
Liver failure resolved via corticosteroids plus plasma exchange in a patient with hepatocellular carcinoma receiving nivolumab immunotherapy: case report
Introduction
Immune checkpoint inhibitors (ICIs) are a remarkable breakthrough in the immunotherapy of many malignancies, from metastatic melanoma and now also to advanced hepatocellular carcinoma (HCC). There have been several approved checkpoint inhibitors, such as programmed cell death receptor 1 (PD-1), programmed death ligand 1 (PD-L1), and cytotoxic T-lymphocyte associated protein 4 (CTLA-4) (1). Nivolumab is one type of PD-1 inhibitor, which has been approved to be used alone or together with other drugs to treat HCC (2).
However, treatment with ICIs may induce various immune-related adverse events (irAEs) in many organs, which are thought to be caused by blocking immune checkpoint and activating nonspecific immunity. irAEs of ICIs often affect the skin, lung, gastrointestinal tract, endocrine, nervous, and musculoskeletal systems. ICI-induced immune-mediated hepatotoxicity (IMH) is an important irAE, and the incidence of reported IMH varies from 0.7–16%, depending on dosage, class of ICIs and whether mono-therapy or combination therapy of ICIs was used. The incidence of IMH of any grade caused by PD-1 is lower (0.7–2.1%) but it is higher in combined CTLA-4/PD-1 (13%) and high dose of CTLA-4 therapies (16%). The incidence of grade 3 and 4 induced by ICIs was up to 11% (3), notably, fulminant liver failure cases of IMH were few (0.1–0.2%) (4,5). The onset of IMH could typically occur during 2 to 12 weeks, or after 1 to 3 doses of ICIs administration. ICI-induced fulminate liver failure is severe, fatal, and with poor prognosis. Corticosteroids is recommended in treatment guidelines of IMH in HCC (6). Other non-corticosteroid treatment methods were still attempted in studies and new strategies are needed to be explored in this liver failure. However, little is known about the combined therapy of corticosteroids and plasma exchange (PE) in IMH, especially in fulminate liver failure induced by nivolumab. Herein, we report a case of nivolumab-induced liver failure in HCC successfully treated with corticosteroid plus PE, who had an excellent and rapid recovery. We present the following case in accordance with the CARE reporting checklist (available at https://aob.amegroups.com/article/view/10.21037/aob-21-65/rc).
Case presentation
A 47-year-old male patient with a history of chronic hepatitis B (CHB) came to the outpatient department of intervention in our hospital with a feeling of fullness in the right upper abdomen on 17 January 2020, he did not get regular physical check-ups and receive any therapy on CHB before. He was examined by ultrasound scan and a large heterogeneous irregular mass was reported in the right hepatic lobe. Subsequently, the multi-slice enhanced computed tomography (CT) scan confirmed this mass as a large primary HCC in the liver. Moreover, extensive cancerous emboli were also found in the main trunk, left and right branches of portal vein, superior mesenteric vein and its branches, Figure 1A. Dynamic representative images of the CT scan are shown in Figure 1A-1C. The level of serum alpha-fetoprotein (AFP) was 112,522 ng/mL at that time, and the dynamic changes in serum AFP over time are shown in Figure 2A. Because of the typical characteristics of HCC in CT images (hyper-enhancement in arterial phase and early wash-out in venous phase), the tumor biopsy was not performed. He was admitted on 19 January 2020, and further routine tests showed that he had positive HBsAg, HBeAg, and HBcAb, and a minor elevation of liver function [alanine aminotransferase (ALT): 40 U/L, aspartate aminotransferase (AST): 69 U/L]. His HBV DNA load was 2.35E+6 IU/mL and anti-HBV therapy with tenofovir alafenamide fumarate (TAF) was initiated. The patient was diagnosed as HCC, cirrhosis, with a Child-Pugh score of 5, and without ascites and hepatic encephalopathy. Therefore, according to the Barcelona Clinic Liver Cancer (BCLC) classification, the tumor stage of BCLC-C was assigned. He underwent the first transcatheter arterial chemoembolization (TACE) treatment for the tumor in the left hepatic lobe with lobaplatin (50 mg) and pirarubicin hydrochloride (40 mg) plus Hepasphere on 20 January 2020, and he started on sorafenib on 22 January 2020. The patient experienced only mild side effects involving the skin (peeling) that did not affect the activities of daily life and sorafenib was not interrupted.
