
A narrative review: the role of DOCK2 in immune-related diseases, hematopoietic or vascular diseases and solid tumor
Introduction
Dedicator of cytokinesis 2 (DOCK2) belongs to the DOCK protein family, which contains 11 members, named DOCK1 (DOCK180) to DOCK11. According to the difference of their structure and activated substrates, DOCK family proteins can be divided into four subgroups-DOCK-A, DOCK-B, DOCK-C and DOCK-D, and DOCK2 belongs to DOCK-A subgroup, which contains another two proteins-DOCK1 and DOCK5 (1).
There are two types of guanine-nucleotide exchange factors (GEFs) that can activate Rho small GTPases—traditional Dbl-GEFs and non-traditional DOCK-GEFs, and DOCK2 belongs to the latter (2). Traditional Dbl-GEFs contains two conserved domains, the Dbl homology (DH) domain and the pleckstrin homology (PH), the former having the catalytic activity of GTPases (3,4) and the latter interacting with phospholipid , which may be related to the binding of GEFs to membrane (5,6). However, the DOCK proteins do not contain either of these domains. Instead, their N-terminal regions contain DOCK homology region 1 (DHR-1) binding to PIP3 to mediate DOCK membrane localization, and their C-terminal regions contain DOCK homology region 2 (DHR-2) to catalyse the activation of small GTPases (7-10). In addition to DHR-1 and DHR-2, there is still another domain located in the N-terminal of DOCK2, Src-homology 3 (SH3), which is a domain unique to DOCK-A and DOCK-B subgroups, but not DOCK-C and DOCK-D, and it can interact with proteins containing proline-rich sequences such as engulfment and cell motility (ELMO) (9,11). Besides, DOCK2 has a segment of polybasic amino acids (PAA) at the C-terminal, and its interaction with phosphatidic acid (PA) may also be involved in DOCK2 localization (12,13) (Figure 1).
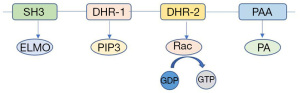
As GEFs, the main function of DOCK protein family is to mediate the activation of Rho small GTPases by catalyzing GDP-GTP conversion (7). Rho small GTPases family is composed of 4 members—RhoA, Cdc42, Rac and less known Rnd (14,15). DOCK2 mainly activates Rac and then activated Rac participates in the regulation of various cellular functional activities, such as cytoskeleton remodeling and cytokine secretion (11,16-19).
DOCK2 is mainly expressed in hematopoietic tissues, especially immune cells such as lymphocytes, neutrophils and plasmacytoid dendritic cells (pDC), so it plays an important role in the regulation of the immune system (20-22). However, DOCK2 is also expressed in other tissues such as prostate cancer and myeloma, and may affect their prognosis (23,24). In addition, under some circumstances, DOCK2 can be induced to express. For example, increased platelet-derived growth factor-BB (PDGF-BB) release after vascular injury can induce the expression of DOCK2 in smooth muscle cell (SMC) and participate in the regulation of vascular remodeling (25).
Dock2 biallele deficiency can cause severe combined immunodeficiency, which is clinically characterized by early onset, invasive infection, and it may be related to T cell, B cell, and NK cell dysfunction caused by DOCK2 deficiency (26). However, with the deepening of research on DOCK2, more and more studies have shown that DOCK2 not only affects immune function, but also is closely related to the occurrence and development of a variety of diseases. This article focuses on the relationship between DOCK2 and diseases and reveals its role in immune-related diseases, hematopoietic and vascular diseases, and tumors. A deeper understanding of DOCK2’s role in the development and progression of disease is conducive to the discovery of new therapeutic targets and methods to improve the survival of some patients. We present the following article in accordance with the Narrative Review reporting checklist (available at https://aob.amegroups.com/article/view/10.21037/aob-21-5/rc).
DOCK2 and immune-related diseases
DOCK2 is expressed in immune cells and can regulate the development (27-29), migration (20,30,31), activation (32) and some other processes of immune cells. Therefore, DOCK2 plays an important role in the occurrence and development of a variety of immune-related diseases, including immunodeficiency diseases and autoimmune diseases.
