigatran | Non-valvular AF, treatment of PE, DVT | No routine laboratory monitoring, fixed dose | Antidote idarucizumab expensive and not always available, PCC as an alternative, dabigatran cannot be used in kidney failure | diluted thrombin time (dTT) calibrated for dabigatran, ecarin clotting assay (ECA) |
| Rivaroxaban | Non-valvular AF, treatment of PE, DVT, prevention of atherothrombosis | No routine laboratory monitoring, fixed dose, once-daily dosing | Antidote andexanet alpha, expensive and not readily available, PCC as an alternative | Anti-Xa calibrated for rivaroxaban |
| Apixaban | Non-valvular AF, treatment of PE, DVT | No routine laboratory monitoring, fixed dose | Antidote andexanet alpha expensive and not readily available, PCC as an alternative | Anti-Xa calibrated for apixaban |
| Edoxaban | Non-valvular AF | No routine laboratory monitoring, fixed dose, once-daily dosing | Antidote andexanet alpha expensive and not readily available, PCC as an alternative | Anti-Xa calibrated for edoxaban |
ACT, activated clotting time; AF, atrial fibrillation; Anti-Xa, anti-factor Xa assay; APTT, activated partial thromboplastin time; dTT, diluted thrombin time; DVT, deep vein thrombosis; ECA, ecarin clotting assay; HIT, heparin-induced thrombocytopenia; INR, international normalized ratio; LMWH, low molecular weight heparin; PCC, prothrombin complex concentrate; PE, pulmonary embolism; TT, thrombin time; UFH, unfractionated heparin.
Chromogenic anti-FXa assay can be calibrated with known concentrations of FXa inhibitors (FXaI) to provide a functional assay for estimating concentration. However, all FXa-inhibiting drugs, including indirect FXa inhibitors, such as heparin, elicit a response. A calibrated assay is said to be specific for the given FXaI, and the clinician must ask for the specific assay compatible with the current FXaI in use (not just request a FXa assay). This is important as anti-FXa sensitivities to different FXaIs vary. As an example, edoxaban amounts to the strongest anti-FXa inhibition in the low molecular weight heparin (LMWH) calibrated assays. On the other hand FXaIs can be quite reliably traced and excluded with LMWH assay (11,17,18), which is particularly relevant where the specific assay is not available for 24/7.
Antidotes for DOACs have been launched with a delay. Idarucizumab, a monoclonal antibody against dabigatran without any influence on thrombin, was licensed by the EMA and was available in Finland in late 2015 when we nationally launched web-based practical guidance to clinicians to approach problematic emergency cases (19). FXaI antidote andexanet alpha, effective against all the FXaIs, became available in EU only recently (20). The pricing will be unfortunately very high, so a rapid nation-specific health economic analysis will be needed prior the potential use of andexanet. Until now, the reversal of the FXaIs has been successfully performed with 4-factor prothrombin complex concentrates (PCC) (21).
DOAC reversal is indicated under acute situations, where it is not feasible to wait for drug elimination. These include drug overdose, acute kidney or liver failure, major bleed, trauma or urgent procedure with significant bleeding risk. Approximately 2–4% of patients on anticoagulation therapy encounter a major bleed and a further 2% require urgent procedure(s) while anticoagulated (22). In 2018, in the Helsinki and Uusimaa Hospital District, idarucizumab was used 120 times. More detailed analyses of these cases revealed that laboratory analyses (blood count, DOAC concentrations) were assessed only in a third of the patients, despite national guidelines from 2015 on DOAC on reversal, as idarucizumab is directly available to clinicians from the hospital pharmacy.
While typical DOAC concentrations during treatment are known, based on previous patient studies, actual concentrations observed in real-life patients may widely vary, 10- or even 100-fold at both peak and trough. Peak and trough levels for DOACs dabigatran, rivaroxaban and apixaban obtained in selected patient sample studies are shown in Tables 2-4. In a recent study, the mean peak level of edoxaban was 301 ng/mL (range, 60–569 ng/mL) and trough level was 39 ng/mL (range, 13–110 ng/mL) in 48 AF patients with an edoxaban OD dose of 60 mg (37).
Table 2
| Study | Indication | Dose | Number of patients | Peak level (ng/mL) | Trough level (ng/mL) | Measurement method | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | Range | Mean | Median | Range | |||||||
| Antovic |
Atrial fibrillation | Not reported | 70 | <0.5–586 | LC-MS/MS | ( |
||||||
| Reilly |
Atrial fibrillation | 110 mg BID | 4,583 | 126 | 133 | 1–745 | 65 | 66 | 1–608 | LC-MS/MS | ( |
|
| Reilly |
Atrial fibrillation | 150 mg BID | 4,600 | 175 | 184 | 2–1,000 | 91 | 93 | 1–809 | LC-MS/MS | ( |
|
| Skeppholm |
Atrial fibrillation | 110 mg BID | 17 | 60 | 23–168 | LC-MS/MS | ( |
|||||
| Skeppholm |
Atrial fibrillation | 150 mg BID | 73 | 52 | 8–188 | LC-MS/MS | ( |
|||||
| Chan |
Atrial fibrillation | 110–150 mg BID | 100 | 155 | <30–722 | dTT | ( |
|||||
| Shimomura |
Atrial fibrillation | 110 mg BID | 259 | 79 | dTT | ( |
||||||
| Shimomura |
Atrial fibrillation | 150 mg BID | 57 | 128 | dTT | ( |
||||||
| Freyburger |
Hip or knee replacement | 150–220 mg OD | 40 | 105 | dTT | ( |
||||||
| Herrmann |
Hip or knee replacement | 150 mg BID | 17 | 129 | 68 | dTT | ( |
|||||
| Samama |
Hip or knee replacement | 150–220 mg OD | 65 | 51 | 0–324 | dTT | ( |
|||||
| Schellings |
Hip or knee replacement | 220 mg OD | 40 | 81 | 12 | LC-MS/MS | ( |
|||||
| Testa |
Various | 110 mg BID | 90 | 170 | 30–650 | 120 | 10–400 | dTT | ( |
|||
| Testa |
Various | 150 mg BID | 70 | 180 | 30–550 | 120 | 10–500 | dTT | ( |
|||
| Lin |
Various | 110 mg BID | 38 | ( |
||||||||
| Lin |
Various | 150 mg BID | 8 | ( |
||||||||
BID, twice daily; dTT, diluted thrombin time; LC-MS/MS, liquid chromatography tandem mass spectrometry.
