
Provision of human leukocyte antigen (HLA) selected platelets: 10-year experience of a regional blood centre
Introduction
Profound thrombocytopenia is a common manifestation of many haematological disorders and occurs after myelosuppressive chemotherapy. Platelet transfusion might be indicated to control or prevent bleeding complications in these patients. Platelet transfusion refractoriness (PTR), defined as repeatedly suboptimal post-transfusion platelet count increments, is of significant concern because these patients are susceptible to life-threatening bleeding and are associated with poorer outcomes (1,2). Non-immune causes like infection, bleeding, drugs and splenomegaly are attributable to most cases of PTR. With repeated transfusions of blood components or multiple pregnancies, patients may be alloimmunized to class I human leukocyte antigens (HLA) or human platelet antigens (HPA) expressed on platelets, resulting in immune-mediated PTR (2,3). Alloimmunization to HLA antigen is more common than that to HPA antigen and is thought to be the most important cause of immune-mediated PTR (4). In this situation, timely provision of HLA-selected platelets by blood centre may improve platelet count increments and save lives. However, it requires rapid identification of suitable donors who could come for plateletpheresis. Moreover, the cost of HLA-selected platelets is significantly higher than that of random donor platelets (5). Although it has been a standard practice to support PTR patients with the product, only limited reports evaluated the clinical effectiveness of HLA-selected platelet transfusion and the service provision from the perspective of blood centres. A retrospective study was undertaken to review the supply of HLA-selected platelets, including the number of units issued and the degree of matching from a regional blood centre over a 10-year period. The predictors of satisfactory response to HLA-selected platelet transfusions were also assessed. We present this article in accordance with the STROBE reporting checklist (available at https://aob.amegroups.com/article/view/10.21037/aob-22-11/rc).
Methods
Setting, study centre and participants
In this retrospective study, HLA-selected platelet transfusions performed between May 2010 and April 2020 were included for analysis. All HLA-selected platelet units were provided by the Hong Kong Red Cross Blood Transfusion Service (BTS), the only blood centre in the city which served a population of 7.5 million people in 2020. Most platelets provided by the BTS are ABO group-matched random donor units prepared from 350 or 450 ml of whole blood collection, which contain on average (64.7±17.1)×109 or (84.5±20.3)×109 platelets per unit respectively. The number of platelets in one adult dose constituting 4 random donor units is no less than 2.7×1011. Each apheresis platelet unit contains >3×1011 platelets. There was no change in the method of collection, processing and storage of platelet units that would affect the quantity of platelets per unit and the quality of the product during the study period.
PTR
One-hour corrected platelet count increment (CCI) is given by the formula [platelet count increment 1 hour after platelet transfusion (/µL) × body surface area (m2)/unit content (1011)] and expressed in terms of m2/µL. PTR is defined by 1-hour CCIs less than 7,500 m2/µL after transfusion of ABO blood group-matched platelets in two occasions (6). Clinicians could request for HLA-selected platelets via hospital blood banks for patients with PTR.
Data collection
Data were retrieved from the computer system of the BTS. Data collection included patients’ demographics and 1-hour CCIs after transfusion of ABO blood group-matched random donor platelets at the time of requesting for HLA-selected platelets. Investigations for immune-mediated PTR were not mandatory when requesting for HLA-selected platelets. Results of anti-HLA class I antibody and anti-HPA antibody testing were provided by the referring units if available. Platelet serology testing was performed by Luminex assay, enzyme-linked immunosorbent assay, direct and/or indirect monoclonal antibody immobilization of platelet antigens (MAIPA) assay based methods as decided by the referring centres. Clinicians were required to check 1-hour CCIs and returned the results to BTS after every HLA-selected platelet transfusion.