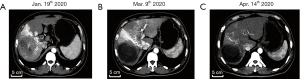
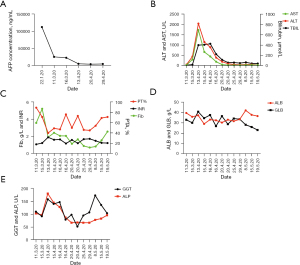
The levels of AFP decreased significantly and it was 25,984 ng/mL on 11 March 2020 (Figure 2A). The images of multi-slice CT scan showed a dramatic reduction in HCC burden (Figure 1B). By 13 March 2020, he received the second TACE with the same chemotherapy drugs as the first treatment of TACE. On 17 March 2020, a dose of nivolumab (140 mg) was intravenously administered as a single injection in addition to daily sorafenib, and at that time the Child-Pugh score of the patient was still 5. Approximately 2 weeks later after the nivolumab treatment, the patient gradually presented dark urine, and general discomfort including fatigue and skin yellowing from 1 April 2020 at home, without fever, clay-colored stools, and itchy skin. However, he did not return to our hospital until 13 April 2020. At the outpatient clinic, the patient was cooperative, conscious, and calm during physical examination, with no objective disorientation. Except for hepatomegaly, skin and scleral jaundice, his body temperature, blood pressure and cardiac frequency were normal and other significant alterations were not found. The results of blood tests indicated total bilirubin (TBIL) 399.4 µmol/L [upper limit of normal (ULN): 23.9 µmol/L], AST: 1,731 U/L (ULN: 35 U/L), ALT: 2,042 U/L (ULN: 35 U/L), gamma-glutamyltransferase (GGT): 158 U/L (ULN: 40 U/L), prothrombin activity (PTA): 40%, prothrombin time (PT)/international normalized ratio (INR): 1.9, fibrinogen (Fib) concentration: 2.11 g/L. Changes in these features over time are shown in Figure 2B-2E.
The patient was hospitalized into the intervention department immediately on 13 April 2020. Liver failure was diagnosed initially and sorafenib was stopped at the same time. Serologic evaluation, namely Epstein Barr virus (EBV) DNA, cytomegalovirus (CMV) DNA, human immunodeficiency virus (HIV), hepatitis A and hepatitis C virus were all negative. Although the HEV-lgG was positive, both HEV-lgM, and HEV-RNA were negative. Further investigations of autoimmune liver disease and Wilson disease were performed and these were all excluded. A more obvious reduction of tumor burden was shown by multi-slice CT scan than that obtained on 9 March 2020 (Figure 1C). However, the levels of TBIL continually increased to 406 µmol/L on 15 April 2020, and the patient was transferred to our liver disease department.
Nivolumab-induced fulminate liver failure, an important irAE of ICIs, was diagnosed on the first day in the liver disease department, and therapy with 120 mg intravenous methylprednisolone (2 mg/kg) was started immediately. The PE was performed on the second day and subsequently, a rapid alleviation of liver injury was observed (ALT decreased to 313 U/L and TBIL dropped to 210.8 µmol/L). In order to enhance the efficacy of the above combination treatment, the second PE treatment was administrated on 20 April 2020. His liver function recovered in further (ALT decreased to 98 U/L and TBIL declined to 60.7 µmol/L). Therefore, the dose of methylprednisolone was gradually reduced to 80 and 60 mg/day with progressive tapering, and the therapeutic approaches are shown in Figure 3A.
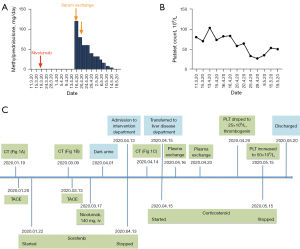
After almost 10 days of corticosteroid therapy combined with twice PE treatments, the liver function of the patient was obviously recovered (ALT decreased to 62.0 U/L, TBIL decreased to 62.3 µmol/L, PTA: 46%). Then the dosage of oral prednisolone was continuously reduced and lasted for 31 days in total. However, we noticed that the platelet (PLT) count of the patient decreased dramatically from 103×109/L to 25×109/L on 29 April, Figure 3B. However, the D-dimer and 3P tests were normal, both of his hemoglobin (HGB) and white blood cells (WBC) were within the normal levels, and the patient showed no signs of bleeding. The result of bone marrow puncture showed that the maturation of megakaryocytes was impaired, although its number increased. The patient received subcutaneous injection of recombinant human thrombogenin daily for 2 weeks, and the count of PLT increased to 50×109/L and remained stable at this level. On the 20 May 2020, the patient felt better and complained no obvious discomfort after above combined treatment. And he was discharged under the following conditions: ALT 35 U/L, TBIL 43.1 µmol/L, and PTA 71%. The timeline of the disease process is shown in Figure 3C.