Table 3
| Study | Indication | Dose | Number of patients | Peak level (ng/mL) | Trough level (ng/mL) | Measurement method | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | Range | Mean | Median | Range | |||||||
| Freyburger |
Hip or knee replacement | 10 mg OD | 40 | 117 | Anti-Xa | ( |
||||||
| Herrmann |
Hip or knee replacement | 10 mg OD | 15 | 133 | 5 | Anti-Xa | ( |
|||||
| Freyburger |
Hip or knee replacement | 10 mg OD | 51 | 113 | 7 | LC-MS/MS | ( |
|||||
| Samama |
Hip or knee replacement | 10 mg OD | 41 | 142 | 0–412 | Anti-Xa | ( |
|||||
| Schellings |
Hip or knee replacement | 10 mg OD | 40 | 125 | 17 | LC-MS/MS | ( |
|||||
| Arachillage |
Thromboembolism | 20 mg OD | 105 | 280 | Anti-Xa | ( |
||||||
| Testa |
Various | 20 mg OD | 37 | 210 | 50–450 | 30 | 5–140 | Anti-Xa | ( |
|||
| Testa |
Various | 15 mg OD | 34 | 180 | 60–410 | 20 | 5–120 | Anti-Xa | ( |
|||
| Suwa |
Atrial fibrillation | 15 mg OD | 90 | 348 | 150–610 | 28 | 0–100 | Anti-Xa | ( |
|||
| Suwa |
Atrial fibrillation | 10 mg OD | 46 | 270 | 120–480 | 26 | 0–120 | Anti-Xa | ( |
|||
LC-MS/MS, liquid chromatography tandem mass spectrometry; OD, once daily.
Table 4
| Study | Indication | Dose | number of patients | Peak level (ng/mL) | Trough level (ng/mL) | Measurement method | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | Range | Mean | Median | Range | |||||||
| Freyburger |
Hip or knee replacement | 2.5 mg BID | 51 | 76 | 45 | LC-MS/MS | ( |
|||||
| Testa |
Various | 5 mg BID | 73 | 200 | 110–440 | 140 | 30–400 | Anti-Xa | ( |
|||
| Testa |
Various | 2.5 mg BID | 26 | 160 | 50–300 | 100 | 20–280 | Anti-Xa | ( |
|||
| Suwa |
Atrial fibrillation | 5 mg BID | 58 | 237 | 100–550 | 108 | 30–190 | Anti-Xa | ( |
|||
| Suwa |
Atrial fibrillation | 2.5 mg BID | 61 | 157 | 60–480 | 68 | 10–170 | Anti-Xa | ( |
|||
BID, twice daily; dTT, diluted thrombin time; LC-MS/MS, liquid chromatography tandem mass spectrometry.
VKAs (warfarin)
Warfarin is the traditional oral anticoagulant with decades of experience for its use. The clinical indications of warfarin include: (I) stroke prevention in all forms of AF, including valvular and under mechanical valvular replacement, (II) treatment of DVT and PE (including complicated, hemodynamically unstable PE), and other rare locations of thrombosis (e.g., cerebral sinus and portal vein thrombosis), although initial heparin bridging is used when treating acute thrombosis, (III) secondary prevention of thrombotic events after myocardial infarction and (IV) prevention of DVT in the setting of surgery.
Warfarin has wide intra- and interindividual variation in its metabolism. The active S enantiomer of warfarin exhibits significant metabolic variation, the variants contributing to increased warfarin sensitivity, include CYP2C9*2 (c.430C>T) and *3 (c.1075A>C) and VKORC1 c.-1639G>A variant (38,39). In addition, the amount of vitamin K in the diet, cigarette smoking, alcohol consumption and many medications interact with warfarin effect, mandating regular laboratory monitoring upon warfarin use (40). The International Normalized Ratio (INR), derived from PT and standardized between laboratories using the International Sensitivity Index (ISI), is used to monitor warfarin effect (41). The INR treatment target is usually 2.0–3.0, with higher treatment target of 2.5–3.5 used in patients with mechanical heart valve replacement, corresponding to higher warfarin dose in these patients (42). It is noteworthy that no DOACs have been approved for use with the mechanical heart valves, and we are not aware of any such ongoing studies.
Quality of warfarin anticoagulation for patients has been measured according to time in therapeutic range (TTR), especially in research settings for over two decades. In the Rosendaal method of calculating TTR, the INR is assumed to change linearly between dates of measurement and total number of days in the therapeutic range is then calculated as a percentage (43). TTR can be used to assess the quality of therapy at individual patient level or at treatment center’s—typical TTRs in warfarin patients range from 55–60% in USA and central Europe to over 70% in Sweden (44-48). TTR should only be used for long-term warfarin treatment when the patient is in a clinically stable condition, without interruptions in warfarin treatment. During acute illness or trauma, hospitalization with warfarin interruptions and heparin use, TTR is decreased, but this offers very limited information on warfarin suitability to a given patient. We offer a TTR calculator freely available on the Internet, including guidance in its rational use. Patients benefit from well-performing centers overall, centers with higher TTR during warfarin fare better with DOAC anticoagulation as well (49). While transitioning from warfarin to DOACs, the challenge is maintaining the high quality and adequate frequency of clinical follow-ups, when laboratory monitoring is no longer necessary (i.e., when on DOACs).
