
Anticoagulation therapy in France: state-of-the-art in 2020
Introduction
Anticoagulant drugs are mainly prescribed for treatment and prevention of venous thromboembolism (VTE) and for stroke and systemic embolism prevention in non-valvular atrial fibrillation (NVAF) patients. Prevalence of NVAF in France is estimated between 1% to 2% of the general population (1), and increases with aging from less than 1% in people below 60 years of age to at least 8% in those above 80 years (2). As well, the incidence and the burden of VTE, which includes pulmonary embolism and deep vein thrombosis are important and increase with age. The incidence of VTE in the French population was estimated at 184 per 100,000 subjects in 2011 with a mortality rate of 6.2% over a 12-month follow-up (3). Consequently, the number of patients receiving anticoagulant therapy per year is considerable in France, as worldwide, and has a substantial impact on the overall cost of health care. Anticoagulated French patients were estimated above 3 million in 2013 (4). We hence propose in this review to go over the available anticoagulant drugs, their indications and doses, their monitoring strategies, their perioperative management as well as their reversal as proposed by French expert groups.
Anticoagulant drugs: availability and indications
Heparin derivatives
Unfractionated heparin (UFH) is marketed in France as sodium (Heparin sodium Panpharma since March 1977 and Heparin Choay since December 1986) or calcium (Heparin calcium Panpharma since February 1978) salts. UFH is administered either by a continuous intravenous (IV) infusion or subcutaneously (SC) every 8 or 12 hours, respectively. Four low molecular weight heparin (LMWH) compounds are commercialized in France: enoxaparin (Lovenox® since April 1987 and Crusia® since July 2017), tinzaparin (Innohep® since October 1991), dalteparin (Fragmine® since December 1987) and nadroparin (Fraxiparine® since March 1985 and Fraxodi® since May 1998). Heparin derivatives are indicated for treatment of acute phase VTE, extra-cerebral arterial embolisms, acute coronary syndromes (ACS), hemodialysis and VTE prevention. UFH remains the anticoagulant of choice during cardiothoracic surgery with extracorporeal circulation and in case of extracorporeal membrane oxygenation. In most indications, a bolus of 80 IU/kg or 5,000 IU UFH is recommended before administration of IV or SC UFH. The bolus is then followed by an initial dose of 18 IU/kg/h (IV) or 500 IU/kg/24 h in 2 to 3 injections per day (SC) (5). UFH doses are then adjusted preferably according to anti-Xa activity (see below).
LMWH are used either twice daily (100 IU/kg enoxaparin or dalteparin, 85 IU/kg nadroparin) or once daily (171 IU/kg nadroparin, 175 IU/kg tinzaparin, 150 IU/kg enoxaparin).
In 2002, the French authorities issued a contraindication to the use of curative dose of LMWH in patients with severe renal impairment defined as a creatinine clearance (CrCl) calculated using Cockcroft and Gault formula, <30 mL/min. In 2017, modifications of the summaries of product characteristics (SmpC) of enoxaparin and tinzaparin have been proposed (6): enoxaparin may be used in patients with CrCl between 15 and 30 mL/min with dose reduction; full dose tinzaparin may be used in patients with CrCl between 20 and 30 mL/min, with peak anti-Xa measurement to detect accumulation (target anti-Xa: 0.5 to 1.5 IU/mL). Enoxaparin (4,000 IU od), dalteparin (5,000 IU od) and tinzaparin (4,500 IU od) are also prescribed for VTE prevention in medical ill patients. All the 4 available LMWH compounds are prescribed for VTE prevention following surgeries with doses depending on the surgery and the patient’s VTE risk (7).
Vitamin K antagonists (VKA)
Three VKA, two coumarin (warfarin and acenocoumarol) and one indane-dione (fluindione) derivatives are currently commercialized in France. Warfarin (Coumadine®) is available in 2 and 5 mg tablets. Acenocoumarol (Sintrom® and Minisintrom®) is available in 4 and 1 mg tablets, respectively. Fluindione (Previscan®) is available in 20 mg tablet in France (8). Outside France, fluindione exists only in Luxembourg and Switzerland. Fluindione is the most prescribed VKA in France since decades, but in February 2019, the French National Authority for Health prohibited the prescription of fluindione in VKA naïve patients due to the risk of immuno-allergic, nephro- and hepato-toxic side effects during the first 6 months of its administration (9). VKA were until recently the primary cause of hospitalization due to drug adverse events in France, inducing about 5,000 deaths per year due to bleedings (10). Since the commercialization of direct oral anticoagulants (DOAC), the prevalence of VKA treatment has been continuously decreasing (11). Indeed, a recent repeated cross-sectional study conducted between 2011 and 2016 revealed a steady decrease in VKA use from 56.6% to 40.8% of all NVAF included patients (12). French patients treated with VKA were estimated around 928,772 in the beginning of October 2016 (11) with 82% receiving fluindione, 13% warfarin and 5% acenocoumarol. Among all oral anticoagulant new users in 2016, 33.7% were prescribed a VKA according to Maura et al. (12). Thus in 2016, VKA remained in France the most prescribed oral anticoagulant for NVAF patients aged above 75 years and for those with a history of arterial thrombotic events or with a high hemorrhagic risk (12). This was also the case for patients included in the non-interventional cross-sectional multicenter French study of routine clinical practice (PAROS study; published in 2019) in which VKA therapy was more common than DOAC among patients with higher bleeding risk and/or worse renal function (13).
French authorities recommended coumarin derivatives or DOAC as first-line anticoagulant drugs for stroke and systemic embolism prevention in patients with NVAF, while DOAC were recommended over VKA in eligible patients by the European Society of Cardiology (14). Noticeably, VKA remain the unique oral anticoagulants recommended in atrial fibrillation (AF) patients with mechanical heart valves in France (8).