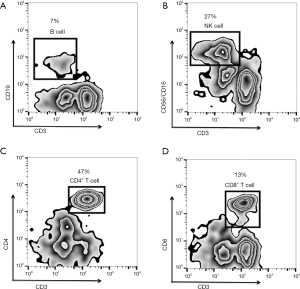
Cellular immunity and serum cytokine levels were evaluated by flow cytometry and ELISA test on the first day of the patients transferring to the liver disease department. The results showed that the proportions of CD3−CD19+ B cells and CD3−CD56+/CD16+ NK cells were normal, at 7% and 27%, respectively. Although the frequency of CD4+ T cells was normal (47%), the frequency of CD8+ T cells decreased (13%) and the ratio of CD4+ to CD8+ T cells increased (3.62). The results of flow cytometry analysis are shown in Figure 4. The level of serum TNF-α was 10.9 pg/mL (ULN: 8.1 pg/mL), serum interleukin (IL)-6 was 6.4 pg/mL (ULN: 7.0 pg/mL), and serum IL-10 was 10.10 pg/mL (ULN: 9.1 pg/mL).
The patient received another three times TACE treatments after discharge. He is still on follow-up every month until 2021 July 21th, and his liver function [ALT, TBIL and albumin (ALB)] kept normal during outpatient visits. The CT scan showed that the size of the tumor in liver decreased to 66 mm × 56 mm, with AFP 1,573 ng/mL.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Currently, ICIs are widely used in tumor treatment, including nivolumab for advanced HCC (2). IMH is a well-known irAEs associated with ICI therapy, of which fulminant liver failure is sever and fatal (4). The case presented herein is noteworthy as the patient rapidly developed liver failure after the nivolumab treatment. However, early recognition and combined therapy of PE and the recommended corticosteroid got a success in treating this case.
The mechanism of IMH induced by ICIs is not well understood. It is already known that ICIs could reverse the dysfunction of immune cells by blocking the interaction between immune checkpoint molecules and their ligands, breaking the exhaustion status of immune cells and enhancing the defense capacity of immune system (7). In animal models, ICIs were shown to be able to promote the activation and infiltration of T cells in the liver, suggesting that increased autoimmune inflammation to hepatocytes induced by ICI-induced T cell activation might be one of the mechanisms involved in IMH (8,9). In addition, genetic factors may also have an important role in the occurrence of IMH during ICIs treatment (10). Currently, several biomarkers were reported to be associated with the development of IMH, such as IL-6, soluble CD163, and CXCL5 (11). Honjo et al. (12) reported a severe syndrome caused by cytokine release in a patient with pleomorphic carcinoma of the lung, which resulted in fulminant purpura despite a successful response to nivolumab therapy, but elevated serum IL-1β, IL-6, IL-10, TNF-α, IFN-γ, and G-CSF levels were found. In our case, although the serum levels of IL-6 were normal, the levels of IL-10 and TNF-α were significantly elevated, indicating the increase of immune inflammation induced by nivolumab therapy in this patient, which might partly contribute to the liver injury and decreased PLT counts. Studies found that infiltrating lymphocytes in the injured liver tissue of IMH induced by ICIs were mainly CD8+ T cells, which was consistent with the proposed mechanism that the restored cytotoxic effects of CD8+ T cells drive IMH immune reactions (9,13). In this patient, we also examined the phenotype of peripheral blood immune cells, as shown in Figure 4, and the result showed there was a lower proportion of CD8+ T cells and an increased CD4+/CD8+ ratio, which is contrary to the above studies, which warrants further evaluation.
The guidelines of ICI-induced irAEs recommends oral prednisone 0.5–1.0 mg/kg/day for grade 2 IMH, and intravenous methylprednisone 1–2 mg/kg/day for grade 3 and 4 IMH (6). Non-corticosteroid immunosuppressive therapies, such as mycophenolate mofetil (13), cyclosporine and antithymocyte globulin (ATG) (14), have been reported in cases of steroid-refractory IMH. Although most of the IMHs induced by ICIs could recover, liver failure is still a fatal irAEs with very poor prognosis. Therefore, new strategies are urgently needed.