INR self-testing and self-management of warfarin are suitable options for motivated patients; even in the era of DOACs there is a special need. Not surprisingly, TTR values are best in patients participating in the self-management programs (50). When of high quality, self-testing performs well and is reliable, safe and efficient, increases patient safety and satisfaction, and decreases complications and even mortality (51,52). Professional training for patient selection is crucial, as appropriate skills and the ability to commit to the program are needed. We have offered a patient self-monitoring service since 2015. The program includes patient selection and training by the anticoagulation nurse and a laboratory technician, so that the patient acquires competency in both warfarin dosing and point-of-care (POC) INR measurement. The demand to serve new patients is currently higher than our capacity, and some primary healthcare units are being trained for this purpose as well. At the moment, the remuneration of self-management (devices, test strips and lancets) varies according to the different municipalities, which limits the adoption of the model nationwide.
Heparins
Heparins are of animal origin, from porcine and bovine gut mucosa, that is another reason why the synthetic anticoagulants appear important in the anticoagulation armamentarium. The unfractionated heparins (UFH) with chains of 10–20 kDa in molecular weight are administered in cardiac and vascular surgery setting and during plasma exchange. Therein contact activation demands an anticoagulant acting both at the level of thrombin and other coagulation factors. The development and subcutaneous administration of LMWH was a major milestone in the 1970s.
The strengths of heparin include its subcutaneous (sc) administration route, when oral route is not reliable; its anti-inflammatory actions and relatively short half-life, 90 min for UFH and 4–6 hours for LMWH depending on the dosing (53). Dabigatran and edoxaban when used to manage venous thromboembolism, requires use of LMWH first for a few days, prior to their initiation. LMWH is the drug of choice for cancer patients at vulnerable phases of chemotherapies and surgery, including pathological fractures (54). Also, pediatric and pregnant patients continue to benefit from this safe strategy to manage and prevent thrombosis. For hospitalized medically ill patients, LMWH as a thromboprophylactic therapy has the most solid evidence of balanced safety and efficacy, while DOACs failed in this indication, with the exception of betrixaban. Also, high risk warfarin treated patients having cardiac valvular replacement need LMWH bridging. The limitation of heparin is its immunological side effects, including heparin-induced thrombocytopenia, with even paradoxical thrombosis. The smallest effective unit of heparin is pentasaccharide, fondaparinux, which binds to antithrombin and as a synthetic mode of parenteral FXa inhibition offers an option to some heparin-intolerant patients.
In specific indications, LMWH and fondaparinux can be measured with the chromogenic anti-FXa assay. Like the FXaIs mentioned previously, the assay requires specific calibration. The aim of the monitoring of these drug effects is to acquire high enough dosing in thrombophilic patients and vice versa, in vulnerable organ-function compromised patients to avoid bleeding complications.
Summary of the anticoagulation options with their advantages and disadvantages are given in Table 1.
Clinical use of oral anticoagulation and laboratory testing in different clinical situations
Oral anticoagulation in cardiology
In heart disease, the most common indications for anticoagulation are AF, mechanical heart valve replacement and severe mitral stenosis. A DOAC is the drug of choice in most new patients, except for those with a mechanical heart valve, moderate to severe mitral stenosis or advanced renal failure (55). Individual risk assessment for stroke (CHA2DS2VASc) and bleeding (HAS-BLED) are guiding the choice of anticoagulation. Yet, there are problems with these scores. At a broad level, some of the scores are semiquantitative, and more attention is needed for a comprehensive understanding of the individual patient’s coagulation status. It is important to assess patient history on a prolonged time period, as limiting attention to only past six months has been shown to lead to underestimation of risk in CHA2DS2VASc score (56). Iron deficiency anemia is often overlooked, while it is a correctable risk factor for bleeds. Hypertension predisposes to intracerebral hemorrhage, as well as other bleeds, and is commonly present in cardiac patients.
Clinical scoring systems account for patient age, frailty, renal and liver dysfunction to varying degrees, while all significantly affect DOAC response. In acute coronary syndrome, dual antithrombotic therapy seems to be as effective as triple antithrombotic therapy, but is not surprisingly associated with significantly lower major bleeding risk (57). Anticoagulants may also be used in cerebrovascular disorders unrelated to AF (e.g., in cerebral venous thrombosis, basilar artery occlusion, or carotid or vertebral artery dissection). However, evidence on the use of DOACs in these special circumstances is still relatively weak. Critical studies are underway (58), and first line drugs are LMWH and warfarin. Use of DOACs is likely to increase along with new evidence as it is gained.
Oral anticoagulation and renal impairment
The incidence of chronic renal disease is increasing along with the aging population. For many clinical situations, knowledge on safety and efficacy of medications is lacking. Renal insufficiency exposes the risk of both bleeding and thrombosis complications, and while anticoagulation appears indicated, it is simultaneously challenging. Risk assessment tools with CHA2DS2VASc and HAS-BLED are not validated in these patients, while renal impairment is a relevant concern in cardiac patients (57). Clinical decisions on anticoagulation should be individualized with the help of laboratory testing. In case of mild and moderate renal insufficiency, with glomerular filtration rate (GFR) less than 30 mL/min, the dose of DOAC must be lowered. Patients on anticoagulation should be followed regularly, and more frequently when renal function declines, as the safety, the choice of management (need of dialysis), the drug and the dose of anticoagulation therapy should be re-evaluated.