Donors and HLA-selected platelet products
HLA-selected platelets were collected from donors by apheresis method. BTS did not have HLA-typed platelets in the inventory. When a request for HLA-selected platelets was initiated, potential donors were identified and called for plateletpheresis in the Hong Kong Bone Marrow Donor Registry (HKBMDR) which is also operated by the BTS. Donors recruited before June 2019 had intermediate or high resolution typing of HLA-A and HLA-B antigens whilst those recruited afterwards had high resolution typing performed by next generation sequencing based method by the Transplantation and Immunogenetics laboratory of Queen Mary Hospital, Hong Kong. In year 2020, there were more than 140,000 donors in the registry. If HLA-A and HLA-B antigens expressed by a donor, who could be homozygous, also exist in the recipient, the pair is considered as “4 out of 4” matched (7). Partially matched products were supplied only if there were no “4 out of 4” matched donors. ABO blood group matching was not considered when looking for potential donors. Electronic instead of physical platelet crossmatches were performed before issue. HLA matching in all donor-recipient pairs was independently and retrospectively reviewed by experts in histocompatability and immunogenetics.
Outcome measures
Primary outcomes of the study included the total number of HLA-selected platelet units supplied by the BTS, the degree of HLA match in donor-recipient pairs and the response of HLA-selected platelet transfusion. The secondary outcome was identifying the factors which influence platelet count increment after HLA-selected platelet transfusion.
Statistical analysis
Descriptive statistics were used to summarize the characteristics of patients, degree of HLA match and 1 hour-CCIs after random donor platelet and HLA-selected platelet transfusions respectively. The findings of platelet serology testing, if available, were also described. The first transfusion event of each donor-recipient pair was included for further analysis of the response to HLA-selected platelet transfusion. First, 1 hour-CCIs after HLA-selected platelet and random donor platelet transfusions were compared. Then, the difference in 1-hour CCIs after HLA-selected platelet and random donor platelet transfusions (i.e., 1-hour CCI HLA selected platelets – 1-hour CCI random donor platelets) between patients who were and were not refractory to random donor platelets was compared. We only selected patients who had PTR to random donor platelets to study the effect of ABO blood group compatibility, patient’s sex and the presence of anti-HLA class I antibodies to 1-hour CCI after HLA-selected platelet transfusion. All comparisons were performed by mixed linear models allowing a random intercept per patient to adjust for the effect of within-subject correlation. Available-case analysis was used when dealing with missing data. Statistical analysis was performed by R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org/). Results were considered significant if P<0.05.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Local ethics committee approval was not sought since the present study was a retrospective quality improvement project using anonymized registry data.
Results
In the 10-year period, 1,080 units of HLA-selected platelets were collected from 393 donors and transfused to 147 patients from 12 hospitals. The male to female ratio was 54:46 and the mean age was 48±21 years. Almost all patients suffered from serious haematological diseases-acute myeloid leukaemia (47%), other myeloid disorders including myelodysplastic syndrome and myelofibrosis (26%), acute lymphoblastic leukaemia (10%) and severe aplastic anaemia (7%). One-hour CCIs after random donor platelet transfusions were not available in 5 patients. In the remaining 142 patients, the mean 1-hour CCI after random donor platelet transfusions was 3,154±4,234 m2/µL upon enrolment of the study. The clinical characteristics of male and female patients receiving HLA-selected platelets are respectively summarized in Table 1.