DOAC
Three DOAC, one thrombin (dabigatran) and two factor Xa inhibitors (xabans: rivaroxaban and apixaban) are currently commercialized in France. DOAC doses and indications are summarized in Table 1. Rivaroxaban 2.5 mg bid was approved by the European Medicines Agency (EMA) and the French National Agency for Medicines and Health Products Safety (ANSM) in association with aspirin and clopidogrel (P2Y12 inhibitor) for treatment of ACS in patients with low hemorrhagic risk and no previous stroke or transient ischemic attack; however its usage remains very limited in France for this indication (15).
Table 1
| Indications | Dabigatran etexilate (Pradaxa®) | Rivaroxaban (Xarelto®) | Apixaban (Eliquis®) |
|---|---|---|---|
| Presentation | 75, 110 & 150 mg; capsules | 10, 15 & 20 mg; tablets | 2.5 & 5 mg; tablets |
| VTE prevention following THRS or TKRS | 220 mg od; 150 mg od*; THRS 28–35 days; TKRS 10 days; reimbursable at 30%; since March 2008 | 10 mg od; THRS 35 days; TKRS 14 days; reimbursable at 65%; since September 2008 | 2.5 mg bid; THRS 32–38 days; TKRS 10–14 days; reimbursable at 65%; since May 2011 |
| VTE treatment and prevention | 150 mg bid; 110 mg bid+; not reimbursable; since June 2014 | 15 mg bid for 3 weeks then 20 mg od; reimbursable at 65%; since November 2012 | 10 mg bid for 7 days then 5 mg bid; reimbursable at 65%; since July 2014 |
| Stroke and systemic embolism prevention in NVAF patients$ | 150 mg bid; 110 mg bid+; reimbursable at 30%; since August 2011 | 20 mg od; 15 mg od#; reimbursable at 65%; since December 2011 | 5 mg bid; 2.5 mg bid‡; reimbursable at 65%; since November 2012 |
$, with at least one additional risk factor: previous stroke or transient ischemic attack, age ≥75 years, diabetes, arterial hypertension or symptomatic heart failure; *, if CrCl 30–50 mL/min, age ≥75 years or P-glycoprotein inhibitors; +, if age ≥80 years or verapamil treatment; #, if CrCl 30–49 mL/min; ‡, if at least 2 criteria: age ≥80 years, weight ≤60 kg and/or plasma creatinine ≥133 µM. bid, bis in die—twice daily; DOAC, direct oral anticoagulants; NVAF, non-valvular atrial fibrillation; od, omne in die—once daily; TKRS, total knee replacement surgery; THRS, total hip replacement surgery; VTE, venous thromboembolism.
A high-dimensional propensity score-matched cohort study of the French national healthcare system database followed up new users of dabigatran, rivaroxaban or VKA in NVAF patients in 2013. It revealed that dabigatran and rivaroxaban were at least as effective and safe as VKA (16). Proportion of DOAC prescription among other anticoagulant drugs continuously increased in France from the last trimester of 2012 to the third trimester of 2016 (11).
Based on the French National Health Insurance System database, a recent retrospective population-based cohort study comprising 814,446 NVAF adult patients revealed that by the end of 2015, 61% of patients received DOAC as initial anticoagulant treatment among which 46.0% were on apixaban, 42.5% on rivaroxaban and 11.5% on dabigatran. Patients receiving apixaban were older and had more comorbidities such as high blood pressure and heart or renal failure than those receiving other DOAC (17). DOAC initiators were younger and healthier compared to VKA initiators. DOAC were more frequently prescribed by cardiologists whereas general practitioners still prescribed VKA more frequently as initial anticoagulant therapy for NVAF (17). A second population-based cohort study revealed that in 2016, among 1.1 million NVAF French patients, 66% were receiving DOAC. Among 192,851 anticoagulant initiators in 2015–2016, DOAC were initiated in 66.3% of cases. Reduced doses were prescribed in 40% of DOAC new users, even though it was not always justified. Inappropriate use was identified in many situations such as concomitant intake of drugs that potentiate the hemorrhagic risk in 33% of the cases or DOAC underdosing, despite that the reduced doses of dabigatran (75 mg) and rivaroxaban (10 mg) are not approved in NVAF patients in Europe (12). As shown in the study of Huiart et al. (17), patients receiving apixaban were also older and had more comorbidities than rivaroxaban- and dabigatran-treated patients in the present one (12). The PAROS cross-sectional multicenter French study of routine clinical practice conducted between January and August 2016 revealed that among 2,027 included patients, 84.8% received DOAC (apixaban was initiated in 38.6% of the cases, rivaroxaban in 36.2% and dabigatran in 10%) and 15.1% VKA (13). Seventy-nine percent of patients treated with apixaban had doses consistent with the summaries of product characteristics (SmpC); underdosing was the most frequent inconsistency, mainly observed in elderly patients despite normal weight and renal function (13). As in the study of Maura et al. (12), apixaban was more common among older patients with a higher bleeding risk and decreased renal function than rivaroxaban and dabigatran (13). The NAXOS study, a nationwide observational retrospective study of a cohort generated from the French national healthcare insurance database, will provide new routine clinical practice data on the effectiveness and safety profiles of apixaban vs. other DOAC and VKA (18).