Dabigatran is 80% excreted by kidneys, and it is cumulating to extravascular tissues during renal dysfunction. If GFR is <30 mL/min, dabigatran is contraindicated. If GFR is 30–50 mL/min, a lower dose of 110 mg BID must be chosen. However, renal extraction is lower for FXaIs, for apixaban, 27%, rivaroxaban 50% and edoxaban 35%. In renal impairment, apixaban is currently preferred, with the lower dose of 2.5 mg BID recommended in case of creatinine >133 mmol/L, elderly patients (>80 yrs) and small weight (<60 kg) (59). Finally, renal patients are subjected to the most severe complications of drug interactions, which tend to be increasingly identified (8).
Oral anticoagulation and surgery or invasive procedures and trauma
The management of chronic anticoagulation therapy in patients undergoing surgical procedures or trauma needs careful planning. Surgery, invasive procedures and trauma all have associated bleeding risks that are increased by the anticoagulant administered for prevention of thromboembolism. At the same time, interrupting anticoagulation transiently increases the risk of thromboembolism at least due to surgery-triggered exposure of vascular collagen and tissue factor. Warfarin therapy may be continued if the bleeding risk is manageable. However, warfarin should be stopped during high bleeding risk procedures and the physicians should carefully consider the need for bridging with LMWH with either full or prophylactic dose. According to the latest findings, bridging anticoagulation may be of no benefit in preventing thromboembolism and may increase the incidence of bleeding in patients with moderate thrombotic risk. In the Bridge study, forgoing heparin bridging anticoagulation in AF patients was non-inferior to perioperative LMWH bridging (60). However, patients included were low risk and bridging is recommended in patients with mechanical heart valve or other high risk for thromboembolism (61). Thus, for patients with high thromboembolic risk, bridging with LMWHs remains a valid strategy. The use of DOACs, during perioperative situations, is increasing in clinical practice, although without evidence.
Acute bleeding complication and oral anticoagulation
During major bleeds, anticoagulation should be either stopped and/or reversed, depending on the severity and localization of the bleed and the half-life of the anticoagulant. The timing of the last intake of the anticoagulant is relevant information, especially in the case of DOACs. Warfarin may be reversed with vitamin K and PCC, dabigatran by idarucizumab, and FXaIs with andexanet alpha (19-21); however, a clearly limiting factor is that specific antidotes are available in the major hospitals only. PCC can be used if antidote is not available or cost constraints preclude its use. Supportive care includes single dose of iv tranexamic acid and replacement of appropriate blood products according to laboratory analysis. Regardless of the severity of bleeding, other risk factors should be treated, i.e., anemia, thrombocytopenia, and hypertension. Possible renal or liver dysfunction must also be assessed, since impairment of hemostasis and drug accumulation potentiates the worsening of hemostasis during acute bleed.
Oral anticoagulation and thrombophilia
Currently for patients with hereditary thrombophilia, DOACs are not usually the primary anticoagulant recommended under these conditions (9-11,59). In the RE-MEDY trial, small subgroup analysis, dabigatran seemed noninferior to warfarin in thrombophilia patients (protein C or S deficiency, antithrombin deficiency, antiphospholipid antibodies, FV Leiden or prothrombin variant heterozygous mutation) (62). Rivaroxaban was assessed as noninferior to warfarin in phospholipid antibody syndrome (APS) in a phase III trial (63); however, the most severe APS patients did not benefit from rivaroxaban in comparison with warfarin (64). Overall, the evidence for use of DOACs in these rare conditions remains limited, with much of the data still based on case reports (65). For the time being, the EMA is evaluating APS data of DOAC treatments, and warfarin and heparin remain the mainstay.
Oral anticoagulation and laboratory testing
While anticoagulation therapy has changed dramatically, one of the major alterations is also the change in laboratory practice. Although the number of DOAC users is steadily increasing, the remuneration policies in each country are the main limiting factor for more widespread use. Since 2–3% of the population is now using anticoagulation therapy, the impact on the laboratory is remarkable (66). Clinicians in the Northern countries are increasingly preferring DOACs over warfarin. However, when DOACs were launched a decade ago, one of the main requirements and concerns were how to measure the effect and level of anticoagulation (13). Clinicians were initially hesitant to start such a drug, the effect of which could not be assessed.
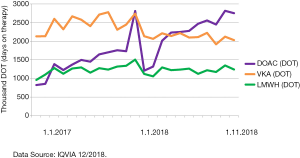
Quite quickly, we learned that routine coagulation tests PT and APTT were of little use in case of DOACs (13). There are large variations in sensitivity of different methods and reagents used. Owren type PT/INR, as used in the Nordic countries, is insensitive to DOACs and FV deficiency (impaired liver function), and this is still a matter of education for our clinicians (16). APTT may show an effect of dabigatran, whereas FXaIs affect the APTT less, or do not affect the APTT (15,16,67-69). More specific assays were quickly needed, and HUSLAB laboratory services was the first laboratory in Finland to set up the specific assays for dabigatran, rivaroxaban and apixaban, and offer them 24/7 for clinical use (since May 2017). The number of requested tests is still small, but increasing, the growth being fastest for apixaban, which came to the market later but is rapidly catching up (Figure 3). At the same time, number of INR tests has steadily declined annually by 10–15% since 2015. Yet, we also note that other coagulation testing, i.e., PT, APTT, fibrinogen, is increasing. In Finland, laboratory personnel, trained laboratory technicians, perform phlebotomy and take almost all the blood samples. The changes in coagulation test ordering practices widely impact on requirements of out-patient clinic blood sampling design also in the future.