Table 1
| Demographics | Male, N=80 | Female, N=67 | P value |
|---|---|---|---|
| Age (years), mean ± SD | 47±21 | 49±21 | 0.67 |
| Diagnosis | |||
| Acute myeloid leukaemia | 32 [40] | 37 [55] | |
| Other myeloid disorders | 22 [28] | 16 [23] | |
| Acute lymphoblastic leukaemia | 7 [9] | 7 [10] | |
| Plasma cell myeloma | 1 [1] | 1 [1] | |
| Non-Hogkin lymphoma | 6 [8] | 1 [1] | |
| Severe aplastic anaemia | 9 [11] | 2 [2] | |
| Others | 3 [4] | 3 [4] | 0.18 |
| Pre-transfusion CCI (m2/µL), mean ± SD | 3,549±5,154 | 2,686±2,744 | 0.21 |
| 1 hour CCI with HLA selected platelets | 14,575±8,898 | 17,757±9,303 | 0.04 |
| Immunological tests for PTR | 29 | 25 | |
| Anti-HLA class I antibody | 21 [72] | 20 [80] | 0.74 |
| Anti-HPA alloantibody | 1 [3] | 0 [0] | 1 |
Data presented as number [percentage] unless otherwise specified. SD, standard deviation; CCI, corrected count increment; HLA, human leukocyte antigen; PTR, platelet transfusion refractoriness; HPA, human platelet antigen.
Median number of HLA-selected platelet units received by each patient was 4 [interquartile range (IQR), 2–9]. All except one issued units were “4 out of 4” matched for the recipients’ HLA-A and B antigens. One HLA-B antigen was mismatched in the remaining transfusion episode, but anti-HLA antibodies were not against the donor’s mismatched antigen. Mean 1-hour CCI after HLA-selected platelet transfusions was 16,865±8,876 m2/µL. Overall, 1-hour CCIs ≥7,500 m2/µL were achieved in 910 episodes (84%) (Figure 1).
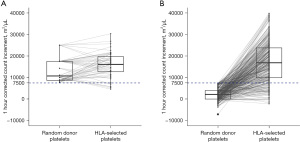
The first transfusion event of 419 donor-recipient pairs were retrieved for further analysis. The mean 1-hour CCI after HLA-selected platelet transfusions was 16,830±9,114 m2/µL, which was 13,316 m2/µL [95% confidence interval (CI): 12,326–14,306 m2/µL] higher than that with random donor platelet transfusions (Figure 2). At the time of requesting for HLA-selected platelets, PTR was not substantiated (1-hour CCIs ≥7,500 m2/µL post-random donor platelet transfusions) in 13 patients, whom involved 55 donor-recipient pairs. The mean difference in 1-hour CCIs after HLA-selected and random donor platelet transfusions of these patients was 2,204±5,481 m2/µL, which was 11,134 m2/µL (95% CI: 6,122–16,135 m2/µL) less than the remaining 364 transfusions given to 129 patients with PTR (Figure 1).
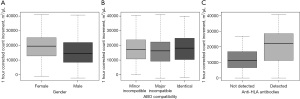
The 364 HLA-selected platelet transfusion events of 129 patients who were refractory to random donor platelets were further selected to study the factors affecting 1-hour CCIs. The mean 1-hour CCI after HLA-selected platelet transfusion of male patients was 3,592 m2/µL (95% CI: 1,270–7,145 m2/µL) less than that of female patients (Figure 2A).
Considering the ABO blood group matching of all donor-recipient pairs, 109 (30%) were ABO-identical, 85 (23%) had minor incompatibility, and 166 (46%) had major incompatibility. The corresponding median 1-hour CCIs were 18,174 (IQR: 10,370–24,968) m2/µL, 17,183 (IQR: 10,803–23,823) m2/µL and 16,312 (IQR: 9,267–22,346) m2/µL respectively. ABO matching data were not available in the remaining 4 pairs. One-hour CCIs were not significantly different with the ABO blood group compatibility (Figure 2B).
Platelet serology testing was performed in 54 patients (37%) who had PTR. Anti-HLA class I antibodies were positive in 41 (76%) recipients (104 transfusions) and negative in 13 (24%) recipients (29 transfusions) respectively. Mean 1-hour CCI post-HLA-selected platelet transfusion was 8,727 m2/µL (95% CI: 595–12,970 m2/µL) higher in patients who demonstrated anti-HLA class I antibody than those who did not (Figure 2C). One-hour CCI was less than 7,500 m2/µL in 60 HLA-selected platelet transfusions involving 36 patients. Anti-HPA antibody was identified in one patient among 13 who had platelet serology testing performed.