Recently, an observational study assessed the initial anticoagulant treatment patterns at baseline (±30 days of diagnosis) in patients with objectively confirmed VTE included in the prospective international non-interventional Global Anticoagulant Registry in the Field (GARFIELD)-VTE. It revealed that more than half of the patients (52.2%) in Europe were given DOAC (19). Six hundred and one French patients were included among which 59.4% received DOAC either alone or after parenteral therapy (19). A prospective monocentric observational study of DOAC patients admitted to emergency departments between August 2013 and April 2014 included 198 patients among which 68.7% were treated by rivaroxaban, 30.8% by dabigatran and 0.5% by apixaban. It showed that 25.8% of included patients suffered from DOAC side effects. Eighteen percent had hemorrhagic complications, 44.4% of which were categorized as major and 7.8% had thrombotic complications. In 16.2% of included patients, DOAC treatment was not consistent with the SmpC: of these, 22% were wrong initial indications and 78% were incorrect dosages (20).
In 2016, the ANSM published post-marketing data on dabigatran side effects reported by the Regional Pharmacovigilance Centers. In total, 1,624 notifications were recorded between December 2008 and August 2013, among which 49.4% were linked to hemorrhagic events (48.3% gastrointestinal, 11.7% muscular, 11% in the urinary tract, 9% cerebral and 8.3% epistaxis), 10.3% to arterial TE and 7% to VTE (21). Concerning rivaroxaban, 1,566 notifications were recorded during the same period, among which 52% were linked to hemorrhagic events (24% gastrointestinal, 11% neurologic, 8% in the urinary tract and 8% subcutaneous and muscular), 21% to TE and 6% to hematologic adverse events (21). Up till now, no post-marketing French data are available for apixaban.
Others
Other anticoagulants are available in France and have restricted indications. They are often delivered in hospital settings and/or are not widely available. They mainly include indirect (antithrombin mediated) FXa (fondaparinux; Arixtra® since March 2002) or FXa and thrombin (danaparoid; Orgaran® since July 1996) inhibitors, and direct thrombin inhibitors (argatroban; Arganova® since June 2011 and bivalirudine; Bivalirudine Accord since January 2018) all administered parenterally.
Danaparoid is given through IV or SC routes and is mainly indicated for prevention or treatment of thrombosis in type II heparin-induced thrombocytopenia (HIT). Argatroban is mainly indicated to treat type II HIT and bivalirudin is used parentally in the treatment of ST-segment elevation myocardial infarction (STEMI) patients having percutaneous coronary intervention.
Fondaparinux is given at fixed dose without any monitoring, and injected SC at a dose of 5 mg/24 h in <50 kg, 7.5 mg/24 h in 50–100 kg and 10 mg/24 h in >100 kg patients. The French Society of Vascular Medicine recommends fondaparinux 2.5 mg od for 45 days in case of initial or first recurrent isolated symptomatic superficial vein thrombosis with a thrombus over 5 cm long and located more than 3 cm from the saphenofemoral junction (22). A treatment for at least 3 months is suggested if it is located less than 3 cm from the saphenofemoral junction in the absence of any bleeding risk. For patients with CrCl of 20–30 mL/min, tinzaparin in replacement to fondaparinux is suggested at a prophylactic dose (22). Fondaparinux is also preferred to other anticoagulant drugs in Non-STEMI (NSTEMI) patients in association to aspirin and P2Y12 inhibitors as specified in the European Heart Rhythm Association guideline (23). Fondaparinux is contraindicated in patients with CrCl <30 mL/min (curative dose) and CrCl <20 mL/min (prophylactic dose).
Monitoring of anticoagulant therapy
Since the anticoagulation response to UFH greatly varies among patients, treatment at therapeutic dose should be regularly monitored and the dose adjusted using preferably a chromogenic anti-FXa assay with a target value between 0.30 and 0.70 IU/mL in most indications. If unavailable, an activated partial thromboplastin time (aPTT) assay may be used: aPTT therapeutic range at each institution should be adapted to the responsiveness of the reagent and coagulometer used in order to correspond to plasma heparin levels of 0.3 to 0.7 IU/mL (24).
Monitoring of IV UFH is performed 6 h following the onset of treatment and 4 to 6 h following any dose change. In case of SC UFH treatment, monitoring is performed 6 or 4 h after injection when administered every 12 or 8 h, respectively. Platelet count should be measured two to three times a week from day 4 to day 14 of treatment, then once a week for 1 month if heparin therapy is continued in order to rule-out any HIT (25).
While monitoring is not recommended in patients treated with LMWH at therapeutic dose, anti-Xa measurement may be considered in special situations such as patients with renal failure or elderly ones in order to detect accumulation. Over-dosage is defined as peak anti-Xa >1.5 IU/mL (tinzaparin), ~>1.4 IU/mL (enoxaparin), >1.8 IU/mL (nadroparin od) (5). Platelet count should not be monitored with LMWH except in post-operative and post-traumatic context or in case of important comorbidities or recent treatment with UFH (during the last 6 months).
VKA treatment is monitored using prothrombin time with a result expressed as international normalized ratio (INR). The latter is comprised between 2.0 and 3.0 for most indications. In specific cases, INR values between 2.5 and 3.5 are targeted. The first INR value is determined following the third VKA intake, and the second determination is done 3 to 6 days thereafter. INR is determined afterwards at least weekly during initiation of anticoagulant therapy then at least monthly when anticoagulation is stable. Following every dose change, INR is controlled 3 days afterwards and repeated 1 to 2 times per week until stabilization. A safe and accurate warfarin initiation dosing algorithm specifically devoted to the elderly has been validated and is currently used (26,27). Noteworthy, anticoagulant clinics are poorly developed in France: general practitioners and cardiologists directly manage patients under VKA therapy. Point-of-care devices are available in France for INR rapid testing. The cost of these devices is covered by the French national health insurance only for patients with mechanical heart valve (28). INR testing is also mandatory for a reliable management of the switch from VKA to DOAC; here, DOACs can be started when INR is below 2 for apixaban and dabigatran and below 2.5 for rivaroxaban in case of VTE treatment or prevention or below 3 in NVAF patients (4,23). Inversely, when a switch from DOAC to VKA is performed, an overlapping of 3 days with dabigatran intake is required in patients with CrCl above 50 mL/min and of 2 days in those with CrCl between 30 and 50 mL/min. As for xabans, they are concomitantly administered with VKA for 2 days, and then until INR is over 2.0. It is to mention that INR should be performed just before DOAC administration in order to limit their interference.
