
Anticoagulation therapy in Slovakia
Introduction
Anticoagulant treatment is predominantly used for inhibiting blood coagulation in patients who are at increased risk of thrombotic complications, such as individuals with non-valvular atrial fibrillation (NVAF), prosthetic heart valves or with the risk of development of venous thromboembolism (VTE) and cardiovascular disease. Due to the disadvantages of traditional anticoagulant drugs, as published in the literature worldwide, a novel generation of anticoagulant agents entitled direct oral anticoagulants (DOACs) has been established (1).
This manuscript provides an overview on the basic characteristics and indications for the use of dabigatran, rivaroxaban, apixaban, edoxaban, warfarin, unfractionated heparin (UFH) and fondaparinux in Slovakia in terms of the prevention and treatment of VTE.
Dabigatran
Dabigatran was firstly developed for orthopaedic surgery. One of the primary indications for the use of dabigatran, representing a direct thrombin inhibitor, is the primary prevention of VTE following orthopaedic surgery. The drug is administered in patients after any elective total knee arthroplasty in the dose of 110 mg twice daily. The treatment should begin 1–4 hours after the end of the surgical intervention with one capsule and continue in the dosage of 2 capsules a day thereafter for a total length of treatment of 10 days. Following elective total hip arthroplasty, the treatment with dabigatran should instead continue for 28–35 days.
In selected groups of patients undergoing total knee or hip arthroplasty, the daily dose of dabigatran ought to be reduced to 150 mg daily (divided to 2 administrations of the 75 mg capsule). These are those individuals with moderate dysfunction of the kidneys (creatinine clearance 30–50 mL/min.), those simultaneously taking verapamil, amiodarone or chinidin (as mild or moderate inhibitors of P-glycoprotein) and all patients older than 75 years. In cases where adequate haemostasis cannot be ensured, the surgical intervention should be postponed, if possible. If the treatment cannot start after surgery on the day of the surgery, then administration ought to begin with 2 capsules once daily (2).
Dabigatran was also the first direct thrombin inhibitor for stroke prophylaxis in atrial fibrillation. In the case of prevention of ischaemic stroke and systemic embolization in adult patients with NVAF with one or more risk factors [previous ischaemic stroke or transitory ischaemic attack, age ≥75 years, chronic heart failure (New York Heart Association class ≥2), diabetes mellitus, arterial hypertension], the recommended dose of dabigatran is 150 mg twice daily.
For the treatment of the deep venous thrombosis (DVT) and pulmonary embolism (PE) and the prevention of recurrent DVT and PE in adult patients, the suggested dose is 150 mg twice daily, after the initial use of a parenteral anticoagulant (LMWH) for at least 5 days. The short-term treatment (minimally 3 months) should be based on the presence of the transient risk factors (e.g., recent surgical intervention, trauma, immobilization), whereas long-term use should be preserved for those with persistence of risk factors or development of an idiopathic DVT or PE.
However, in selected groups of patients, despite a previous VTE episode, a reduced dose of dabigatran (110 mg capsule given twice daily) should be considered: specifically, patients 80 years or older and individuals taking simultaneously verapamil. In some patients, the risk of bleeding versus benefit from the treatment of VTE should be assessed for alternate daily administered dose of dabigatran 300 vs. 220 mg: patients aged 75–80 years, those with moderately reduced kidney function, with gastritis, oesophagitis or gastroesophageal reflux disease and other subjects with an increased risk of bleeding (3).
Idarucizumab is a specific antidote for dabigatran, and is given to adult patients in case of inevitable urgent surgical interventions and life-threatening bleeding in the dose 2.5 g/50 mL administered as a bolus injection twice (5.0 g i.v. before surgery/episode) (4).
Rivaroxaban
Rivaroxaban is a direct anti-Xa agent and used in the prevention of ischaemic stroke and systemic embolism in patients with one or more risk factors (previous ischaemic stroke or transitory ischaemic attack, age ≥75 years, chronic congestive heart failure, diabetes mellitus, arterial hypertension) in a daily dose 20 mg.