PE is a treatment option for immune-related diseases, as it has the ability to remove toxic and inflammatory molecules from the serum (15-18). Studies have also found that PE could improve acute liver failure survival by attenuating innate immune activation and increasing the proportion of Treg cells (19). There have been many reports about PE therapy for organ toxicity induced by ICIs, such as bullous pemphigoid, myasthenia, myositis, encephalitis, and thrombotic thrombocytopenic purpura (20-24). However, few liver failure cases induced by ICIs have been reported that were treated using PE. The exception is Riveiro-Barciela et al. (25), who reported a success therapy using immunosuppressant drugs plus PE for treating a melanoma patient with liver failure induced by nivolumab. Further, nivolumab is an ideal target of PE (molecular weight 143,597 Da). Nonetheless, combined steroid and PE therapy for nivolumab-induced liver failure has never been reported in HCC patients with a history of chronic liver disease or cirrhosis. Therefore, we performed PE for this patient during the early hospitalization with 2,000 mL of fresh plasma and his liver function showed a rapid improvement, which might due to the above benefit of PE. In addition, combination treatment might mitigate other problems caused by HCC, for example, reducing inflammatory cytokine production by HCC tissue and serum endotoxin levels, which may aggravate liver injury. Our current liver failure case induced by nivolumab reached a rapid recovery using this treatment strategy, which added the effect of corticosteroids and PE. This treatment may be of certain clinical significance for patients at the early stage of liver failure induced by nivolumab in HBV-related HCC, and might provide some information on cell immunity and cytokines in these patients.
In conclusion, our case raises an important issue for the application of corticosteroids plus PE to rapidly block liver failure progression induced by ICIs and promote liver recovery, suggesting that PE may be a feasible treatment option for nivolumab-induced liver failure in HCC. However, we only describe one successful case and examined simple immune features in this patient, more studies are needed to confirm this therapeutic strategy and explore the dynamic immunity change. Moreover, much more attention needed to be paid to the liver failure caused by ICIs, and early detection and prevention are also necessary to avoid liver failure occurrence.
Patient perspective
I experienced a great relief of dark urine and skin yellowing, and my appetite became very good. The fatigue disappeared and my strength is recovered.
Acknowledgments
Funding: This work was supported by the Research and Development Planned Project in Key Areas of Guangdong Province (grant number 2019B110233002) and the Guangzhou Science and Technology Project (grant number 202002030431).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://aob.amegroups.com/article/view/10.21037/aob-21-65/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aob.amegroups.com/article/view/10.21037/aob-21-65/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Johnson DB, Nebhan CA, Moslehi JJ, et al. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol 2022;19:254-67. [Crossref] [PubMed]
- Chen LT, Martinelli E, Cheng AL, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with intermediate and advanced/relapsed hepatocellular carcinoma: a TOS-ESMO initiative endorsed by CSCO, ISMPO, JSMO, KSMO, MOS and SSO. Ann Oncol 2020;31:334-51. [Crossref] [PubMed]
- Parlati L, Vallet-Pichard A, Batista R, et al. Incidence of grade 3-4 liver injury under immune checkpoints inhibitors: A retrospective study. J Hepatol 2018;69:1396-7. [Crossref] [PubMed]
- Bhave P, Buckle A, Sandhu S, et al. Mortality due to immunotherapy related hepatitis. J Hepatol 2018;69:976-8. [Crossref] [PubMed]
- Peeraphatdit TB, Wang J, Odenwald MA, et al. Hepatotoxicity From Immune Checkpoint Inhibitors: A Systematic Review and Management Recommendation. Hepatology 2020;72:315-29. [Crossref] [PubMed]
- Haanen JBAG, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv264-6. [Crossref] [PubMed]