DOAC treatment does not require monitoring. However, specific anti-IIa and anti-Xa assays are commercialized and available to assess dabigatran and xabans plasma levels in specific situations such as urgent invasive procedures, hemorrhage or acute liver or renal failure. A French study performed in 30 laboratories using 4 dabigatran and 5 rivaroxaban/apixaban calibrated assays on 3 analyzers revealed an inter-laboratory coefficient of variation below 18% for concentrations above 100 ng/mL and higher for concentrations around 40 ng/mL. Therefore, calibrated DOAC assays commercialized allow reliable measurement of anticoagulant plasma concentrations with a relatively good between-laboratory agreement even though improvement of their performances is required especially for low concentrations assessment (29). Safety thresholds of anti-Xa and anti-IIa DOAC levels have been previously discussed in the literature. A threshold of 50 ng/mL has been proposed by the subcommittee on control of anticoagulation of the International Society on Thrombosis and Hemostasis (ISTH) and the French Working group on perioperative hemostasis (GIHP) to warrant antidote administration in case of serious bleeding in DOAC patients. As well a safety hemostatic threshold of 30 ng/mL is considered in case of high bleeding risk surgery (30,31). As for IV thrombolysis for acute ischemic stroke in patients on DOAC, joint propositions from the French Vascular Neurology Society and the French Study Group on Hemostasis and Thrombosis (GFHT) and based on DOAC concentrations were issued in 2018 (32).
Apart from anticoagulant effect monitoring, a structured follow-up of DOAC patients before initiation and at least annually are recommended by the European Heart Rhythm Association (23). Monitoring consists of assessing the hemoglobin level, platelets count, full coagulation panel and renal and hepatic functions. The recheck interval depends on patient comorbidities (23). Rechecking should be done yearly, every 6 months for patients above 75 years of age or every CrCl/10 months in renal insufficient patients. Switching between heparin derivatives and DOAC is relatively simple to manage. DOAC can be started when the next dose of LMWH or SC UFH is due or 2 to 4 h after IV UFH discontinuation. Inversely, UFH or LMWH can be initiated when the next DOAC dose is due (23). However, in this later case monitoring of UFH is complex due to the interference of xaban on UFH anti-Xa and conversely.
Fondaparinux presents little inter- and intra-subject variability therefore its monitoring is not required. Anticoagulant monitoring is generally not required in danaparoid patients except in particular cases such as renal failure, extreme body weight or old patients (>75 years). If performed, a specific anti-Xa assay is required with a target range comprised between 0.5 and 0.8 IU/mL (33). Treatment with argatroban is monitored by a specific chromogenic substrate assay or an aPTT assay with a target ratio between 1.5 and 3 times the control aPTT (34). Bivalirudin may be monitored by the activated clotting time if required.
Anticoagulants reversal agents and bleeding management
Despite its poor therapeutic index, protamine remains the unique commercialized antidote of heparin derivatives. Protamine induces hemodynamic side effects, increases histamine plasma concentration and has an anticoagulant activity; this explains why the dose of protamine is limited to 1 mg per 100 IU heparin and protamine-to-heparin ratio must not be above 1.1; otherwise bleeding risk would be significantly increased (32,35). Protamine completely neutralizes the thrombin-inhibitory activity of LMWH but only partially anti-Xa activity. Molecular size as well as the sulfonation degree makes it impossible to completely neutralize LMWH anticoagulant activity (36).
Major bleeding in patients while on VKA therapy is managed by co-administration of prothrombin complex concentrate (PCC, 25 IU/kg IV or dose based on INR if rapidly available) plus vitamin K (10 mg per os) in order to rapidly achieve an INR below 1.5 and maintain a normal coagulation profile. INR should be measured at least 30 min after the infusion (37). If INR remains above 1.5, a second dose of PCC should be administered and INR is rechecked 6 h later (37). Indeed, the synthesis of new functional clotting factors by the liver following vitamin K administration in VKA patients requires at least 6 h and more likely 12 to 24 h to significantly lower the INR. The French prospective observational cohort EPAHK study showed that guideline-concordant VKA reversal with PCC and vitamin K within 8 hours after admission was associated with a significant decrease in 7-day mortality (38,39). According to the French guidelines, management of VKA over-dosage without bleeding is based on INR value and INR target range and is summarized in Table 2 (40).
Table 2
| INR measured value | INR target 2–3 | INR target 2.5–4.5 |
|---|---|---|
| INR <4 | No intervention | No intervention |
| INR 4–6 | Omit 1 dose | No intervention |
| INR 6–10 | Stop VKA treatment; vitamin K 1–2 mg per os | Omit 1 dose; discuss vitamin K administration |
| INR >10 | Stop VKA treatment; vitamin K 5 mg per os | Stop VKA treatment; hospitalization/discussion with a specialist |
INR, international normalized ratio; VKA, vitamin K antagonists.
Bleeding during anticoagulant therapy remains a crucial issue with DOAC. Idarucizumab is available for dabigatran reversal since February 2016 in case of urgent surgery or life-threatening bleeding. Andexanet-alfa is not yet available in France, although in March 2019, the EMA human medicines committee (CHMP) recommended granting a conditional marketing authorization in the European Union for andexanet-alfa use in adult patients receiving xabans with life threatening or uncontrolled bleeding. Although these specific antidotes are available or imminent, they are very expensive in comparison to those of heparin derivatives and VKA.