For the treatment of DVT and PE, and prevention of their recurrence, the recommended dose at the start of therapy following the acute DVT or PE is 15 mg twice daily during the first three weeks followed by a daily dose of 20 mg (5).
Rivaroxaban may also be used for primary prevention of VTE in adult patients undergoing an elective surgical intervention (total hip or knee arthroplasty). The recommended dose is 10 mg administered once daily. The starting dose should be used 6–10 hours after the intervention and the duration of thromboprophylaxis is 5 weeks for hip and 2 weeks for knee arthroplasty (6).
Apixaban
Apixaban is another anti-Xa agent, and also used for primary, as well as secondary thromboprophylaxis. In the case of primary prevention of VTE in individuals undergoing elective hip and knee arthroplasty, the starting dose is 2.5 mg, and should be administered 12–24 hours after surgery and subsequently twice daily. The length of treatment is 32–38 days in case of hip and 10–14 days in case of knee arthroplasty.
Apixaban in an increased dose of 5 mg twice daily should be used for primary prevention of ischaemic stroke and systemic embolism in patients with NVAF with one or more risk factors [previous ischaemic stroke or transitory ischaemic attack, age ≥75 years, symptomatic chronic heart failure (New York Heart Association class ≥2), diabetes mellitus, arterial hypertension] (7).
For at risk patients (i.e., those with 2 or more features from the following: age ≥80 or ≤60 years or serum creatinine ≥1.5 mg/dL (133 micromol/L), the recommended dose is 2.5 mg used twice daily.
For the treatment of DVT and PE, the recommended dose is 10 mg administered twice daily during the first 7 days and subsequently 5 mg twice daily.
In subjects with secondary prevention of recurrence of DVT and PE, a dose of 2.5 mg given twice daily is suggested. This protocol may follow the recommendation to use apixaban in the dosage 5 mg twice daily or any other anticoagulant drug for 6 months (8).
Edoxaban
Edoxaban was the last approved DOAC in Slovakia (in 2016). It is another anti-Xa agent and is indicated in primary prevention of ischaemic stroke and systemic embolism in patients with one or more risk factors (previous ischaemic stroke or transitory ischaemic attack, age ≥75 years, chronic congestive heart failure, diabetes mellitus, arterial hypertension) in the dose of 60 mg, administered once daily.
In patients with acute DVT and PE, after the initial use of a parenteral anticoagulant (LMWH) for at least 5 days, the recommended daily dose of edoxaban is 60 mg once daily.
In the subjects with NVAF or VTE, a reduced dose of edoxaban 30 mg daily is used in the presence of one or more risk factors: moderate or severe renal dysfunction (creatinine clearance 15–50 mL/min), body weight ≤60 kg, concomitant use of the inhibitors of P-glycoprotein, such as cyclosporine, dronedarone, erythromycin or ketoconazole (9).
Reversal of direct anti-Xa agents
A reversal agent for patients with life-threatening bleeding episodes treated with direct inhibitors of activated coagulation factor X (i.e., for the reversal of the action of rivaroxaban, apixaban or edoxaban)—andexanet alpha—has not yet been approved by the European Medicines Agency, although it is available in some other countries.
Warfarin
Warfarin is a vitamin K antagonist (VKA) and represents one of the first oral anticoagulants to be used for treatment and thromboprophylaxis of DVT and PE, for the primary prevention of ischaemic stroke and systemic embolism in the presence of NVAF, artificial valve or associated disorders, for the treatment and secondary prevention of transitory ischaemic attack and ischaemic stroke, as well as for secondary prevention of myocardial infarction and its related ischaemic stroke or systemic embolism. In the case of prevention of thromboembolic events with coexistence of artificial heart valve, the targeted International Normalized Ratio (INR) should be in the range 2.5–3.5; otherwise, the INR ought to be between 2.0–3.0.