Anticoagulation treatment increases the risk of bleeding complications, especially in the elderly, patients with cancer or with renal and liver dysfunction. To minimize the risk of bleeding, all anticoagulated patients should have regular follow-ups at minimum once, but in case of co-morbidities more frequently, at least 2–4 times annually (8,55).
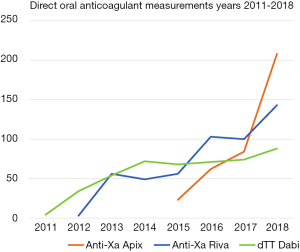
Laboratory testing during such visits may help to assess the thrombotic burden, i.e., thrombophilia panel, high fibrinogen and FVIII, and elevated D-dimer (70-72) (Figure 4). Thrombotic and bleeding tendencies and complications, adverse effects, adherence, blood pressure, renal and liver function, comorbidities and drug interactions, possible anemia and thrombocytopenia, as well as the appropriateness (dose and product) of the current anticoagulation should be repeatedly addressed during regular follow-ups. This assessment should be repeated at each patient clinical visit.

Specific, calibrated anticoagulant measurement tests are needed in special clinical situations to capture the extent of anticoagulation, in terms of timing of the drug dosing and blood sampling. The effective levels vary widely, with overlapping peaks and troughs at a steady state. Under special complications, it is advisable to assess whether the level is on-or off-therapy range. The level of anticoagulation should be optimized where there is potential for over-anticoagulation: acute bleeding, preoperatively overdosing, renal failure, low BMI, acute medical illness, drug interactions and in the elderly patients, and in cases of under-anticoagulation: treatment failure, obesity, malabsorption and drug interactions (73,74).
In case of trauma, acute procedure and need of antidote, the test is requested to save time and help decision-making at critical moments (Figure 4). For many laboratories, it is difficult to keep up 24/7 services for few requests and the distances to the nearest laboratory will increase compared with standard INR availability. While point-of-care INR and activated clotting time (ACT) have some sensitivity to rivaroxaban, the authors opine that POC testing of DOACs is unlikely to offer solutions for the near future (75-78), although POC tests specifically targeted to DOACs are emerging (79,80). It is also noteworthy, that national and local conditions will dictate the access to (e.g., special) assays to measure DOAC effects, depending on the part of the Hospital District the patients reside in.
Novel global approaches for anticoagulation management feasibly include viscoelastic testing and thrombin generation. Viscoelastic tests, the TEG® and ROTEM® are well suited for acute assessment of fibrin deficiency and massive bleeding complications in the emergency rooms or operating theaters. For both warfarin and DOACs, viscoelastic assays have shown conflicting data on detecting anticoagulation, and they are not recommended for monitoring (81,82). Thrombin generation assays have the potential to detect any anticoagulant, but their use is currently still limited to the research laboratory (83). Novel standardized methods, including the Stago ST Genesia® will probably become clinical routine in the near future (84).
Conclusions
In the future of anticoagulation therapy, individual decisions and practices will be even more common. Basic laboratory testing, including blood cell counts with hematocrit, hemoglobin, leukocyte and platelet count, creatinine and estimated GFR and liver enzymes (ALT) as well as function tests (prealbumin, albumin, PT, lipids) and basic metabolic assays (pH, electrolytes and cations) are crucial in the risk assessment, but will also increasingly become a part of the regular follow-up of anticoagulated patients. The number of INR tests continues to decrease, as the usage of warfarin declines. DOACs will mainly replace warfarin, imposing additional requirements for specific testing under acute clinical situations, such as major bleeds, thrombosis and emergency surgery or trauma. Pediatric and pregnant populations as well as cancer patients will require the gathering of more evidence as to how to most optimally secure their anticoagulation management.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Emmanuel J. Favaloro) for the series “Anticoagulant and antithrombotic therapy: globally applied according to local geographical selection criteria” published in Annals of Blood. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob.2019.07.01). The series “Anticoagulant and antithrombotic therapy: globally applied according to local geographical selection criteria” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Salmela B, Joutsi-Korhonen L, Armstrong E, et al. Active online assessment of patients using new oral anticoagulants: bleeding risk, compliance, and coagulation analysis. Semin Thromb Hemost 2012;38:23-30. [Crossref] [PubMed]
- Lassila R, Armstrong E, Halinen M, et al. Uusien antikoagulanttien hallittu käyttöönotto. Suom Lääkäril 2011;66:2753-62.