Despite the lack of robust data, the European Heart Rhythm Association and the GIHP have proposed the use of activated PCC (aPCC; 30–50 IU/kg IV) or non-activated (PCC; 50 IU/kg IV) for life-threatening bleeding or when immediate hemostatic support is required in xaban-treated patients (23,41). These are also considered in patients under dabigatran when idarucizumab is not available especially since recent data from the French Pharmacovigilance database suggested that idarucizumab was not superior to aPCC or PCC in terms of fatality rate (17.6% vs. 18.6%) (42). The choice between PCC and aPCC depends on their availability, the clinical situation and the experience of the physicians (23).
A prospective multicenter observational cohort study in France and Belgium of 732 patients with severe bleeding while treated with dabigatran (28%), rivaroxaban (64%) or apixaban (7.2%) was conducted between June 2013 and November 2015 on behalf of GIHP to describe the management strategies and outcomes. Thirty-seven percent of the bleeds were gastrointestinal bleedings and 24% intracranial. PCC or aPCC were administered in 38% of the cases and the mortality by day 30 was 14% (43). In patients with acute major bleeding associated with the use of a xaban and treated with andexanet-alfa, the mortality rate was of 14% within 30 days after enrollment; mortality rate was of 18.8% and 18.9% in patients treated with dabigatran and receiving idarucizumab for uncontrolled bleeding or an urgent procedure, respectively (44,45). Recombinant FVIIa is no longer included in the French guidelines for DOAC management in context of bleeding or urgent invasive procedure owing to its uncertain benefit-risk balance.
The GIHP proposed in 2016 an algorithm for dabigatran management in patients with hemorrhagic events (Figure 1) (41). The management depends on the type and localization of bleeding and on DOAC concentration. This algorithm is also used by extension in xaban-treated patients. When idarucizumab is administered, it is recommended to measure dabigatran concentration before and 12 to 18 hours after in order to determine if a subsequent second dose of 5 g is needed (38).
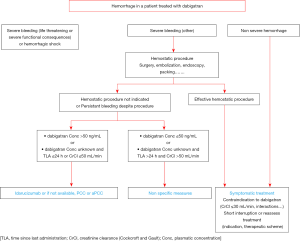
Perioperative management of anticoagulant therapy
Every year, 10% to 15% of anticoagulant patients require urgent surgeries or invasive procedures (46). Elective invasive procedures with a high to moderate hemorrhagic risk require interruption of VKA therapy 5 days before without LMWH bridging. In patients with concomitant high thrombotic risk (patients with mechanical heart valves, a history of stroke or VTE in the preceding 3 months or more than 2 VTE with at least one idiopathic), perioperative bridging therapy is recommended. Therefore, LMWH is started 2 days following VKA stop and the last dose is given 24 hours before the procedure. If INR is still above the value for the procedure (cut-off of 1.5 or 1.3), 2.5 mg of vitamin K is given orally. In case of expedited surgery with high bleeding risk, 5 mg vitamin K with or without PCC is administered to reach an INR of <1.5 (<1.3 in neurosurgery) (38). Procedures with a low risk of hemorrhage does not necessitate treatment discontinuation (38). When discontinued, VKA therapy should be resumed at least 6 hours after the end of the procedure. When necessary, LMWH is added to VKA 24 hours following interventions with low bleeding risk and 48 to 72 hours after those with high bleeding risk (47).
In case of elective surgery at low bleeding risk in patients on DOAC, the GIHP suggested that patients interrupt DOAC the night before irrespective of the type of drug and to resume therapy 6 hours or more after the end of the invasive procedure (Figure 2). For invasive procedures at high bleeding risk, it was suggested to interrupt rivaroxaban and apixaban 3 days before. Dabigatran should be interrupted according to the renal function, 4 and 5 days if CrCl is higher than 50 mL/min and between 30 and 50 mL/min, respectively. For invasive procedures at very high bleeding risk such as intracranial neurosurgery or neuraxial anesthesia, longer interruption times were suggested. Finally, bridging with parenteral anticoagulation and measurement of DOAC concentrations should no longer routinely be used (30). The objective of the proposed management strategy is to ensure a minimal pre-procedural DOAC concentration (48). In case of urgent invasive procedure, algorithms for the management of dabigatran-treated patients are summarized in Figures 3,4 (41). The same are used by extension in patients receiving xaban. Full dose (curative) can be reintroduced 24 to 72 hours following the procedures. If venous thromboprophylaxis is mandatory, heparin derivatives or fondaparinux can be administered at least 6 hours after the procedure. DOAC is thereafter reintroduced at least 12 hours following the last SC LMWH. In the PAUSE study, a standardized perioperative DOAC therapy interruption and resumption strategy for elective surgery in AF patients, based on DOAC pharmacokinetic properties, procedure-associated bleeding risk, and CrCl levels without heparin bridging or coagulation function testing was evaluated. This strategy, very similar to those proposed by the GIHP (DOAC omitted for 1 day before a low-bleeding-risk procedure and 2 days before a high-bleeding-risk procedure) was associated with low rates of major bleeding and arterial TE (48,49).
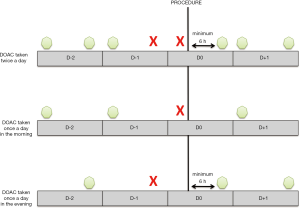
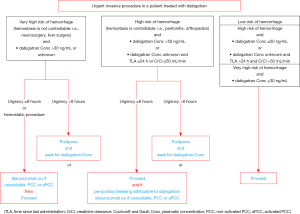
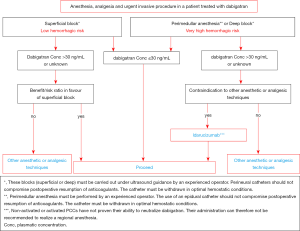
Anticoagulation in specific population groups
Despite their improved ease of use compared to VKA and heparin derivatives, DOAC need to be managed cautiously in patients with particular clinical profile with regards to weight, renal and hepatic function, drug-drug interactions and risks of bleeding.