Congenital DOCK2 biallele mutation can cause severe combined immune deficiency (CID), and it is autosomal recessive. Its clinical characteristics are susceptible to a variety of bacteria and viruses at a young age, and the infection is aggressive and difficult to control. The molecular mechanism has not been fully elucidated. However, in such patients, the number of T cells decreases and the activation of Rac1 and the polymerization of actin in T cells are abnormal, and the secretion of interferon by NK cells is also impaired. Abnormalities in the number or function of various immune cells may partly contribute to CID (33). Some case has also reported that DOCK2 deficient patient may have increased IgM, but more case data are needed to support this conclusion (34).
DOCK2 also affects the pathogenesis and disease severity of systemic lupus erythematosus by regulating the secretion of typeⅠinterferon. After the stimulation of Toll like receptor (TLR) 7 or TLR9 by RNA or DNA, respectively, plasmacytoid dendritic cells (pDC) are activated (35-38). Then DOCK2 activates Rac1 in a TLR-independent manner, the latter phosphorylates IκB kinase (IKK) α, then activated IKKα promotes the activation and nuclear transposition of transcription factor interferon regulatory factor (IRF) 7, thereby promoting the secretion of type Ⅰ interferon (IFN) (18). Through its N-terminal interaction with DOCK2, B cell adaptor for PI3K (BCAP) synergistic promotes Rac1 activation and IKKα phosphorylation, and also participates in the secretion of typeⅠinterferon and the development of lupus disease (39). However, DOCK2 only participates in a branch of interferon secretion regulation pathway. After TLR7 or TLR9 are stimulated, MyD88 can be recruited and activated, and then the downstream IKKα and IRF7 can be activated (40). In addition, other molecules, such as TRAF3 and AP, are also involved in the regulation of interferon secretion (41). The etiology of lupus disease is still unclear, and DOCK2 may only play a partial regulatory role in its development.
DOCK2 is also associated with the pathogenesis of Alzheimer’s disease. Normally, DOCK2 is low expressed in brain tissue; however, the number of cells expressing DOCK2 in the brain of Patients with Alzheimer’s disease is significantly increased. In addition, the expression of DOCK2 in microglia promotes the secretion of TNFα and MCP-1 after LPS stimulation, which exacerbates the inflammatory damage of neurons and may promote the development of Alzheimer’s disease. Another evidence is that the offspring of DOCK2 knockout mice bred with Alzheimer’s mice have reduced amyloid beta-deposits in the hippocampus, compared with the control group (42). In conclusion, DOCK2 may, to some extent, promote the development of Alzheimer’s disease through immune-related injuries.
Besides, DOCK2 is involved in the inflammatory response and graft rejection. When DOCK2 was deficient, Rac could not be activated. After TCR was stimulated, IL-4R migration from the cell surface to lysosomes was blocked, resulting in impaired IL-4R degradation and enhanced TH2 immune effect, which could lead to more severe blepharitis in mice and longer duration of inflammation after infection (43). Studies have shown that DOCK2 defect in transplant recipients can prolong the survival time of cardiac allograft in their body. The mechanism is not clear, but it may be related to reduced T cell activation and infiltration into transplanted tissue (44).
In general, DOCK2 is essential to maintain the normal function of the immune system. DOCK2 abnormalities are involved in the occurrence and development of a variety of immune-related diseases, such as CID, systemic lupus erythematosus, Alzheimer’s disease, etc., but more studies are needed to reveal their pathogenesis.
DOCK2 and hematopoietic or vascular diseases
DOCK2 is significantly expressed in hematopoietic cells and is essential for the normal function and development of hematopoietic cells (20,45-47). In malignant diseases of hemopoietic system, DOCK2 has a high probability of abnormality and significantly affect the prognosis of the disease.