- Helin TA, Lemponen M, Hjemdahl P, et al. From laboratory to clinical practice: Dabigatran effects on thrombin generation and coagulation in patient samples. Thromb Res 2015;136:154-60. [Crossref] [PubMed]
- Helin TA, Virtanen L, Manninen M, et al. Effects of thromboprophylactic doses of apixaban and rivaroxaban on coagulation and thrombin generation in association with total hip replacement. J Thromb Thrombolysis 2017;43:562-9. [Crossref] [PubMed]
- Anand SS, Bosch J, Eikelboom JW, et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet 2018;391:219-29. [Crossref] [PubMed]
- Laube ES, Yu A, Gupta D, et al. Rivaroxaban for Stroke Prevention in Patients With Nonvalvular Atrial Fibrillation and Active Cancer. Am J Cardiol 2017;120:213-7. [Crossref] [PubMed]
- Melloni C, Dunning A, Granger CB, et al. Efficacy and Safety of Apixaban Versus Warfarin in Patients with Atrial Fibrillation and a History of Cancer: Insights from the ARISTOTLE Trial. Am J Med 2017;130:1440-1448.e1. [Crossref] [PubMed]
- Heidbuchel H, Berti D, Campos M, et al. Implementation of non-vitamin K antagonist oral anticoagulants in daily practice: the need for comprehensive education for professionals and patients. Thromb J 2015;13:22. [Crossref] [PubMed]
- EMA. EPAR Summary for the public: Pradaxa. EMA/54606/2012. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/pradaxa#product-information-section
- EMA. Xarelto Summary of Product Characteristics. EMEA/H/C/000944 -IB/0040/G 2009. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/xarelto#product-information-section
- EMA. EPAR Summary for the Public Lixiana. EMEA/H/C/002629 2017. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/lixiana#product-information-section
- Cohen AT, Harrington RA, Goldhaber SZ, et al. Extended Thromboprophylaxis with Betrixaban in Acutely Ill Medical Patients. N Engl J Med 2016;375:534-44. [Crossref] [PubMed]
- Baglin T, Hillarp A, Tripodi A, et al. Measuring Oral Direct Inhibitors (ODIs) of thrombin and factor Xa: A recommendation from the Subcommittee on Control of Anticoagulation of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 2013; [Epub ahead of print]. [Crossref] [PubMed]
- Favaloro EJ, Pasalic L, Curnow J, et al. Laboratory Monitoring or Measurement of Direct Oral Anticoagulants (DOACs): Advantages, Limitations and Future Challenges. Curr Drug Metab 2017;18:598-608. [Crossref] [PubMed]
- Hillarp A, Strandberg K, Baghaei F, et al. Effects of the oral, direct factor Xa inhibitor edoxaban on routine coagulation assays, lupus anticoagulant and anti-Xa assays. Scand J Clin Lab Invest 2018;78:575-83. [Crossref] [PubMed]
- Helin TA, Pakkanen A, Lassila R, et al. Laboratory assessment of novel oral anticoagulants: method suitability and variability between coagulation laboratories. Clin Chem 2013;59:807-14. [Crossref] [PubMed]
- He L, Kochan J, Lin M, et al. Determination of edoxaban equivalent concentrations in human plasma by an automated anti-factor Xa chromogenic assay. Thromb Res 2017;155:121-7. [Crossref] [PubMed]
- Becker RC, Yang H, Barrett Y, et al. Chromogenic laboratory assays to measure the factor Xa-inhibiting properties of apixaban--an oral, direct and selective factor Xa inhibitor. J Thromb Thrombolysis 2011;32:183-7. [Crossref] [PubMed]
- Pollack CV Jr, Reilly PA, Eikelboom J, et al. Idarucizumab for Dabigatran Reversal. N Engl J Med 2015;373:511-20. [Crossref] [PubMed]
- Siegal DM, Curnutte JT, Connolly SJ, et al. Andexanet Alfa for the Reversal of Factor Xa Inhibitor Activity. N Engl J Med 2015;373:2413-24. [Crossref] [PubMed]
- Samuelson BT, Cuker A. Measurement and reversal of the direct oral anticoagulants. Blood Rev 2017;31:77-84. [Crossref] [PubMed]
- Tornkvist M, Smith JG, Labaf A. Current evidence of oral anticoagulant reversal: A systematic review. Thromb Res 2018;162:22-31. [Crossref] [PubMed]
- Antovic JP, Skeppholm M, Eintrei J, et al. Evaluation of coagulation assays versus LC-MS/MS for determinations of dabigatran concentrations in plasma. Eur J Clin Pharmacol 2013;69:1875-81. [Crossref] [PubMed]
- Reilly PA, Lehr T, Haertter S, et al. The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: the RE-LY Trial (Randomized Evaluation of Long-Term Anticoagulation Therapy). J Am Coll Cardiol 2014;63:321-8. [Crossref] [PubMed]
- Skeppholm M, Hjemdahl P, Antovic JP, et al. On the monitoring of dabigatran treatment in "real life" patients with atrial fibrillation. Thromb Res 2014;134:783-9. [Crossref] [PubMed]
- Chan NC, Coppens M, Hirsh J, et al. Real-world variability in dabigatran levels in patients with atrial fibrillation. J Thromb Haemost 2015;13:353-9. [Crossref] [PubMed]
- Shimomura D, Nakagawa Y, Kondo H, et al. Relationship between plasma dabigatran concentration and activated partial thromboplastin time in Japanese patients with non-valvular atrial fibrillation. J Arrhythm 2015;31:183-8. [Crossref] [PubMed]
- Freyburger G, Macouillard G, Labrouche S, et al. Coagulation parameters in patients receiving dabigatran etexilate or rivaroxaban: two observational studies in patients undergoing total hip or total knee replacement. Thromb Res 2011;127:457-65. [Crossref] [PubMed]
- Herrmann R, Thom J, Wood A, et al. Thrombin generation using the calibrated automated thrombinoscope to assess reversibility of dabigatran and rivaroxaban. Thromb Haemost 2014;111:989-95. [Crossref] [PubMed]
- Samama MM, Guinet C, Le Flem L, et al. Measurement of dabigatran and rivaroxaban in primary prevention of venous thromboembolism in 106 patients, who have undergone major orthopedic surgery: an observational study. J Thromb Thrombolysis 2013;35:140-6. [Crossref] [PubMed]
- Schellings MW, Boonen K, Schmitz EM, et al. Determination of dabigatran and rivaroxaban by ultra-performance liquid chromatography-tandem mass spectrometry and coagulation assays after major orthopaedic surgery. Thromb Res 2016;139:128-34. [Crossref] [PubMed]
- Testa S, Tripodi A, Legnani C, et al. Plasma levels of direct oral anticoagulants in real life patients with atrial fibrillation: Results observed in four anticoagulation clinics. Thromb Res 2016;137:178-83. [Crossref] [PubMed]
- Lin SY, Tang SC, Kuo CH, et al. Factors affecting serum concentration of dabigatran in Asian patients with non-valvular atrial fibrillation. J Formos Med Assoc 2019;118:1154-60. [Crossref] [PubMed]
- Freyburger G, Macouillard G, Khennoufa K, et al. Rivaroxaban and apixaban in orthopaedics: is there a difference in their plasma concentrations and anticoagulant effects? Blood Coagul Fibrinolysis 2015;26:925-33. [PubMed]
- Arachchillage DR, Mackie IJ, Efthymiou M, et al. Rivaroxaban limits complement activation compared with warfarin in antiphospholipid syndrome patients with venous thromboembolism. J Thromb Haemost 2016;14:2177-86. [Crossref] [PubMed]
- Suwa M, Morii I, Kino M. Rivaroxaban or Apixaban for Non-Valvular Atrial Fibrillation- Efficacy and Safety of Off-Label Under-Dosing According to Plasma Concentration. Circ J 2019;83:991-9. [Crossref] [PubMed]
- Testa S, Dellanoce C, Paoletti C, et al. Edoxaban plasma levels with non-valvular atrial fibrillation: Inter and intra-individual variability, correlation with coagulation screening test and renal function. Thromb Res 2019;175:61-7. [Crossref] [PubMed]
- Daly AK, Day CP, Aithal GP. CYP2C9 polymorphism and warfarin dose requirements. Br J Clin Pharmacol 2002;53:408-9. [Crossref] [PubMed]
- Owen RP, Gong L, Sagreiya H, et al. VKORC1 pharmacogenomics summary. Pharmacogenet Genomics 2010;20:642-4. [Crossref] [PubMed]
- Holbrook A, Schulman S, Witt DM, et al. Evidence-Based Management of Anticoagulant Therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e152S-84S.