At the beginning of treatment with warfarin with INR <1.2, as well as in patients with average weight (around 70 kg according to the summary of the characteristic features of the drug), warfarin is administered in the dose 10 mg daily for three days, with control by INR measurement on the fourth and subsequent days of treatment (10).
UFH
In the routine prevention of thromboembolic complications, UFH is administered subcutaneously in the dose 5,000 IU 2 or 3 times daily with no need for laboratory monitoring. However, in individuals with an increased risk of thrombosis, control of dosing using the activated partial thromboplastin time (aPTT) and tailoring of the dose to be 1.2–1.3 times the reference time is suggested.
For the treatment of acute thrombosis, subcutaneous administration of UFH is subjected to higher levels, for example approximating 1.5–2.5 of the control of aPTT for the assessment of which the blood is taken in the middle of the interval between the injections.
At the beginning of treatment of the thromboembolic events, normally we administer a bolus dose of 5,000–10,000 IU of UFH intravenously and in the subsequent continual infusion we give approximately 1,000 IU/hour. The most convenient approach is to start with a dosage of 18–20 IU/kg of the body weight per hour both in adults and in children. This dose may then be modified according to aPTT with targeted value 1.5–2.5 of the reference range. Blood sampling for the control by aPTT is initially recommended to be done each 6 hours and then once daily after achieving the intended values.
In patients undergoing vascular surgery, the suggested approach is intravenous infusion with a dose of 20,000 IU of UFH in 1,000 mL of infusion solution using a rate of administration of 15–25 drops per minute.
When performing haemodialysis with systemic heparinization, UFH is initially administered at the dose of 10,000 IU (administration in the form of a combination of the injections of 3,000–5,000 IU) under the control using an aPTT. The dose can also be administered as a continuous infusion of 20,000 IU/100 mL of infusion solution. In older patients (at the age of 65 years or more), the dose of UFH is recommended to be reduced (the reduced dose, however, is not specified in the summary of the characteristic features of the drug, so it should be modified according to the actual results and clinical state of the patient individually) (11).
Fondaparinux
The representative of the synthetic pentasaccharide inhibitors in our country is predominantly fondaparinux, which is used for prevention of venous thromboembolic episodes in patients undergoing orthopaedic surgery of the lower limbs, comprising surgery for hip fracture, as well as total hip arthroplasty, for the prevention of VTE in individuals with need for abdominal surgery and in all patients, where there is a risk of VTE because of immobilisation (e.g., due to severe heart failure, diseases of the respiratory tract or infectious disease).
In patients undergoing orthopaedic or intraabdominal surgical interventions, the recommended dose is 2.5 mg once daily administered subcutaneously at least 6 hours after the surgery up until the disappearance of the risk of thrombotic complications (usually until the patient’s discharge—being up to 5–9 days after the intervention). In patients following surgery for hip fracture, prolongation of treatment for a further 24 days is recommended. For thromboprophylaxis, the suggested dose is 2.5 mg once daily (12).
A summary of all of the antithrombotic drugs, their indications, doses and recommended length of the treatment is provided in Table 1 (2-12).