- Shankar B, Zhang J, Naqash AR, et al. Multisystem Immune-Related Adverse Events Associated With Immune Checkpoint Inhibitors for Treatment of Non-Small Cell Lung Cancer. JAMA Oncol 2020;6:1952-6. [Crossref] [PubMed]
- Affolter T, Llewellyn HP, Bartlett DW, et al. Inhibition of immune checkpoints PD-1, CTLA-4, and IDO1 coordinately induces immune-mediated liver injury in mice. PLoS One 2019;14:e0217276. [Crossref] [PubMed]
- Llewellyn HP, Arat S, Gao J, et al. T cells and monocyte-derived myeloid cells mediate immunotherapy-related hepatitis in a mouse model. J Hepatol 2021;75:1083-95. [Crossref] [PubMed]
- Boilève A, Carlo MI, Barthélémy P, et al. Immune checkpoint inhibitors in MITF family translocation renal cell carcinomas and genetic correlates of exceptional responders. J Immunother Cancer 2018;6:159. [Crossref] [PubMed]
- Nakamura Y. Biomarkers for Immune Checkpoint Inhibitor-Mediated Tumor Response and Adverse Events. Front Med (Lausanne) 2019;6:119. [Crossref] [PubMed]
- Honjo O, Kubo T, Sugaya F, et al. Severe cytokine release syndrome resulting in purpura fulminans despite successful response to nivolumab therapy in a patient with pleomorphic carcinoma of the lung: a case report. J Immunother Cancer 2019;7:97. [Crossref] [PubMed]
- Ito T, Ishigami M, Yamamoto T, et al. Clinical course of liver injury induced by immune checkpoint inhibitors in patients with advanced malignancies. Hepatol Int 2021;15:1278-87. [Crossref] [PubMed]
- Spänkuch I, Gassenmaier M, Tampouri I, et al. Severe hepatitis under combined immunotherapy: Resolution under corticosteroids plus anti-thymocyte immunoglobulins. Eur J Cancer 2017;81:203-5. [Crossref] [PubMed]
- Damsgaard J, Larsen FS, Ytting H. Reversal of Acute Liver Failure Due to Wilson Disease by a Regimen of High-Volume Plasma Exchange and Penicillamine. Hepatology 2019;69:1835-7. [Crossref] [PubMed]
- David S, Bode C, Putensen C, et al. Adjuvant therapeutic plasma exchange in septic shock. Intensive Care Med 2021;47:352-4. [Crossref] [PubMed]
- Siemieniuk RA, Bartoszko JJ, Díaz Martinez JP, et al. Antibody and cellular therapies for treatment of covid-19: a living systematic review and network meta-analysis. BMJ 2021;374: [Crossref] [PubMed]
- Veeramachaneni H, Subramanian RM. Combined High-Dose Continuous Renal Replacement Therapy and Plasma Exchange in the Management of Severe Multiorgan System Dysfunction Associated With Acute Liver Failure. Hepatology 2021;74:1124-6. [Crossref] [PubMed]
- Sugiura H, Matsuoka KI, Sando Y, et al. Plasma exchange eliminates residual mogamulizumab but does not warrant prompt recovery of peripheral Treg levels. Transfus Apher Sci 2019;58:472-4. [Crossref] [PubMed]
- Ridpath AV, Rzepka PV, Shearer SM, et al. Novel use of combination therapeutic plasma exchange and rituximab in the treatment of nivolumab-induced bullous pemphigoid. Int J Dermatol 2018;57:1372-4. [Crossref] [PubMed]
- Psimaras D, Velasco R, Birzu C, et al. Immune checkpoint inhibitors-induced neuromuscular toxicity: From pathogenesis to treatment. J Peripher Nerv Syst 2019;24:S74-85. [Crossref] [PubMed]
- Kao JC, Liao B, Markovic SN, et al. Neurological Complications Associated With Anti-Programmed Death 1 (PD-1) Antibodies. JAMA Neurol 2017;74:1216-22. [Crossref] [PubMed]
- Safa H, Johnson DH, Trinh VA, et al. Immune checkpoint inhibitor related myasthenia gravis: single center experience and systematic review of the literature. J Immunother Cancer 2019;7:319. [Crossref] [PubMed]
- Dickey MS, Raina AJ, Gilbar PJ, et al. Pembrolizumab-induced thrombotic thrombocytopenic purpura. J Oncol Pharm Pract 2020;26:1237-40. [Crossref] [PubMed]
- Riveiro-Barciela M, Muñoz-Couselo E, Fernandez-Sojo J, et al. Acute liver failure due to immune-mediated hepatitis successfully managed with plasma exchange: New settings call for new treatment strategies? J Hepatol 2019;70:564-6. [Crossref] [PubMed]
Cite this article as: Gu Y, Zhang Y, Huang Y, Bi Y, Li Z, Zhu J. Liver failure resolved via corticosteroids plus plasma exchange in a patient with hepatocellular carcinoma receiving nivolumab immunotherapy: case report. Ann Blood 2023;8:9.