After CXCL12 stimulates the multiple myeloma receptor CXCR4, DOCK2 activates Rac1, and then promotes the integrinα4β1 of myeloma cells to bind to other cells that express VCAM-1, enhancing the adhesion ability of myeloma cells, and promoting their homing to bone marrow (24,48). Myeloma cells also express another receptor, sphingosine-1-phosphate receptor 1 (S1P1). After the stimulation of its ligand sphingosine-1-phosphate (S1P), the affinity of integrinα4β1 is up-regulated, and its binding with VCAM1 is more efficient. In addition, S1P is also involved in the activation of DOCK2-Rac1 pathway (49). These two pathways synergistically regulate the progression of multiple myeloma.
With the induction of Wnt5a, the proline-rich domain (PRD) in the cytoplasm of receptor tyrosine kinase-like orphan receptor 1 (ROR1) interacts with SH3 domain of DOCK2 which activates Rac1 and Rac2 to promote the proliferation of chronic lymphocytic leukemia (CLL). However, DOCK2 do not affect the chemotaxis migration of CLL cells promoted by Wnt5 (50). Abnormal Wnt5a-ROR1-DOCK2-Rac pathway may be one of the causes of disease progression in CLL patients with high ROR1 expression.
Internal tandem duplication (ITD) mutations of FMS-like tyrosine kinase-3 (FLT3) in acute myeloid leukemia (AML) cells are common, and lead to poor prognosis of AML patients. However, the reduction of DOCK2 expression in FLT3 ITD mutated leukemia cells can inhibit the proliferation of AML cells and improve their sensitivity to cytosine arabinoside, thus prolonging the survival time of patients. The mechanism has not been fully elucidated, but DOCK2 has been observed to interact with FLT3 (51), which may change the biological effect of FLT3 mutation.
DOCK2 activates Rac, and the activated Rac phosphorylates ERK, which then starts the signal cascade reaction and promotes the proliferation of B-cell lymphoma (52).
In addition, when DOCK2 is deficient, NK cells cannot normally form lytic synapses to kill leukemia cells due to the lack of Rac activation, thus leading to the weakening of anti-tumor immunity (53).
DOCK2 is also associated with other diseases in addition to hematopoietic malignancies. In patients with type A hemophilia lacking factor VIII (FVIII), DOCK2 gene polymorphism caused by DOCK2 single nucleotide mutations may lead to increased autoantibodies and cause FVIII resistance, which may lead to a decrease in the effect of alternative therapy (54). Compared with the mouse inflammatory response model caused by low-dose LPS, the expression of DOCK2 in the mouse sepsis model caused by high-dose LPS was decreased, suggesting that the normal expression of DOCK2 has a positive effect on the prognosis of sepsis (55). But more research is needed on how DOCK2 can improve the prognosis of sepsis.
DOCK2 may also be associated with vascular abnormalities after vascular injury, and vascular abnormalities are associated with a variety of diseases, such as atherosclerosis and restenosis after angioplasty. The mechanism is that, after blood vessel injury, platelet-derived growth factor-BB (PDGF-BB) induces the expression of DOCK2 in the medial smooth muscle cell (SMC) of the blood vessels. DOCK2 reduces the expression of myocardial protein and its binding with serum response factor (SRF), resulting in decreased expression of SMC markers, thus modulating phenotypic transformation of SMC from contractibility to decreased elasticity (25,56).
The above evidence proves that DOCK2 plays an irreplaceable role in a variety of hematopoietic malignant diseases. Because DOCK2 is selectively highly expressed in hematopoietic cells and relatively low in other tissues, it has the potential to become one of the potential therapeutic targets for hematopoietic malignant diseases and may also provide new ideas for the treatment of these diseases. In addition, DOCK2 is also associated with hemophilia, septicemia and abnormal blood vessels after injury, indicating that DOCK2 functions are relatively complex, and more studies are needed to reveal its pathogenic mechanism.
DOCK2 and solid tumor
With the development of gene sequencing technology and epigenetics, DOCK2 has been found to be closely related to the development and prognosis of a variety of tumors. Abnormal expression, mutation or modification of DOCK2 may cause changes in tumor behavior.