Until more data are available, obese patients are managed according to the ISTH guidance statements which suggest not to use DOAC in patients with BMI >40 kg/m2 or weight >120 kg and to check peak and trough drug-specific levels if DOAC are used in these patients and to change to VKA if the level is found to be below the expected range (50). Prospective studies of DOAC use after bariatric surgery are very limited and available information comes mostly from retrospective studies of small number of patients and numerous case reports. VKA is still preferred over DOAC in such patients as they can be monitored with the INR.
In case of severe renal failure (CrCl <30 mL/min) dabigatran is the unique DOAC contraindicated whereas in France as in all the European countries, xabans can be used with caution in patients with CrCl between 15 and 29 mL/min with the reduced dose regimen (23). In patients with end-stage chronic kidney disease (CrCl <15 mL/min) or on dialysis, VKA (INR 2 to 3) are the unique recommended oral anticoagulant drug in France. Since June 2017, enoxaparin can be prescribed in patients with a CrCl ≥15 mL/min. All three DOAC are contraindicated in patients with hepatic disease associated with coagulopathy that results in a clinically relevant bleeding risk (23).
In patients with VTE associated to antiphospholipid antibody syndrome (APLS), VKA is the best option for long term treatment especially for triple positive APLS (positive lupus anticoagulant associated to positive anticardiolipin and anti-β2-glycoprotein antibodies) as well as in APLS associated to arterial TE (51). An INR value of 2 to 3 is targeted. For patients with recurrent arterial or venous thrombosis despite adequate treatment, addition of low-dose aspirin, increase of INR target to 3 to 4 or switch to LMWH may be considered according to the updated guidelines of the European League Against Rheumatism (52). In women with prior obstetric APLS, combination treatment with low-dose aspirin and prophylactic dose of LMWH during pregnancy is recommended (52). Oral anticoagulant treatment choice in simple positive venous APLS remained controversial since the evidence of DOAC efficacy and safety compared to VKA is limited by small samples and short follow-up retrospective studies (53). In contrast, the use of DOAC is now proposed in the management of patients with acute HIT, not only by the American Society of Hematology (54), but also more recently by the GIHP (25).
In pregnant women, the mainstay of anticoagulant treatment is LMWH with no need of monitoring. In breastfeeding mothers, warfarin is the recommended oral anticoagulant therapy that can be started 2 to 5 days post-partum. DOAC are contraindicated.
Optimal therapy of VTE in cancer patients is still uncertain. The French Society for Vascular Medicine suggests treatment with LMWH with no subsequent switch to any other anticoagulant drugs for patients with cancer in the absence of contraindication to LMWH (22). However, according to the 2018 guidelines of the ISTH and the 2019 guidelines of the International Initiative on Thrombosis and Cancer (ITAC) academic working group, DOAC can be used especially in patients with low bleeding risk, in the absence of drug-drug interactions with current systemic therapy, and of gastrointestinal cancers or cancers at risk of bleeding from genitourinary tract, bladder or nephrostomy tubes, or with duodenal ulcers, gastritis, esophagitis or colitis (55,56).
Conclusions
Both in France and worldwide, DOAC usage continues to grow-up as a replacement of VKA-heparin derivatives therapies. Further data are still needed to warrant favorable benefit-risk balance of DOAC compared to VKA in special populations and for management of DOAC in urgent situations.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Emmanuel J. Favaloro) for the series “Anticoagulant and antithrombotic therapy: globally applied according to local geographical selection criteria” published in Annals of Blood. The article has undergone external peer review.
Conflicts of Interest: The series “Anticoagulant and antithrombotic therapy: globally applied according to local geographical selection criteria” was commissioned by the editorial office without any funding or sponsorship. Isabelle Gouin-Thibault and Virginie Siguret: Bristol-Myers Squibb/Pfizer, Bayer Healthcare AG and Boehringer Ingelheim; Pierre Albaladejo: Bayer Healthcare AG, Bristol-Myers Squibb/Pfizer, Sanofi Aventis, Portola, Aspen; Yves Gruel: Bayer Healthcare AG, Sanofi Aventis, Aspen, LFB, Stago; The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Charlemagne A, Blacher J, Cohen A, et al. Epidemiology of atrial fibrillation in France: extrapolation of international epidemiological data to France and analysis of French hospitalization data. Arch Cardiovasc Dis 2011;104:115-24. [Crossref] [PubMed]
- Agence nationale de sécurité du médicament et des produits de santé. Les nouveaux anticoagulants oraux dans la fibrillation auriculaire: ce qu’il faut savoir. 2012.
- Bouée S, Emery C, Samson A, et al. Incidence of venous thromboembolism in France: a retrospective analysis of a national insurance claims database. Thromb J 2016;14:4-12. [Crossref] [PubMed]
- Agence nationale de sécurité du médicament et des produits de santé. Les anticoagulants en France en 2014: état des lieux, synthèse et surveillance. 2014.
- Gouin-Thibault I, Lecompte T, Sie P, et al. Anticoagulants usuels: maniement et gestion des complications. EMC - AKOS (Traité de Médecine) 2013;8:1-8.