- van den Besselaar AM. Standardization of the prothrombin time in oral anticoagulant control. Haemostasis 1985;15:271-7. [PubMed]
- Helin TA, Joutsi-Korhonen L, Asmundela H, et al. Warfarin dose requirement in patients having severe thrombosis or thrombophilia. Br J Clin Pharmacol 2019;85:1684-91. [PubMed]
- Rosendaal FR, Cannegieter SC, van der Meer FJ, et al. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost 1993;69:236-9. [Crossref] [PubMed]
- Kaatz S. Determinants and measures of quality in oral anticoagulation therapy. J Thromb Thrombolysis 2008;25:61-6. [Crossref] [PubMed]
- Wallentin L, Yusuf S, Ezekowitz MD, et al. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet 2010;376:975-83. [Crossref] [PubMed]
- Lehto M, Niiranen J, Korhonen P, et al. Quality of warfarin therapy and risk of stroke, bleeding, and mortality among patients with atrial fibrillation: results from the nationwide FinWAF Registry. Pharmacoepidemiol Drug Saf 2017;26:657-65. [Crossref] [PubMed]
- Raatikainen MJP, Penttila T, Korhonen P, et al. The quality of warfarin therapy and CHA2DS2-VASc score associate with the incidence of myocardial infarction and cardiovascular outcome in patients with atrial fibrillation: data from the nationwide FinWAF Registry. Eur Heart J Cardiovasc Pharmacother 2018;4:211-9. [Crossref] [PubMed]
- Penttilä T, Lehto M, Niiranen J, et al. Differences in the risk of stroke, bleeding events, and mortality between female and male patients with atrial fibrillation during warfarin therapy. Eur Heart J Cardiovasc Pharmacother 2019;5:29-36. [Crossref] [PubMed]
- Wallentin L, Lopes RD, Hanna M, et al. Efficacy and safety of apixaban compared with warfarin at different levels of predicted international normalized ratio control for stroke prevention in atrial fibrillation. Circulation 2013;127:2166-76. [Crossref] [PubMed]
- Heneghan C, Ward A, Perera R, et al. Self-monitoring of oral anticoagulation: systematic review and meta-analysis of individual patient data. Lancet 2012;379:322-34. [Crossref] [PubMed]
- Regier DA, Sunderji R, Lynd LD, et al. Cost-effectiveness of self-managed versus physician-managed oral anticoagulation therapy. CMAJ 2006;174:1847-52. [Crossref] [PubMed]
- Heneghan CJ, Garcia-Alamino J, Spencer EA, et al. Self-monitoring and self-management of oral anticoagulation. Cochrane Database Syst Rev 2016;7:CD003839 [PubMed]
- Garcia DA, Baglin TP, Weitz JIFCCP, et al. Parenteral Anticoagulants: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e24S-43S.