DOCK2 regulates prostate cancer in a variety of ways. On the one hand, DOCK2 is highly methylated in prostate cancer tissues and hypomethylated in other tissues including benign and malignant tumors and blood cells. Moreover, hypermethylation of DOCK2 is positively correlated with some adverse prognostic indicators of prostate cancer, such as high prostate-specific antigen (PSA), large tumor volume, positive surgical margin, and positive lymph node metastasis, suggesting DOCK2 hypermethylation may lead to poor prognosis of prostate cancer (57). On the other hand, DOCK2 affects cell proliferation in PC3 cell lines of prostate cancer cells. After CXCL13 stimulates its receptor CXCR5, DOCK2 knockdown will cause decreased Rac activation and then affect the JNK signaling pathway, thus reducing PC3 proliferation. However, DOCK2 knockdown have little influence on AKT or ERK1/2 activation mediated by CXCL13 in PC3 cell line, which is important to cell proliferation and survival (58), and DOCK2 knockdown also have little influence on the migration ability of PC3 cell line. But strangely, in another cell line of prostate cancer, LNCaP, DOCK2 is barely expressed and it also does not affect LNCaP cell invasion and proliferation mediated by CXCL13 (23). But the mechanism by which DOCK2 acts differently in the two lines of prostate cancer cells remains unclear.
DOCK2 is highly expressed in the tumor tissues of patients with early colorectal cancer, and more CD8+ T lymphocytes are recruited to infiltrate into the tumor tissues, thus prolonging the overall survival time of patients. Moreover, the high expression of DOCK2 was negatively correlated with tumor size and invasion (59). Therefore, the high expression of DOCK2 may improve the prognosis of colorectal cancer.
A series of bioinformatics analysis shows that DOCK2 has a high mutation rate in a variety of tumors, such as esophageal adenocarcinoma (60), colorectal cancer (61) and intraductal papillary mucinous neoplasms of the pancreas (62), but the mutated domain and relevant mechanisms are still unclear. Besides, when Piwi like RNA-mediated gene silencing 1 gene (PIWIL1) was knocked out, the invasion and migration ability of gastric cancer cells was weakened, and DOCK2 expression was decreased, suggesting that PIWIL1 might be the upstream molecule of DOCK2 in gastric cancer (63).
In conclusion, DOCK2 epigenetic changes, abnormal expression or mutations may affect the occurrence, development and prognosis of tumors. However, due to the different expression levels of DOCK2 in different tumors and its different role, it may be difficult to develop targeted drugs for DOCK2, but DOCK2 may be a predictive indicator for some tumors.
Conclusions
As GEF, DOCK2 participates in multiple signaling pathways and regulates various diseases by activating Rac. DOCK2 is expressed in a variety of immune cells and affects their functions. Therefore, abnormal DOCK2 can cause serious immune deficiency and is also related to autoimmune diseases. DOCK2 also affects the progression of a variety of hematopoietic malignancies. Due to its relatively high expression in hematopoietic cells, DOCK2 has the potential to become a new therapeutic target for leukemia, lymphoma, etc.
However, due to the diversity of downstream molecules of Rac and the complexity of signaling pathways, the pathogenic mechanism of DOCK2 abnormality has not been fully clarified. Cell type is one of the factors that determine the molecular mechanism of DOCK2. In lymphocytes, DOCK2 affects their migration, differentiation and activation, and is indispensable for the maintenance of normal immune function (27,31,32). In tumor cells, abnormal DOCK2 may alter tumor cell behaviors and affect the occurrence and development of tumors (20). The different expression and epigenetic regulation of DOCK2 in different cells may be the reason for the different functions of DOCK2.
Existing studies on DOCK2 mostly focus on its regulation of hematopoietic cells, and partly reveal the mechanism of its regulation on immune functions, which may be related to its relatively high expression in hematopoietic cells. However, some researches have shown that DOCK2 may also be related to the occurrence and development of solid tumors, but most studies only stay at the level of gene sequencing or macroscopic phenomena, and have not clarified the molecular mechanism of how DOCK2 regulates tumor cell behavior. In the future, on the basis of gene sequencing finding that DOCK2 is highly mutated in some solid tumors, we can further explore the molecular mechanism of how DOCK2 regulates tumor cell behavior.