Discussion
Blood centre has its mission to provide safe and high-quality blood components to meet clinical needs in a timely manner. At the same time, the use of scarce and costly products such as HLA-selected platelets has to be justified to ensure efficient and effective utilization of resources. In this retrospective study, we evaluated our experience in providing HLA-selected platelets over a 10-year period, at an aim to identify the strengths and weaknesses of the current practice, and to demonstrate the clinical effectiveness of HLA-selected platelet transfusions.
The BTS issued around 1,000 units of HLA-selected platelets which involved more than 400 donor-recipient pairs in the study period. The products were almost exclusively fully matched with the recipients’ HLA-A and -B loci. To support the HLA-selected platelet service, a large donor pool is necessary. In the past 10 years, the number of voluntary donors in the HKBMDR of BTS has grown from around 80,000 to almost 140,000. Every year, around 5,000 to 10,000 people registered as marrow donors and had HLA typed. In fact, the BTS has been actively recruiting blood donors into the HKBMDR with a requirement that they are healthier and have consented to bone marrow donation. Upon enrolment, donors also consented to be called up by the BTS for HLA-selected platelet donation when necessary. This readily available HLA typed donor pool could increase the likelihood of successful matching and swift supply of HLA-selected platelets. The strategy of recruiting donors from the respective local unrelated bone marrow donor registry streamlines the HLA-selected platelet service, and that could be propagated to other blood centres.
Satisfactory CCI >7,500 m2/µL was achieved in 84% of transfusion episodes, demonstrating the effectiveness of HLA-selected platelets. When trying to identify the predictors of satisfactory response, we found that CCI improvement after HLA-selected platelet transfusion was significantly less in patients without refractoriness to random donor platelets. Although HLA-selected platelets are only indicated in PTR due to HLA alloimmunization, they were issued in instances as per clinicians’ request when CCIs after random donor platelet transfusions were not consistently satisfactory. Our data reinforced that random donor platelets should be continued to support these patients and alternative causes of suboptimal platelet increment should be sought.
Around one third of patients had platelet serology testing performed. Concurring with the findings of a recent registry-based study (8), we showed that 1-hour CCIs after HLA-selected platelet transfusions were significantly higher in patients with anti-HLA antibodies than those who were antibody negative. Our data also suggested that female patients had better CCI response to HLA-selected platelet transfusions than male patients. This is likely due to the higher incidence of HLA alloimmunization as the cause of PTR in the former. Recent studies reported that PTR occurred in 19% to 27% of multiply transfused patients, and 19% to 39% of Chinese patients requiring repeated platelet transfusions developed alloimmunization to HLA class I antigens (3,9-12). PTR due to HLA alloimmunization is thus an important clinical problem in patients with haematological disorders. Our findings consolidated the role of transfusing HLA-selected platelets to overcome PTR in these patients.
It is well known that ABO identical platelet transfusion would result in a modestly better platelet increment (8,13). However, an association between ABO compatibility and 1-hour CCIs after transfusion of HLA-selected platelets could not be found in this study. It has been reported previously that ABO compatibility might not affect platelet increment if the unit is crossmatch compatible (10). The impact of ABO compatibility in the setting of HLA-selected platelet transfusion warrants further study.
Although the platelet increment was satisfactory in most patients in the cohort, some patients did not respond to HLA-selected platelet transfusions. Among the patients who had platelet serology testing performed, only one demonstrated alloantibody against HPA-2b antigen. Because of the smaller genetic variability of the HPA system, its frequency of alloimmunization is less than that of the HLA system (14). Of note, CD36 deficiency, which is also implicated in PTR, is relatively common in Chinese population (15). This ought to be considered when platelet increment did not improve with HLA-selected platelets. HLA-C antigen alloimmunization is also known to be implicated in immune PTR (16), but BTS did not routinely provide HLA-C selected platelets to patients.