Table 1
| Drug and its indication | Dose | Length of treatment |
|---|---|---|
| Dabigatran | ||
| Patients after an elective total knee arthroplasty | 110 mg twice daily | 10 days |
| Patients after an elective total hip arthroplasty | 110 mg twice daily | 28–35 days |
| Patients after the orthopaedic surgery with moderate dysfunction of the kidneys (creatinine clearance 30–50 mL/min), taking simultaneously verapamil, amiodarone or chinidin and all patients older than 75 years | 150 mg once daily | 10/28–35 days |
| Patients with non-valvular atrial fibrillation (NVAF) with one or more risk factors [previous ischaemic stroke or transitory ischaemic attack, age ≥75 years, chronic heart failure (New York Heart Association class ≥2), diabetes mellitus, arterial hypertension] | 150 mg twice daily | Long-term prevention of ischaemic stroke and systemic embolization |
| For the treatment of the deep venous thrombosis (DVT) and pulmonary embolism (PE) and the prevention of recurrent DVT and PE in adult patients | 150 mg twice daily, after the initial use of a parenteral anticoagulant [low molecular weight heparin (LMWH)] for at least 5 days | Minimally 3 months based on the presence of the transient risk factors |
| For the treatment of DVT or PE in patients 80 years or older and individuals taking simultaneously verapamil | 110 mg capsule given twice daily | Minimally 3 months based on the presence of the transient risk factors |
| For the treatment of DVT or PE in patients aged 75–80 years, those with moderately reduced kidney function, with gastritis, oesophagitis or gastroesophageal reflux disease and other subjects with an increased risk of bleeding | 150 mg twice daily/110 mg twice daily | Minimally 3 months based on the presence of the transient risk factors |
| Idarucizumab | ||
| For the reversal of the dabigatran effect in adult patients in case of inevitable urgent surgical interventions and life-threatening bleeding | 2.5 g/50 mL administered as a bolus injection twice (5.0 g i.v. before surgery/episode) | Bolus before surgery/episode |
| Rivaroxaban | ||
| Prevention of ischaemic stroke and systemic embolism | 20 mg once daily | Long-term prevention of ischaemic stroke and systemic embolization |
| Treatment of DVT and PE, and prevention of their recurrence | 15 mg twice daily during the first three weeks followed by a daily dose of 20 mg | Minimally 3 months based on the presence of the transient risk factors |
| Primary prevention of venous thromboembolism (VTE) in adult patients undergoing an elective surgical intervention (total hip or knee arthroplasty) | 10 mg once daily | 5 weeks for hip and 2 weeks for knee arthroplasty |
| Apixaban | ||
| Primary prevention of VTE in individuals undergoing elective hip and knee arthroplasty | 2.5 mg twice daily | 32–38 days in case of hip and 10–14 days in case of knee arthroplasty |
| Primary prevention of ischaemic stroke and systemic embolism in patients with NVAF with 2 or more risk factors mentioned above | 5 mg twice daily | Long-term prevention of ischaemic stroke and systemic embolization |
| At risk patients [i.e., those with 2 or more features from the following: age ≥80 or ≤60 years or serum creatinine ≥1.5 mg/dL (133 micromol/L)] | 2.5 mg twice daily | Long-term prevention of ischaemic stroke and systemic embolization |
| Treatment of DVT and PE | 10 mg administered twice daily during the first 7 days and subsequently 5 mg twice daily | Minimally 3 months based on the presence of the transient risk factors |
| For the secondary prevention of recurrence of DVT and PE | 2.5 mg twice daily | Minimally 3 months based on the presence of the transient risk factors |
| Edoxaban | ||
| Primary prevention of ischaemic stroke and systemic embolism | 60 mg once daily | Long-term prevention of ischaemic stroke and systemic embolization |
| Treatment of DVT and PE | After the initial use of a parenteral anticoagulant (LMWH) for at least 5 days, the recommended daily dose of edoxaban is 60 mg once daily | Minimally 3 months based on the presence of the transient risk factors |