In addition, although DOCK2 is associated with a variety of diseases and has the potential to be a target for treatment of some diseases, no drug targeting DOCK2 has been developed so far. In the future, based on the further study of the mechanism of DOCK2 regulating diseases, some drugs targeting DOCK2 can also be developed appropriately. Only in this way can we really benefit the patients.
In general, DOCK2 is closely related to the occurrence and development of a variety of diseases. Although the knowledge we have learned so far may only be the tip of the iceberg, it provides some clues for us to further explore the function of DOCK2 in the future, and also provides new ideas for the treatment of some patients.
Acknowledgments
Funding: This work was supported by National Key Research and Development Program of China (No. 2018YFC1313400), the National Natural Science Foundation of China (No. 81773110) and the Guangdong Province Science and Technology Plan Project (No. 2017B020227003).
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aob.amegroups.com/article/view/10.21037/aob-21-5/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aob.amegroups.com/article/view/10.21037/aob-21-5/coif). All authors report that this work was supported by National Key Research and Development Program of China (No. 2018YFC1313400), the National Natural Science Foundation of China (No. 81773110.) and the Guangdong Province Science and Technology Plan Project (No. 2017B020227003). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Côté JF, Vuori K. GEF what? Dock180 and related proteins help Rac to polarize cells in new ways. Trends Cell Biol 2007;17:383-93. [Crossref] [PubMed]
- Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: Turning on the switch. Genes Dev 2002;16:1587-609. [Crossref] [PubMed]
- Whitehead IP, Campbell S, Rossman KL, et al. Dbl family proteins. Biochim Biophys Acta 1997;1332:F1-23. [PubMed]
- Erickson JW, Cerione RA. Structural Elements, Mechanism, and Evolutionary Convergence of Rho Protein-Guanine Nucleotide Exchange Factor Complexes. Biochemistry 2004;43:837-42. [Crossref] [PubMed]
- Lemmon MA, Ferguson KM, Schlessinger J. PH domains: Diverse sequences with a common fold recruit signaling molecules to the cell surface. Cell 1996;85:621-4. [Crossref] [PubMed]
- Rossman KL, Cheng L, Mahon GM, et al. Multifunctional roles for the PH domain of Dbs in regulating Rho GTPase activation. J Biol Chem 2003;278:18393-400. [Crossref] [PubMed]
- Côté JF. Identification of an evolutionarily conserved superfamily of DOCK180-related proteins with guanine nucleotide exchange activity. J Cell Sci 2002;115:4901-13. [Crossref] [PubMed]
- Meller N, Irani-Tehrani M, Kiosses WB, et al. Zizimin1, a novel Cdc42 activator, reveals a new GEF domain for Rho proteins. Nat Cell Biol 2002;4:639-47. [Crossref] [PubMed]
- Brugnera E, Haney L, Grimsley C, et al. Unconventional Rac-GEF activity is mediated through the Dock180-ELMO complex. Nat Cell Biol 2002;4:574-82. [Crossref] [PubMed]
- Côté JF, Motoyama AB, Bush JA, et al. A novel and evolutionarily conserved Ptdlns(3,4,5)P3-binding domain is necessary for DOCK180 signalling. Nat Cell Biol 2005;7:797-807. [Crossref] [PubMed]
- Sanui T, Inayoshi A, Noda M, et al. DOCK2 regulates Rac activation and cytoskeletal reorganization through interaction with ELMO1. Blood 2003;102:2948-50. [Crossref] [PubMed]