- Agence nationale de sécurité du médicament et des produits de santé. Lettre aux professionnels de la santé. LOVENOX® (énoxaparine sodique): mise à jour de l’expression du dosage, de sa posologie dans le traitement de la thrombose veineuse profonde (TVP), et de l’embolie pulmonaire (EP) et de son utilisation en cas d’insuffisance rénale sévère - Lettre aux professionnels de santé. 2017.
- Samama CM, Gafsou B, Jeandel T, et al. Prévention de la maladie thromboembolique veineuse postopératoire. Actualisation 2011. Texte court French Society of Anaesthesia and Intensive Care. Guidelines on perioperative venous thromboembolism prophylaxis. Update 2011. Short text. Ann Fran Anesth Réa 2011;30:947-51. [Crossref]
- Haute Autorité de Santé. Haute Autorité de Santé, direction de l‘évaluation médicale, économique et de santé publique. Commission de la transparence. 2018.
- Haute Autorité de Santé. Haute Autorité de Santé, direction de l‘évaluation médicale, économique et de santé publique. 2019.
- Bénard-Laribière A, Miremont-Salamé G, Pérault-Pochat MC, et al. Incidence of hospital admissions due to adverse drug reactions in France: the EMIR study. Fundam Clin Pharmacol 2015;29:106-11. [Crossref] [PubMed]
- Haute Autorité de Santé. Haute Autorité de Santé, direction de l‘évaluation médicale, économique et de santé publique. 2016.
- Maura G, Billionnet C, Drouin J, et al. Oral anticoagulation therapy use in patients with atrial fibrillation after the introduction of non-vitamin K antagonist oral anticoagulants: findings from the French healthcare databases, 2011-2016. BMJ Open 2019;9:e026645 [Crossref] [PubMed]
- Falissard B, Picard F, Mahe I, et al. Apixaban for prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation in France: The PAROS cross-sectional study of routine clinical practice. Arch Cardiovasc Dis 2019;112:400-9. [Crossref] [PubMed]
- Kirchhof P, Benussi S, Kotecha DESC Scientific Document Group, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893-962. [Crossref] [PubMed]
- Cayla G, Leclercq F, Schmutz L, et al. Syndrome coronarien aigu: y a-t-il une place pour les anticoagulants oraux directs? Presse Med 2016;45:919-25. [Crossref] [PubMed]
- Blin P, Dureau-Pournin C, Bénichou J, et al. Comparative real-life effectiveness and safety of dabigatran or rivaroxaban vs. vitamin k antagonists: a high-dimensional propensity score matched new users cohort study in the french national healthcare data system SNDS. Am J Cardiovasc Drugs 2020;20:81-103. [Crossref] [PubMed]
- Huiart L, Ferdynus C, Renoux C, et al. Trends in initiation of direct oral anticoagulant therapies for atrial fibrillation in a national population-based cross-sectional study in the French health insurance databases. BMJ Open 2018;8:e018180 [Crossref] [PubMed]
- Picard F, Van Ganse E, Ducrocq G, et al. EvaluatioN of ApiXaban in strOke and systemic embolism prevention in patients with non-valvular atrial fibrillation in clinical practice Setting in France, rationale and design of the NAXOS: SNIIRAM study. Clin Cardiol 2019;42:851-9. [Crossref] [PubMed]
- Haas S, Ageno W, Weitz JI, et al. Anticoagulation therapy patterns for acute treatment of venous thromboembolism in GARFIELD-VTE patients. J Thromb Haemost 2019;17:1694-706. [Crossref] [PubMed]
- Lafon T, Vallejo C, Hadj M, et al. Misuse and adverse effects of new direct oral anticoagulants: a prospective observational study in patients admitted to an emergency unit of a French university hospital. Therapie 2018;73:209-15. [Crossref] [PubMed]
- Agence nationale de sécurité du médicament et des produits de santé. Compte rendu de séance. Réunion du Comité technique de Pharmacovigilance – CT012016053. 2016.
- Quéré I, Elias A, Maufus M, et al. Unresolved questions on venous thromboembolic disease. Consensus statement of the French Society for Vascular Medicine (SFMV). J Med Vasc 2019;44:28-70. [Crossref] [PubMed]
- Steffel J, Verhamme P, Potpara T, et al. The 2018 European Heart Rythm Association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J 2018;39:1330-93. [Crossref] [PubMed]
- Garcia DA, Baglin TP, Weitz JI, et al. Parenteral anticoagulants: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e24S-43S.
- Gruel Y, De Maistre E, Pouplard C, et al. Diagnosis and management of heparin-induced thrombocytopenia: Proposals from the French Working Group on Perioperative Haemostasis (GIHP) and the French Study Group on Thrombosis and Haemostasis (GFHT), in collaboration with the French Society for Anesthesia and Intensive Care (SFAR). Available online: https://sfar.org/propositions-tih-gihp-gfht-sfar
- Siguret V, Gouin I, Debray M, et al. Initiation of warfarin therapy in elderly medical inpatients: a safe and accurate regimen. Am J Med 2005;118:137-42. [Crossref] [PubMed]
- Moreau C, Pautas E, Gouin-Thibault I, et al. Predicting the warfarin maintenance dose in elderly inpatients at treatment initiation: accuracy of dosing algorithms incorporating or not VKORC1/CYP2C9 genotypes. J Thromb Haemost 2011;9:711-8. [Crossref] [PubMed]
- Azarnoush K, Camilleri L, Aublet-Cuvelier B, et al. Results of the first randomized French study evaluating self-testing of the International Normalized Ratio. J Heart Valve Dis 2011;20:518-25. [PubMed]
- Gouin-Thibault I, Freyburger G, de Maistre E, et al. Evaluation of dabigatran, rivaroxaban and apixaban target-specific assays in a multicenter French study. Thromb Res 2017;158:126-33. [Crossref] [PubMed]
- Albaladejo P, Bonhomme F, Blais N, et al. Management of direct oral anticoagulants in patients undergoing elective surgeries and invasive procedures: updated guidelines from the French working group on perioperative haemostasis (GIHP). Anaesth Crit Care Pain Med 2017;36:73-6. [Crossref] [PubMed]
- Levy JH, Ageno W, Chan NC, et al. When and how to use antidotes for the reversal of direct oral anticoagulants: guidance from the SSC of the ISTH. J Thromb Haemost 2016;14:623-7. [Crossref] [PubMed]
- Touzé E, Gruel Y, Gouin-Thibault I, et al. Intravenous thrombolysis for acute ischaemic stroke in patients on direct oral anticoagulants. Eur J Neurol 2018;25:747-e52. [Crossref] [PubMed]
- Aspen. Orgaran SPC 2019.