We identified several issues regarding the current HLA-selected platelet service. First, not all the requests for HLA-selected platelets were justified. As previously discussed, PTR was not established in a significant minority of patients and our data showed that these patients did not benefit from HLA-selected platelet transfusions. Also, the proportion of patients with platelet serology testing was low. This could be partly attributed to the limited access of the tests, which were only restricted to patients who did not respond to HLA-selected platelets in some centres. Another possible reason was a lack of awareness to the investigations for PTR. It is important to differentiate whether PTR is immune related owing to its impact on clinical management. Moreover, requests for HLA-selected platelets should be withdrawn by clinicians if anti-HLA antibodies could not be demonstrated. When patients did not respond to HLA- and/or HPA-selected platelets, they should be supported by random donor platelets. Local guidelines regarding the workup and management of PTR should be developed. The current practice could also be improved by education, audit and a feedback system.
During the study period, BTS issued around 440,000 adult doses of platelets and among them 54,000 units were prepared by apheresis method. HLA-selected platelets, which were the most commonly used products to support patients with PTR locally, only constituted a small proportion of the total platelet supply. To increase the availability of products to support alloimmunized patients, crossmatch compatible platelets or antigen negative units could be considered in these patients (17). Recently, platelet products selected by eplet-based approach were shown to be non-inferior to standard antigen selected method. This approach might further benefit highly sensitized patients by identifying more units suitable for transfusions (18). The cost-effectiveness of different approaches to support PTR patients warrants further investigations.
Certain limitations exist in this study. First, random donor platelets were shelved and issued on a first-in-first-out basis whilst HLA-selected platelets were usually fresher. This introduced a confounder in the comparison of platelet increments. Second, only a minority of patients had platelet antibody tests performed. Also, the method of antibody analysis was not the same in different centres. These factors could bias our analysis of how antibodies affect platelet increment. Moreover, the strength of anti-HLA and anti-HPA antibodies was not tested. It has been reported that weak anti-HLA antibody levels were not associated with PTR (19), that whether HLA-selected platelets benefit these patients remain to be elucidated.
Conclusions
In conclusion, HLA-selected platelets provided by the BTS in the past ten years were almost exclusively “4 out of 4” matched, which resulted in satisfactory 1-hour CCIs after most transfusions. With the continuous expansion of the unrelated bone marrow donor registry, the chance of successful matching and availability of HLA-selected platelets would increase further. To improve the clinical care of patients with PTR and ensure effective utilization of resources, it is important to promote thorough workup and judicious request for HLA-selected platelets. Considering products other than HLA-selected platelets might benefit more patients with alloimmune PTR.
Acknowledgments
The authors would like to thank all blood donors who made their generous platelet donation. They would also like to acknowledge the staff who involved in blood donations, handled the requests, and processed the collected apheresis platelets.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://aob.amegroups.com/article/view/10.21037/aob-22-11/rc
Data Sharing Statement: Available at https://aob.amegroups.com/article/view/10.21037/aob-22-11/dss