- Stace CL, Ktistakis NT. Phosphatidic acid- and phosphatidylserine-binding proteins. Biochim Biophys Acta 2006;1761:913-26. [Crossref] [PubMed]
- Wang X, Devaiah SP, Zhang W, et al. Signaling functions of phosphatidic acid. Prog Lipid Res 2006;45:250-78. [Crossref] [PubMed]
- Piekny A, Werner M, Glotzer M. Cytokinesis: Welcome to the Rho zone. Trends Cell Biol 2005;15:651-8. [Crossref] [PubMed]
- Chardin P. Function and regulation of Rnd proteins. Nat Rev Mol Cell Biol 2006;7:54-62. [Crossref] [PubMed]
- Nishihara H, Maeda M, Oda A, et al. DOCK2 associates with CrkL and regulates Rac1 in human leukemia cell lines. Blood 2002;100:3968-74. [Crossref] [PubMed]
- Nishihara H, Maeda M, Tsuda M, et al. DOCK2 mediates T cell receptor-induced activation of Rac2 and IL-2 transcription. Biochem Biophys Res Commun 2002;296:716-20. [Crossref] [PubMed]
- Gotoh K, Tanaka Y, Nishikimi A, et al. Selective control of type I IFN induction by the Rac activator DOCK2 during TLR-mediated plasmacytoid dendritic cell activation. J Exp Med 2010;207:721-30. [Crossref] [PubMed]
- Ippagunta SK, Malireddi RKS, Shaw PJ, et al. The inflammasome adaptor ASC regulates the function of adaptive immune cells by controlling Dock2-mediated Rac activation and actin polymerization. Nat Immunol 2011;12:1010-6. [Crossref] [PubMed]
- Fukui Y, Hashimoto O, Sanui T, et al. Haematopoietic cell-specific CDM family protein DOCK2 is essential for lymphocyte migration. Nature 2001;412:826-31. [Crossref] [PubMed]
- Kunisaki Y, Nishikimi A, Tanaka Y, et al. DOCK2 is a Rac activator that regulates motility and polarity during neutrophil chemotaxis. J Cell Biol 2006;174:647-52. [Crossref] [PubMed]
- Gotoh K, Tanaka Y, Nishikimi A, et al. Differential requirement for D0CK2 in migration of plasmacytoid dendritic cells versus myeloid dendritic cells. Blood 2008;111:2973-6. [Crossref] [PubMed]
- El-Haibi CP, Singh R, Sharma PK, et al. CXCL13 mediates prostate cancer cell proliferation through JNK signalling and invasion through ERK activation. Cell Prolif 2011;44:311-9. [Crossref] [PubMed]
- Parmo-Cabañas M, Bartolomé RA, Wright N, et al. Integrin α4β1 involvement in stromal cell-derived factor-1α-promoted myeloma cell transendothelial migration and adhesion: Role of cAMP and the actin cytoskeleton in adhesion. Exp Cell Res 2004;294:571-80. [Crossref] [PubMed]
- Guo X, Shi N, Cui XB, et al. Dedicator of cytokinesis 2, a novel regulator for smooth muscle phenotypic modulation and vascular remodeling. Circ Res 2015;116:e71-80. [Crossref] [PubMed]
- Dimitrova D, Freeman AF. Current Status of Dedicator of Cytokinesis-Associated Immunodeficiency: DOCK8 and DOCK2. Dermatol Clin 2017;35:11-9. [Crossref] [PubMed]
- Jing Y, Kang D, Liu L, et al. Dedicator of cytokinesis protein 2 couples with lymphoid enhancer–binding factor 1 to regulate expression of CD21 and B-cell differentiation. J Allergy Clin Immunol 2019;144:1377-1390.e4. [Crossref] [PubMed]
- Ushijima M, Uruno T, Nishikimi A, et al. The rac activator DOCK2 mediates plasma cell differentiation and IgG antibody production. Front Immunol 2018;9:243. [Crossref] [PubMed]
- Wen Y, Elliott MJ, Huang Y, et al. DOCK2 is critical for CD8+TCR- graft facilitating cells to enhance engraftment of hematopoietic stem and progenitor cells. Stem Cells 2014;32:2732-43. [Crossref] [PubMed]