- Aguettant. Arganova SPC 2019.
- Ni Ainle F, Preston R, Jenkins V, et al. Protamine sulfate down-regulates thrombin generation by inhibiting factor V activation. Blood 2009;114:1658-65. [Crossref] [PubMed]
- Boer C, Meesters MI, Veerhoek D, Vonk ABA. Anticoagulant and side-effects of protamine in cardiac surgery: a narrative review. Br J Anaesth 2018;120:914-27. [Crossref] [PubMed]
- Sokolowska E, Kalaska B, Miklosz J, et al. The toxicology of heparin reversal with protamine: past, present and future. Expert Opin Drug Metab Toxicol 2016;12:897-909. [Crossref] [PubMed]
- Lapostolle F, Siguret V, Martin AC, et al. Vitamin K antagonists and emergencies. Eur J Emerg Med 2018;25:378-86. [Crossref] [PubMed]
- Tazarourte K, Riou B, Tremey B, et al. Guideline-concordant administration of prothrombin complex concentrate and vitamin K is associated with decreased mortality in patients with severe bleeding under vitamin K antagonist treatment (EPAHK study). Crit Care 2014;18:R81. [Crossref] [PubMed]
- Haute Autorité de Santé. Prise en charge des surdosages en antivitamines K, des situations à risque hémorragique et des accidents hémorragiques chez les patients traités par antivitamines K en ville et en milieu hospitalier. Sang Thromb Vaiss 2008; 20 (n° special juillet 2008).
- Albaladejo P, Pernod G, Godier A, et al. Management of bleeding and emergency invasive procedures in patients on dabigatran: updated guidelines from the French working group on perioperative haemostasis (GIHP). Anaesth Crit Care Pain Med 2018;37:391-9. [Crossref] [PubMed]
- Trinh-Duc A, Lillo-Le Louet A, Tellier E, et al. Interpretation of idarucizumab clinical trial data based on spontaneous reports of dabigatran adverse effects in the French Pharmacovigilance database. Thromb Res 2016;146:43-5. [Crossref] [PubMed]
- Albaladejo P, Samama CM, Sié P, et al. Management of severe bleeding in patients treated with direct oral anticoagulants: an observational registry analysis. Anesthesiology 2017;127:111-20. [Crossref] [PubMed]
- Connolly SJ, Crowther M, Eikelboom JW, et al. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med 2019;380:1326-35. [Crossref] [PubMed]
- Pollack CV, Reilly PA, van Ryn J, et al. Idarucizumab for dabigatran reversal - full cohort analysis. N Engl J Med 2017;377:431-41. [Crossref] [PubMed]
- Sherwood MW, Douketis JD, Patel MR, et al. Outcomes of temporary interruption of rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: results from the rivaroxaban once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation (ROCKET AF). Circulation 2014;129:1850-9. [Crossref] [PubMed]
- Witt DM, Clark NP, Kaatz S, et al. Guidance for the practical management of warfarin therapy in the treatment of venous thromboembolism. J Thromb Thrombolysis 2016;41:187-205. [Crossref] [PubMed]
- Godier A, Dincq AS, Martin AC, et al. Predictors of pre-procedural concentrations of direct oral anticoagulants: a prospective multicenter study. Eur Heart J 2017;38:2431-9. [Crossref] [PubMed]
- Douketis JD, Spyropoulos AC, Duncan J, et al. Perioperative management of patients with atrial fibrillation receiving a direct oral anticoagulant. JAMA Intern Med 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Martin K, Beyer-Westendorf J, Davidson BL, et al. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost 2016;14:1308-13. [Crossref] [PubMed]
- Agence nationale de sécurité du médicament et des produits de santé. Lettre aux professionnels de santé. 2019.
- Tektonidou MG, Andreoli L, Limper M, et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis 2019;78:1296-304. [Crossref] [PubMed]
- Dufrost V, Risse J, Reshetnyak T, et al. Increased risk of thrombosis in antiphospholipid syndrome patients treated with direct oral anticoagulants. Results from an international patient-level data meta-analysis. Autoimmun Rev 2018;17:1011-21. [Crossref] [PubMed]
- Cuker A, Arepally GM, Chong BH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv 2018;2:3360-92. [Crossref] [PubMed]
- Khorana AA, Noble S, Lee AYY, et al. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: guidance from the SSC of the ISTH. J Thromb Haemost 2018;16:1891-4. [Crossref] [PubMed]
- Farge D, Frere C, Connors JM, et al. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol 2019;20:e566-81. [Crossref] [PubMed]
Cite this article as: Jourdi G, Mansour A, Vayne C, Godon A, Tacquard C, Siguret V, Albaladejo P, Gruel Y, Gouin-Thibault I; French Study Group on Hemostasis and Thrombosis (GFHT) & French Working group on perioperative hemostasis (GIHP). Anticoagulation therapy in France: state-of-the-art in 2020. Ann Blood 2020;5:3.