| Patients with NVAF or VTE with the presence of one or more risk factors: moderate or severe renal dysfunction (creatinine clearance 15–50 mL/min), body weight ≤60 kg, concomitant use of the inhibitors of P-glycoprotein, such as cyclosporine, dronedarone, erythromycin or ketoconazole | 30 mg once daily | Long-term prevention of ischaemic stroke and systemic embolization in case of NVAF and minimally 3 months based on the presence of the transient risk factors in case of VTE |
| Warfarin | ||
| Prevention of thromboembolic events with coexistence of artificial heart valve | The targeted international normalized ratio (INR) should be in the range 2.5–3.5 | long-term prevention of ischaemic stroke and systemic embolization |
| For treatment and thromboprophylaxis of DVT and PE, for the primary prevention of ischaemic stroke and systemic embolism in the presence of NVAF, for the treatment and secondary prevention of transitory ischaemic attack and ischaemic stroke, as well as for secondary prevention of myocardial infarction and its related ischaemic stroke or systemic embolism | The targeted INR should be in the range 2.0–3.0 | Long-term prevention or minimally 3 months based on the presence of the transient risk factors in case of treatment of VTE |
| Unfractionated heparin (UFH) | ||
| Routine prevention of thromboembolic complications | 5,000 IU 2 or 3 times daily | |
| Treatment of acute thrombosis | Subcutaneous administration is subjected to higher levels, for example approximating 1.5–2.5 of the control of aPTT for the assessment of which the blood is taken in the middle of the interval between the injections | |
| Thromboprophylaxis in patients undergoing vascular surgery | Intravenous infusion with a dose of 20,000 IU of UFH in 1,000 mL of infusion solution using a rate of administration of 15–25 drops per minute | |
| Haemodialysis with systemic heparinization | 10,000 IU (administration in the form of a combination of the injections of 3,000–5,000 IU) under the control using an aPTT. The dose can also be administered as a continuous infusion of 20,000 IU/100 mL of infusion solution | |
| Fondaparinux | ||
| Patients undergoing orthopaedic or intraabdominal surgical interventions | 2.5 mg once daily | Until the disappearance of the risk of thrombotic complications (usually until the patient’s discharge—being up to 5–9 days after the intervention) |
| Patients following surgery for hip fracture | 2.5 mg once daily | 24 days after the patient’s discharge—being up to 5–9 days after the intervention |
| Thromboprophylaxis | 2.5 mg once daily |
aPTT, activated partial thromboplastin time; DVT, deep venous thrombosis; LMWH, low molecular weight heparin, NVAF non-valvular atrial fibrillation; PE, pulmonary embolism; UFH, unfractionated heparin; VTE, venous thromboembolism.
Our experience with anticoagulation therapy with DOACs
We performed a retrospective analysis of patients using DOACs treated at the National Centre for Haemostasis and Thrombosis in Slovakia for VTE. Totally, we included 71 adult patients treated at our centre during a representative 12 months (the last year).
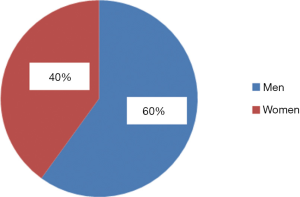
The majority were men (60%), while women comprised 40% (Figure 1).
In terms of indication, DVT had developed in 43 patients, whereas PE was detected in another 28 individuals.
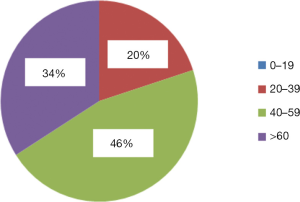
The age of patients was most commonly between 40–59 years (see Figure 2). There were no patients aged ≤19 years.
The average age for the initial development of DVT in men was 55.6 years (range, 37–88 years), and in women 50.04 years (range, 24–79 years). In the group of patients with PE, the average age in men was 45.3 years (range, 25–71 years), and in women 51.9 (range, 20–87) years.
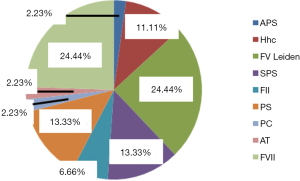
Thrombosis as the selected indication for the use of DOACs developed once (in 54%) or occurred repeatedly (46% of the cases). Sometimes, inherited or acquired thrombophilia was detected and determined to be a contributing cause (Figure 3). A family history was, however, present in only 4 cases.
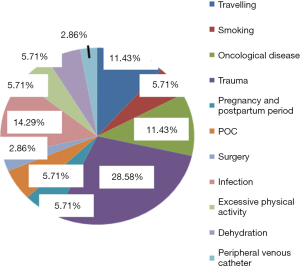
A review of acquired risk factors (circumstances) preceeding the development of VTE episodes in our patient cohort was performed and is outlined in Figure 4.