- Nombela-Arrieta C, Lacalle RA, Montoya MC, et al. Differential requirements for DOCK2 and phosphoinositide-3-kinase γ during T and B lymphocyte homing. Immunity 2004;21:429-41. [Crossref] [PubMed]
- Nombela-Arrieta C, Mempel TR, Soriano SF, et al. A central role for DOCK2 during interstitial lymphocyte motility and sphingosine-1-phosphate-mediated egress. J Exp Med 2007;204:497-510. [Crossref] [PubMed]
- Ackerknecht M, Gollmer K, Germann P, et al. Antigen Availability and DOCK2-Driven Motility Govern CD4 + T Cell Interactions with Dendritic Cells In Vivo . J Immunol 2017;199:520-30. [Crossref] [PubMed]
- Dobbs K, Conde CD, Zhang SY, et al. Inherited DOCK2 deficiency in patients with early-onset invasive infections. N Engl J Med 2015;372:2409-22. [Crossref] [PubMed]
- Alizadeh Z, Mazinani M, Shakerian L, et al. DOCK2 Deficiency in a Patient with Hyper IgM Phenotype. J Clin Immunol 2018;38:10-2. [Crossref] [PubMed]
- Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature 2000;408:740-5. [Crossref] [PubMed]
- Jarrossay D, Napolitani G, Colonna M, et al. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol 2001;31:3388-93. [Crossref] [PubMed]
- Diebold SS, Kaisho T, Hemmi H, et al. Innate Antiviral Responses by Means of TLR7-Mediated Recognition of Single-Stranded RNA. Science 2004;303:1529-31. [Crossref] [PubMed]
- Heil F, Hemmi H, Hochrein H, et al. Species-Specific Recognition of Single-Stranded RNA via Till-like Receptor 7 and 8. Science 2004;303:1526-9. [Crossref] [PubMed]
- Chu T, Ni M, Chen C, et al. Cutting Edge: BCAP Promotes Lupus-like Disease and TLR-Mediated Type I IFN Induction in Plasmacytoid Dendritic Cells. J Immunol 2019;202:2529-34. [Crossref] [PubMed]
- Hoshino K, Sugiyama T, Matsumoto M, et al. IκB kinase-α is critical for interferon-α production induced by Toll-like receptors 7 and 9. Nature 2006;440:949-53. [Crossref] [PubMed]
- Swiecki M, Colonna M. The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol 2015;15:471-85. [Crossref] [PubMed]
- Cimino PJ, Sokal I, Leverenz J, et al. DOCK2 is a microglial specific regulator of central nervous system innate immunity found in normal and Alzheimer’s disease brain. Am J Pathol 2009;175:1622-30. [Crossref] [PubMed]
- Tanaka Y, Hamano S, Gotoh K, et al. T helper type 2 differentiation and intracellular trafficking of the interleukin 4 receptor-α subunit controlled by the Rac activator Dock2. Nat Immunol 2007;8:1067-75. [Crossref] [PubMed]
- Jiang H, Pan F, Erickson LM, et al. Deletion of DOCK2, a regulator of the actin cytoskeleton in lymphocytes, suppresses cardiac allograft rejection. J Exp Med 2005;202:1121-30. [Crossref] [PubMed]
- Nishihara H, Kobayashi S, Hashimoto Y, et al. Non-adherent cell-specific expression of DOCK2, a member of the human CDM-family proteins. Biochim Biophys Acta 1999;1452:179-87. [Crossref] [PubMed]
- Kunisaki Y, Tanaka Y, Sanui T, et al. DOCK2 Is Required in T Cell Precursors for Development of Vα14 NK T Cells. J Immunol 2006;176:4640-5. [Crossref] [PubMed]
- Kikuchi T, Kubonishi S, Shibakura M, et al. Dock2 participates in bone marrow lympho-hematopoiesis. Biochem Biophys Res Commun 2008;367:90-6. [Crossref] [PubMed]
- Azab AK, Azab F, Blotta S, et al. RhoA and Rac1 GTPases play major and differential roles in stromal cell-derived factor-1-induced cell adhesion and chemotaxis in multiple myeloma. Blood 2009;114:619-29. [Crossref] [PubMed]