Peer Review File: Available at https://aob.amegroups.com/article/view/10.21037/aob-22-11/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aob.amegroups.com/article/view/10.21037/aob-22-11/coif). CKL serves as an unpaid editorial board member of Annals of Blood from July 2022 to June 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Local ethics committee approval was not sought since the present study was a retrospective quality improvement project using anonymized registry data.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Forest SK, Hod EA. Management of the Platelet Refractory Patient. Hematol Oncol Clin North Am 2016;30:665-77. [Crossref] [PubMed]
- Stanworth SJ, Navarrete C, Estcourt L, et al. Platelet refractoriness – practical approaches and ongoing dilemmas in patient management. Br J Haematol 2015;171:297-305. [Crossref] [PubMed]
- Kickler T, Kennedy SD, Braine HG. Alloimmunization to platelet-specific antigens on glycoproteins IIb- IIIa and Ib/IX in multiply transfused thrombocytopenic patients. Transfusion 1990;30:622-5. [Crossref] [PubMed]
- Doughty HA, Murphy MF, Metcalfe P, et al. Relative importance of immune and non-immune causes of platelet refractoriness. Vox Sang 1994;66:200-5. [Crossref] [PubMed]
- Freedman J, Gafni A, Garvey MB, et al. A cost-effectiveness evaluation of platelet crossmatching and HLA matching in the management of alloimmunized thrombocytopenic patients. Transfusion 1989;29:201-7. [Crossref] [PubMed]
- Delaflor-Weiss E, Mintz PD. The evaluation and management of platelet refractoriness and alloimmunization. Transfus Med Rev 2000;14:180-96. [Crossref] [PubMed]
- Nahirniak S, Slichter SJ, Tanael S, et al. Guidance on Platelet Transfusion for Patients With Hypoproliferative Thrombocytopenia. Transfus Med Rev 2015;29:3-13. [Crossref] [PubMed]
- Kreuger AL, Mäkelburg ABU, Somers JAE, et al. HLA-matched platelet transfusions are effective only in refractory patients with positive HLA antibody screening. Transfusion 2019;59:3303-7. [Crossref] [PubMed]
- Zhu H, He J, Cai J, et al. Pre-existing anti-HLA antibodies negatively impact survival of pediatric aplastic anemia patients undergoing HSCT. Clin Transplant 2014;28:1225-33. [Crossref] [PubMed]
- Chow MP, Yung CF, Hu HY, et al. HLA antibodies - the cause of platelet alloimmunization in Chinese. Am J Hematol 1992;39:15-9. [Crossref] [PubMed]
- Song T, Zhang Y, Huang J, et al. Transfusion-induced platelet antibodies and regulatory T cells in multiply transfused patients. J Clin Lab Anal 2021;35:e23864. [Crossref] [PubMed]
- Slichter SJ, Davis K, Enright H, et al. Factors affecting posttransfusion platelet increments, platelet refractoriness, and platelet transfusion intervals in thrombocytopenic patients. Blood 2005;105:4106-14. [Crossref] [PubMed]
- Shehata N, Tinmouth A, Naglie G, et al. ABO-identical versus nonidentical platelet transfusion: a systematic review. Transfusion 2009;49:2442-53. [Crossref] [PubMed]
- Curtis BR, McFarland JG. Human platelet antigens – 2013. Vox Sang 2014;106:93-102. [Crossref] [PubMed]
- Wu G, Zhou Y, Li L, et al. Platelet Immunology in China: Research and Clinical Applications. Transfus Med Rev 2017;31:118-25. [Crossref] [PubMed]
- Saito S, Ota S, Seshimo H, et al. Platelet transfusion refractoriness caused by a mismatch in HLA-C antigens. Transfusion 2002;42:302-8. [Crossref] [PubMed]
- Cohn CS. Platelet transfusion refractoriness: how do I diagnose and manage? Hematology Am Soc Hematol Educ Program 2020;2020:527-32. [Crossref] [PubMed]
- Marsh JC, Stanworth SJ, Pankhurst LA, et al. An epitope-based approach of HLA-matched platelets for transfusion: a noninferiority crossover randomized trial. Blood 2021;137:310-22. [Crossref] [PubMed]
- Jackman RP, Deng X, Bolgiano D, et al. Low-level HLA antibodies do not predict platelet transfusion failure in TRAP study participants. Blood 2013;121:3261-6; quiz 3299. [Crossref] [PubMed]
Cite this article as: Chan LKL, Tam KWK, Wong CK, Leung JNS, Kwok JSY, Tsoi WC, Lee CK. Provision of human leukocyte antigen (HLA) selected platelets: 10-year experience of a regional blood centre. Ann Blood 2023;8:22.