Besides the fore mentioned—“classical” forms of VTE (DVT and PE), in our group of patients, DOAC treatment was performed due to the presence of inherited thrombophilia, or due to thrombosis at unusual parts of the circulation—1 patient suffered thrombosis of the cerebral sinuses, 2 were treated with DOACs because of central retinal vein occlusion, and in 2 patients, thrombosis in the splanchnic circulation was diagnosed and 1 patient had thrombosis of the upper extremity.
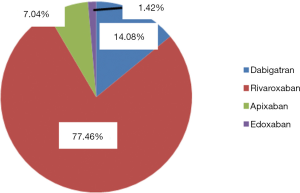
At our National Centre of Haemostasis and Thrombosis, we use most commonly rivaroxaban (77.46% of the patients) with dabigatran and apixaban being used in 10 and 5 patients. Edoxaban is used only in 1 patient (see Figure 5). The choice was driven by the preference of treating physician according to the levels of liver enzymes and parameters of the renal functions and assumption of the compliance of the particular patient.
An overview of the number of the patients taking particular doses of DOACs is presented (Table 2).
Table 2
| DOAC and its dose (mg) | Number of the patients (n) | Average age (years) |
|---|---|---|
| Dabigatran | ||
| Dabigatran 150 mg twice daily | 5 | 47.4 |
| Dabigatran 110 mg twice daily | 5 | 44.15 |
| Rivaroxaban | ||
| Rivaroxaban 20 mg once daily | 44 | 52.2 |
| Rivaroxaban 15 mg once daily | 11 | 49.07 |
| Apixaban | ||
| Apixaban 5 mg twice daily | 5 | 51.33 |
| Edoxaban | ||
| Edoxaban 60 mg once daily | 1 | 64 |
DOAC(s), direct oral anticoagulant(s).
A decreased dose of dabigatran was administered in secondary thromboprophylaxis used more than 6 months after the last thrombotic event due to the persistence of several risk factors for VTE recurrence in one patient, such as prolonged positivity of the markers of the activation of hemostasis (e.g., D-dimer levels), recurrent episodes of VTE in the past, presence of the postthrombotic syndrome, age, etc. The physician decided for this reduced dose, because only this dosage was proven to be effective without the risk of complications (higher dose showed increased anti-IIa activity above the reference range and it was dangerous due to the comorbidities of the patients, such as increased blood pressure and recent bleeding). Another reason to reduce its dose was the combined use of dabigatran and ASA due to the coexistence of sticky platelet syndrome.
A reduced dose of rivaroxaban was used in patients with gastroesophageal reflux, antrum gastritis, hepatal lesion, combined use of rivaroxaban and ASA due to the coexistence of sticky platelet syndrome, chemotherapy in a patient with active ovarian cancer, metrorrhagia, or because of the prolongation of the secondary anticoagulant thromboprophylaxis in a patient with the recidives of DVT and persistence of its risk factors.
The effectiveness of DOACs was evaluated with standard anti-IIa based on the principle of the diluted thrombin time in patients on dabigatran and anti-Xa assays in those on xabans (Table 3). We assessed trough levels because these were believed to provide easier management of patients.
Table 3
| DOAC | Test for the assessment of the effectiveness of DOAC (ng/mL) |
|---|---|
| Dabigatran | Anti-IIa activity measured 12 hours after administration |
| Dabigatran 110 mg twice daily | 47 |
| Dabigatran 150 mg twice daily | 133 |
| Rivaroxaban | Anti-Xa activity measured 24 hours after the administration |
| Rivaroxaban 15 mg daily | 102.3 |
| Rivaroxaban 20 mg daily | 58.9 |
| Apixaban | Anti-Xa activity measured 12 hours after the administration |
| Apixaban 5 mg twice daily | 122.9 |
| Edoxaban | Anti-Xa activity measured 24 hours after the administration |
| Edoxaban 60 mg daily | 62 |
DOACs, direct oral anticoagulants.