- García-Bernal D, Redondo-Muñoz J, Dios-Esponera A, et al. Sphingosine-1-phosphate activates chemokine-promoted myeloma cell adhesion and migration involving α4β1 integrin function. J Pathol 2013;229:36-48. [Crossref] [PubMed]
- Hasan MK, Yu J, Widhopf GF 2nd, et al. Wnt5a induces ROR1 to recruit DOCK2 to activate Rac1/2 in chronic lymphocytic leukemia. Blood 2018;132:170-8. [Crossref] [PubMed]
- Wu M, Hamaker M, Li L, et al. DOCK2 interacts with FLT3 and modulates the survival of FLT3-expressing leukemia cells. Leukemia 2017;31:688-96. [Crossref] [PubMed]
- Wang L, Nishihara H, Kimura T, et al. DOCK2 regulates cell proliferation through Rac and ERK activation in B cell lymphoma. Biochem Biophys Res Commun 2010;395:111-5. [Crossref] [PubMed]
- Sakai Y, Tanaka Y, Yanagihara T, et al. The Rac activator DOCK2 regulates natural killer cell-mediated cytotoxicity in mice through the lytic synapse formation. Blood 2013;122:386-93. [Crossref] [PubMed]
- Naderi N, Yousefi H, Mollazadeh S, et al. Inflammatory and immune response genes: A genetic analysis of inhibitor development in Iranian hemophilia A patients. Pediatr Hematol Oncol 2019;36:28-39. [Crossref] [PubMed]
- Chen M, Chen X, Hu Y, et al. Screening of key genes related to the prognosis of mouse sepsis. Biosci Rep 2020;40:BSR20202649. [Crossref] [PubMed]
- Marmur JD, Poon M, Rossikhina M, et al. Induction of PDGF-responsive genes in vascular smooth muscle: Implications for the early response to vessel injury. Circulation 1992;86:III53-60. [PubMed]
- Bjerre MT, Strand SH, Nørgaard M, et al. Aberrant DOCK2, GRASP, HIF3A and PKFP hypermethylation has potential as a prognostic biomarker for prostate cancer. Int J Mol Sci 2019;20:1173. [Crossref] [PubMed]
- Caraglia M, Marra M, Leonetti C, et al. R115777 (Zarnestra®)/zoledronic acid (Zometa®) cooperation on inhibition of prostate cancer proliferation is paralleled by Erk/Akt inactivation and reduced Bcl-2 and bad phosphorylation. J Cell Physiol 2007;211:533-43. [Crossref] [PubMed]
- Miao S, Zhang RY, Wang W, et al. Overexpression of dedicator of cytokinesis 2 correlates with good prognosis in colorectal cancer associated with more prominent CD8+ lymphocytes infiltration: a colorectal cancer analysis. J Cell Biochem 2018;119:8962-70. [Crossref] [PubMed]
- Dulak AM, Stojanov P, Peng S, et al. Exome and whole-genome sequencing of esophageal adenocarcinoma identifies recurrent driver events and mutational complexity. Nat Genet 2013;45:478-86. [Crossref] [PubMed]
- Yu J, Wu WKK, Li X, et al. Novel recurrently mutated genes and a prognostic mutation signature in colorectal cancer. Gut 2015;64:636-45. [Crossref] [PubMed]
- Furukawa T, Kuboki Y, Tanji E, et al. Whole-exome sequencing uncovers frequent GNAS mutations in intraductal papillary mucinous neoplasms of the pancreas. Sci Rep 2011;1:161. [Crossref] [PubMed]
- Araújo T, Khayat A, Quintana L, et al. Piwi like RNA-mediated gene silencing 1 gene as a possible major player in gastric cancer. World J Gastroenterol 2018;24:5338-50. [Crossref] [PubMed]
Cite this article as: Chen Y, Di M, Tang Y, Xia J. A narrative review: the role of DOCK2 in immune-related diseases, hematopoietic or vascular diseases and solid tumor. Ann Blood 2022;7:32.