- Ratasvuori M, Lassila R, Laitinen M. Venous thromboembolism after surgical treatment of non-spinal skeletal metastases - An underdiagnosed complication. Thromb Res 2016;141:124-8. [Crossref] [PubMed]
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS: The Task Force for the management of atrial fibrillation of the European Society of Cardiology (ESC)Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESCEndorsed by the European Stroke Organisation (ESO). Eur J Cardiothorac Surg 2016;50:e1-88. [Crossref] [PubMed]
- Friberg L. Short lookback periods causing exaggerated stroke risk estimates in atrial fibrillation may expose patients to unnecessary anticoagulant treatment. Pharmacoepidemiol Drug Saf 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267-315. [Crossref] [PubMed]
- Covut F, Kewan T, Perez O, et al. Apixaban and rivaroxaban in patients with cerebral venous thrombosis. Thromb Res 2019;173:77-78. [Crossref] [PubMed]
- EMA. Eliquis Summary of Product Characteristics. EMEA/H/C/002148 -R/0034 2011. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/eliquis#product-information-section
- Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative Bridging Anticoagulation in Patients with Atrial Fibrillation. N Engl J Med 2015;373:823-33. [Crossref] [PubMed]
- Pengo V, Denas G. Optimizing quality care for the oral vitamin K antagonists (VKAs). Hematology Am Soc Hematol Educ Program 2018;2018:332-8. [Crossref] [PubMed]
- Goldhaber SZ, Eriksson H, Kakkar A, et al. Efficacy of dabigatran versus warfarin in patients with acute venous thromboembolism in the presence of thrombophilia: Findings from RE-COVER(R), RE-COVER II, and RE-MEDY. Vasc Med 2016;21:506-14. [Crossref] [PubMed]
- Cohen H, Dore CJ, Clawson S, et al. Rivaroxaban in antiphospholipid syndrome (RAPS) protocol: a prospective, randomized controlled phase II/III clinical trial of rivaroxaban versus warfarin in patients with thrombotic antiphospholipid syndrome, with or without SLE. Lupus 2015;24:1087-94. [Crossref] [PubMed]
- Pengo V, Denas G, Zoppellaro G, et al. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood 2018;132:1365-71. [Crossref] [PubMed]
- Skelley JW, White CW, Thomason AR. The use of direct oral anticoagulants in inherited thrombophilia. J Thromb Thrombolysis 2017;43:24-30. [Crossref] [PubMed]
- Finnish Medical Authority. Drug consumption statistics. 2018. Available online: https://www.fimea.fi/en/web/en/databases_and_registeries/consumption
- Helin TA, Pakkanen A, Lassila R, et al. Effects of apixaban on prothrombin time, activated partial thromboplastin time and anti-Xa assays: a European survey. Clin Chem Lab Med 2017;55:e178-80. [Crossref] [PubMed]
- Hillarp A, Gustafsson KM, Faxalv L, et al. Effects of the oral, direct factor Xa inhibitor apixaban on routine coagulation assays and anti-FXa assays. J Thromb Haemost 2014;12:1545-53. [Crossref] [PubMed]
- Lindahl TL, Baghaei F, Blixter IF, et al. Effects of the oral, direct thrombin inhibitor dabigatran on five common coagulation assays. Thromb Haemost 2011;105:371-8. [Crossref] [PubMed]
- Connors JM. Thrombophilia Testing and Venous Thrombosis. N Engl J Med 2017;377:1177-87. [Crossref] [PubMed]
- Jenkins PV, Rawley O, Smith OP, et al. Elevated factor VIII levels and risk of venous thrombosis. Br J Haematol 2012;157:653-63. [Crossref] [PubMed]
- Bjøri E, Johnsen HS, Hansen JB, et al. D-dimer at venous thrombosis diagnosis is associated with risk of recurrence. J Thromb Haemost 2017;15:917-24. [Crossref] [PubMed]
- Piran S, Traquair H, Chan N, et al. Peak plasma concentration of direct oral anticoagulants in obese patients weighing over 120 kilograms: A retrospective study. Res Pract Thromb Haemost 2018;2:684-8. [Crossref] [PubMed]
- Molteni M, Crippa M, Orenti A, et al. Investigation on Dabigatran Etexilate and Worsening of Renal Function in Patients with Atrial fibrillation: The IDEA Study. Clin Drug Investig 2019;39:355-62. [Crossref] [PubMed]
- van Ryn J, Baruch L, Clemens A. Interpretation of point-of-care INR results in patients treated with dabigatran. Am J Med 2012;125:417-20. [Crossref] [PubMed]
- Mani H, Herth N, Kasper A, et al. Point-of-care coagulation testing for assessment of the pharmacodynamic anticoagulant effect of direct oral anticoagulant. Ther Drug Monit 2014;36:624-31. [Crossref] [PubMed]
- Ebner M, Birschmann I, Peter A, et al. Point-of-care testing for emergency assessment of coagulation in patients treated with direct oral anticoagulants. Crit Care 2017;21:32. [Crossref] [PubMed]
- Ebner M, Peter A, Spencer C, et al. Point-of-Care Testing of Coagulation in Patients Treated With Non-Vitamin K Antagonist Oral Anticoagulants. Stroke 2015;46:2741-7. [Crossref] [PubMed]
- Ansell J, Zappe S, Jiang X, et al. A Novel Whole Blood Point-of-Care Coagulometer to Measure the Effect of Direct Oral Anticoagulants and Heparins. Semin Thromb Hemost 2019;45:259-63. [Crossref] [PubMed]
- Harder S, Santos SMD, Krozer V, et al. Surface Acoustic Wave-Based Microfluidic Coagulation Device for Monitoring Anticoagulant Therapy. Semin Thromb Hemost 2019;45:253-8. [Crossref] [PubMed]
- Nilsson CU, Strandberg K, Reinstrup P. Warfarin monitoring with viscoelastic haemostatic assays, thrombin generation, coagulation factors and correlations to Owren and Quick prothrombin time. Scand J Clin Lab Invest 2018;78:358-64. [Crossref] [PubMed]
- Gosselin RC, Adcock DM, Bates SM, et al. International Council for Standardization in Haematology (ICSH) Recommendations for Laboratory Measurement of Direct Oral Anticoagulants. Thromb Haemost 2018;118:437-50. [Crossref] [PubMed]
- Al Dieri R, de Laat B, Hemker HC. Thrombin generation: what have we learned? Blood Rev 2012;26:197-203. [Crossref] [PubMed]
- Douxfils J, Morimont L, Bouvy C, et al. Assessment of the analytical performances and sample stability on ST Genesia system using the STG-DrugScreen application J Thromb Haemost 2019; [Epub ahead of print]. [Crossref] [PubMed]
Cite this article as: Helin T, Joutsi-Korhonen L, Lassila R. Clinical use and laboratory testing of oral anticoagulation therapy: experience from Finland. Ann Blood 2019;4:17.