The average results of the standard coagulation parameters during the administration of DOACs are summarized in Table 4.
Table 4
| Standard coagulation test | Dabigatran | Rivaroxaban | Apixaban | Edoxaban |
|---|---|---|---|---|
| PT (%) | 99.7 | 89.8 | 85.5 | 100 |
| aPTT (s) | 33.6 | 31.5 | 33.1 | 28.6 |
| TT (s) | 62.8 | 14.7 | 14.6 | 13.8 |
aPTT reference range (22–32 s), PT reference range (75–120%), TT reference range (12–18 s). aPTT, activated partial thromboplastin time; DOACs, direct oral anticoagulants; PT, prothrombin time; TT, thrombin time.
In comparison with the results from the literature, there was no significant prolongation of prothrombin time (PT) in patients taking rivaroxaban. However, thrombin time was prolonged (as expected), in those taking dabigatran (13).
For the assessment of the activation of haemostasis during treatment with DOACs, we assessed D-dimer levels and coagulation factor VIII (FVIII) activity (Table 5).
Table 5
| DOAC and its dosage | D-dimer level (mg/L) | Coagulation factor VIII (FVIII) activity (U/mL) |
|---|---|---|
| Dabigatran | ||
| Dabigatran 110 mg twice daily | 0.40 | 1.99 |
| Dabigatran 150 mg twice daily | 0.32 | 1.62 |
| Rivaroxaban | ||
| Rivaroxaban 15 mg daily | 0.43 | 1.75 |
| Rivaroxaban 20 mg daily | 1.12 | 1.87 |
| Apixaban | ||
| Apixaban 5 mg twice daily | 0.26 | 1.86 |
| Edoxaban | ||
| Edoxaban 60 mg daily | 0.17 | NA |
D-dimer reference range (0–0.50 mg/L), FVIII reference range (0.60–1.50 U/mL). DOACs, direct oral anticoagulants; FVIII, coagulation factor VIII; NA, not available.
Discussion
D-dimer levels were generally in the reference range or increased, but not significantly, with similar conclusions for FVIII activity. This finding may be influenced by the comorbidities of patients, their age and previous episode of VTE. Surprising perhaps was the fact that in our group of patients, men were more affected than women. However, this finding may be influenced by the enhanced physical activity possible more in men and perhaps due to an increased risk of trauma associated with physical work, as confirmed by Figure 4 (trauma is here the most common acquired risk factor).
In the comparison with our reference ranges, average FVIII activity levels were moderately increased. This could be explained by the age, comorbidities and persistence of the prothrombotic tendency in the patients after previous episodes of VTE.
Conclusively, we can state that treatment with DOACs in our group of patients with VTE appears to be effective with levels of anti-IIa and anti-Xa activity within the expected 'within therapy‘ range for the particular DOACS and assays.
Our retrospective analysis has several shortcomings—first of all, it includes only the cohort of the patients treated at our centre, data is retrospective and last but not least, the results of the anti-IIa activity, anti-Xa activity, D-dimer and FVIII levels could be influenced by other comorbidities of the patients and drugs they have taken due to the thrombophilia (e.g., acetylsalicylic acid in the case of the sticky platelet syndrome), concomitant ischaemic heart disease, state after myocardial infarction and other prothrombotic states. For the improvement of such weaknesses, we plan to include more patients to form a basis for a prospective study.
Acknowledgments
Funding: We thank the projects of the Agency for the Support of Research and Development (APVV) APVV-16-0020, Scientific Grant Agency (Vega) 1/0168/16 and Vega 1/0549/19.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Emmanuel J. Favaloro) for the series “Anticoagulant and antithrombotic therapy: globally applied according to local geographical selection criteria” published in Annals of Blood. The article has undergone external peer review.
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/aob.2019.08.02). The series “Anticoagulant and antithrombotic therapy: globally applied according to local geographical selection criteria” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. In our study, patient data were retrieved from the hospital medical record system. The authors declare that the patient’s personal data have been secured.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lippi G, Mattiuzzi C, Cervellin G, et al. Direct oral anticoagulants: analysis of worldwide use and popularity using Google Trends. Ann Transl Med 2017;5:322. [Crossref] [PubMed]
- Pradaxa 110 mg tvrdé kapsuly cps dur (blis.Al/Al) 3x60 ks (180 ks) [published 17 Jan 2013, accessed 1 May 2019]. Available online: https://www.adc.sk/databazy/produkty/spc/pradaxa-110-mg-tvrde-kapsuly-588622.html
- Pradaxa 150 mg tvrdé kapsuly cps dur (blis.Al/Al) 3x60 ks (180 ks) [published 17 Jan 2013, accessed 1 May 2019]. Available online: https://www.adc.sk/databazy/produkty/spc/pradaxa-150-mg-tvrde-kapsuly-643656.html
- Praxbind 2,5 g/50 ml injekčný/infúzny roztok sol ijf (liek.inj.skl) 2x50 ml [published 17 Jan 2015, accessed 1 May 2019]. Available online: https://www.adc.sk/databazy/produkty/spc/praxbind-2-5-g-50-ml-injekcny-infuzny-roztok-819994.html
- Xarelto 15 mg filmom obalené tablety tbl flm 15 mg (blis.PP/Al) 1x28 ks [published 22 May 2013, accessed 1 May 2019]. Available online: https://www.adc.sk/databazy/produkty/spc/xarelto-15-mg-filmom-obalene-tablety-416827.html
- Xarelto tbl flm 10 mg 1x10 ks [published 22 May 2013, accessed 1 May 2019]. Available online: https://www.adc.sk/databazy/produkty/spc/xarelto-551682.html
- ELIQUIS 2,5 mg filmom obalené tablety tbl flm (blis.) 1x60 ks [published 18 May 2011, accessed 1 May 2019]. Available online: https://www.adc.sk/databazy/produkty/spc/eliquis-2-5-mg-filmom-obalene-tablety-766017.html
- ELIQUIS 5 mg filmom obalené tablety tbl flm 1x60 ks [published 18 May 2011, accessed 1 May 2019]. Available online: https://www.adc.sk/databazy/produkty/spc/eliquis-5-mg-filmom-obalene-tablety-501241.html
- Lixiana 60 mg filmom obalené tablety tbl flm 60 mg (blis.PVC/Al) 1x30 ks [published 19 Jun 2015, accessed 1 May 2019]. Available online: https://www.adc.sk/databazy/produkty/spc/lixiana-60-mg-filmom-obalene-tablety-821934.html
- Warfarin PMCS 5 mg tbl 1x100 ks [published 9 Sep 2008, accessed 2 May 2019]. Available online: https://www.adc.sk/databazy/produkty/spc/warfarin-pmcs-5-mg-346497.html
- HEPARIN LÉČIVA sol inj 50 K (liek. inj. skl.) 1x10 ml [published 28 Nov 2006, accessed 2 May 2019]. Available online: https://www.adc.sk/databazy/produkty/spc/heparin-leciva-286260.html
- Arixtra 2,5 mg/0,5 ml sol inj (striek.inj.napl.skl. s autom.zabezp.syst.) 20x0,5 ml [published 21 Mar 2007, accessed 2 May 2019]. Available online: https://www.adc.sk/databazy/produkty/spc/arixtra-2-5-mg-0-5-ml-520419.html
- Favaloro EJ, Lippi G. Interference of direct oral anticoagulants in haemostasis assays: high potential for diagnostic false positives and false negatives. Blood Transfus 2017;15:491-4. [PubMed]
Cite this article as: Stanciakova L, Dobrotova M, Plamenova I, Holly P, Bolek T, Samos M, Kubisz P, Stasko J. Anticoagulation therapy in Slovakia. Ann Blood 2019;4:22.


